

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DNA polymerase III PolC-type (Bacillus subtilis) | BDBM21688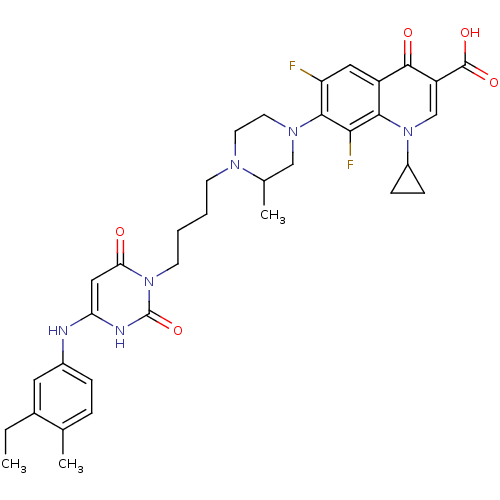 (1-cyclopropyl-7-[4-(4-{4-[(3-ethyl-4-methylphenyl)...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 19 | -10.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Microbiotix | Assay Description Reaction velocities were determined at each dGTP concentration and used to create double reciprocal plots of velocity versus dGTP concentration. The ... | Antimicrob Agents Chemother 51: 119-27 (2007) Article DOI: 10.1128/AAC.01311-05 BindingDB Entry DOI: 10.7270/Q2988599 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8503 ((2R)-2-[(4-fluoro-3-methylbenzene)sulfonamido]-N-h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc. Curated by ChEMBL | Assay Description Competitive inhibition of Bacillus anthracis recombinant lethal factor expressed in Escherichia coli by linear double-reciprocal plot analysis | Bioorg Med Chem 22: 419-34 (2013) Article DOI: 10.1016/j.bmc.2013.11.009 BindingDB Entry DOI: 10.7270/Q2QV3NZ9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| DNA polymerase III PolC-type (Bacillus subtilis) | BDBM21686 (3-(4-hydroxybutyl)-6-(3-ethyl-4-methylanilino)urac...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 63 | -9.98 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Microbiotix | Assay Description Reaction velocities were determined at each dGTP concentration and used to create double reciprocal plots of velocity versus dGTP concentration. The ... | Antimicrob Agents Chemother 51: 119-27 (2007) Article DOI: 10.1128/AAC.01311-05 BindingDB Entry DOI: 10.7270/Q2988599 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM23274 ((2E)-3-(2,4-dichlorophenyl)-N-hydroxyprop-2-enamid...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix Inc Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum toxin BoNT/A light chain | J Med Chem 53: 2264-76 (2010) Article DOI: 10.1021/jm901852f BindingDB Entry DOI: 10.7270/Q2HQ40V7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50242333 ((S)-2-{(S)-2-[(S)-6-Amino-2-((2S,3R)-2-{(S)-2-[(S)...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc. Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum botulinum neurotoxin type A light chain | Bioorg Med Chem 19: 7338-48 (2011) Article DOI: 10.1016/j.bmc.2011.10.062 BindingDB Entry DOI: 10.7270/Q2FF3SS3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50444728 (CHEMBL3098990) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 478 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc. Curated by ChEMBL | Assay Description Non-competitive inhibition of Bacillus anthracis lethal factor using 3.12 uM MCA-KKVYPYPME[dnp]K amide as substrate after 30 mins by Eadie-Hofstee pl... | Bioorg Med Chem 22: 419-34 (2013) Article DOI: 10.1016/j.bmc.2013.11.009 BindingDB Entry DOI: 10.7270/Q2QV3NZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50444748 (Aminoquinuride) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc. Curated by ChEMBL | Assay Description Competitive inhibition of Bacillus anthracis lethal factor | Bioorg Med Chem 22: 419-34 (2013) Article DOI: 10.1016/j.bmc.2013.11.009 BindingDB Entry DOI: 10.7270/Q2QV3NZ9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50444767 (CHEMBL3099016) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc. Curated by ChEMBL | Assay Description Inhibition of Bacillus anthracis lethal factor by fluorescence assay | Bioorg Med Chem 22: 419-34 (2013) Article DOI: 10.1016/j.bmc.2013.11.009 BindingDB Entry DOI: 10.7270/Q2QV3NZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50308031 (CHEMBL591192 | N-(7-(7-aminoheptylamino)heptyl)-5-...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix Inc Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum toxin BoNT/A light chain | J Med Chem 53: 2264-76 (2010) Article DOI: 10.1021/jm901852f BindingDB Entry DOI: 10.7270/Q2HQ40V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50444770 (CHEMBL3099017) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc. Curated by ChEMBL | Assay Description Inhibition of Bacillus anthracis lethal factor | Bioorg Med Chem 22: 419-34 (2013) Article DOI: 10.1016/j.bmc.2013.11.009 BindingDB Entry DOI: 10.7270/Q2QV3NZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50444735 (CHEMBL3099031) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc. Curated by ChEMBL | Assay Description Non-competitive inhibition of Bacillus anthracis lethal factor assessed as MCA-KKVYPYPME[dnp]K amide cleavage after 30 mins by Eadie-Hofstee plot ana... | Bioorg Med Chem 22: 419-34 (2013) Article DOI: 10.1016/j.bmc.2013.11.009 BindingDB Entry DOI: 10.7270/Q2QV3NZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50444735 (CHEMBL3099031) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc. Curated by ChEMBL | Assay Description Non-competitive inhibition of Bacillus anthracis lethal factor using 3.12 uM MCA-KKVYPYPME[dnp]K amide as substrate after 30 mins by Eadie-Hofstee pl... | Bioorg Med Chem 22: 419-34 (2013) Article DOI: 10.1016/j.bmc.2013.11.009 BindingDB Entry DOI: 10.7270/Q2QV3NZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50444728 (CHEMBL3098990) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc. Curated by ChEMBL | Assay Description Non-competitive inhibition of Bacillus anthracis lethal factor assessed as MCA-KKVYPYPME[dnp]K amide cleavage after 30 mins by Eadie-Hofstee plot ana... | Bioorg Med Chem 22: 419-34 (2013) Article DOI: 10.1016/j.bmc.2013.11.009 BindingDB Entry DOI: 10.7270/Q2QV3NZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase III subunit alpha (Bacillus subtilis) | BDBM21686 (3-(4-hydroxybutyl)-6-(3-ethyl-4-methylanilino)urac...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.17E+5 | -5.45 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Microbiotix | Assay Description Reaction velocities were determined at each dGTP concentration and used to create double reciprocal plots of velocity versus dGTP concentration. The ... | Antimicrob Agents Chemother 51: 119-27 (2007) Article DOI: 10.1128/AAC.01311-05 BindingDB Entry DOI: 10.7270/Q2988599 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha subunit B (Bos taurus (bovine)) | BDBM21688 (1-cyclopropyl-7-[4-(4-{4-[(3-ethyl-4-methylphenyl)...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >4.82E+5 | >-4.70 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Microbiotix | Assay Description Reaction velocities were determined at each dGTP concentration and used to create double reciprocal plots of velocity versus dGTP concentration. The ... | Antimicrob Agents Chemother 51: 119-27 (2007) Article DOI: 10.1128/AAC.01311-05 BindingDB Entry DOI: 10.7270/Q2988599 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase III subunit alpha (Escherichia coli) | BDBM21688 (1-cyclopropyl-7-[4-(4-{4-[(3-ethyl-4-methylphenyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >4.82E+5 | >-4.60 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Microbiotix | Assay Description Reaction velocities were determined at each dGTP concentration and used to create double reciprocal plots of velocity versus dGTP concentration. The ... | Antimicrob Agents Chemother 51: 119-27 (2007) Article DOI: 10.1128/AAC.01311-05 BindingDB Entry DOI: 10.7270/Q2988599 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase III subunit alpha (Bacillus subtilis) | BDBM21688 (1-cyclopropyl-7-[4-(4-{4-[(3-ethyl-4-methylphenyl)...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >4.82E+5 | >-4.60 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Microbiotix | Assay Description Reaction velocities were determined at each dGTP concentration and used to create double reciprocal plots of velocity versus dGTP concentration. The ... | Antimicrob Agents Chemother 51: 119-27 (2007) Article DOI: 10.1128/AAC.01311-05 BindingDB Entry DOI: 10.7270/Q2988599 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase subunit gamma-1 (Homo sapiens (Human)) | BDBM21688 (1-cyclopropyl-7-[4-(4-{4-[(3-ethyl-4-methylphenyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.84E+5 | -4.49 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Microbiotix | Assay Description Reaction velocities were determined at each dGTP concentration and used to create double reciprocal plots of velocity versus dGTP concentration. The ... | Antimicrob Agents Chemother 51: 119-27 (2007) Article DOI: 10.1128/AAC.01311-05 BindingDB Entry DOI: 10.7270/Q2988599 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase subunit gamma-1 (Homo sapiens (Human)) | BDBM21686 (3-(4-hydroxybutyl)-6-(3-ethyl-4-methylanilino)urac...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.01E+6 | >-4.25 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Microbiotix | Assay Description Reaction velocities were determined at each dGTP concentration and used to create double reciprocal plots of velocity versus dGTP concentration. The ... | Antimicrob Agents Chemother 51: 119-27 (2007) Article DOI: 10.1128/AAC.01311-05 BindingDB Entry DOI: 10.7270/Q2988599 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha subunit B (Bos taurus (bovine)) | BDBM21686 (3-(4-hydroxybutyl)-6-(3-ethyl-4-methylanilino)urac...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.01E+6 | >-4.25 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Microbiotix | Assay Description Reaction velocities were determined at each dGTP concentration and used to create double reciprocal plots of velocity versus dGTP concentration. The ... | Antimicrob Agents Chemother 51: 119-27 (2007) Article DOI: 10.1128/AAC.01311-05 BindingDB Entry DOI: 10.7270/Q2988599 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase III subunit alpha (Escherichia coli) | BDBM21686 (3-(4-hydroxybutyl)-6-(3-ethyl-4-methylanilino)urac...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.01E+6 | >-4.15 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Microbiotix | Assay Description Reaction velocities were determined at each dGTP concentration and used to create double reciprocal plots of velocity versus dGTP concentration. The ... | Antimicrob Agents Chemother 51: 119-27 (2007) Article DOI: 10.1128/AAC.01311-05 BindingDB Entry DOI: 10.7270/Q2988599 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein (REBOV) | BDBM574780 (US11459308, Compound 3729) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Recombinant vesicular stomatitis viruses (VSVs) (serotype Indiana) expressing eGFP and EBOV, SUDV, or BDBV GP in place of VSV G, as well as those exp... | Citation and Details BindingDB Entry DOI: 10.7270/Q28G8PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein (REBOV) | BDBM574776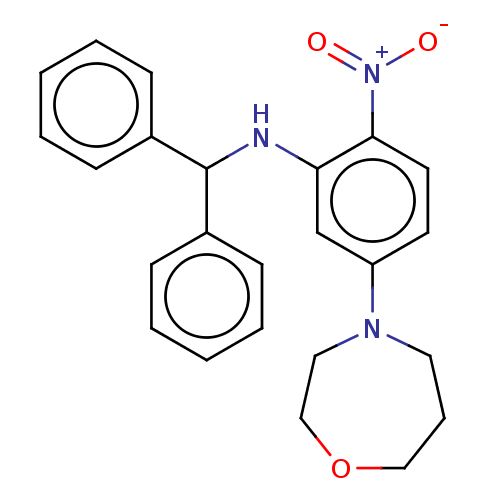 (US11459308, Compound 3700) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Recombinant vesicular stomatitis viruses (VSVs) (serotype Indiana) expressing eGFP and EBOV, SUDV, or BDBV GP in place of VSV G, as well as those exp... | Citation and Details BindingDB Entry DOI: 10.7270/Q28G8PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein (REBOV) | BDBM574777 (US11459308, Compound 3748) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Recombinant vesicular stomatitis viruses (VSVs) (serotype Indiana) expressing eGFP and EBOV, SUDV, or BDBV GP in place of VSV G, as well as those exp... | Citation and Details BindingDB Entry DOI: 10.7270/Q28G8PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein (REBOV) | BDBM574786 (US11459308, Compound 4336) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Recombinant vesicular stomatitis viruses (VSVs) (serotype Indiana) expressing eGFP and EBOV, SUDV, or BDBV GP in place of VSV G, as well as those exp... | Citation and Details BindingDB Entry DOI: 10.7270/Q28G8PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM23274 ((2E)-3-(2,4-dichlorophenyl)-N-hydroxyprop-2-enamid...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix Inc Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum toxin BoNT/A light chain | J Med Chem 53: 2264-76 (2010) Article DOI: 10.1021/jm901852f BindingDB Entry DOI: 10.7270/Q2HQ40V7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Envelope glycoprotein (ZEBOV) | BDBM574785 (US11459308, Compound 4335) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The authentic filoviruses Ebola virus/H. sapiens-tc/COD/1995/Kikwit-9510621 (EBOV/Kik-9510621; ‘EBOV-Zaire 1995’) (Jahrling et al., J. Infect. Dis., ... | Citation and Details BindingDB Entry DOI: 10.7270/Q28G8PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50308030 (5-chloro-7-((4-ethoxyphenyl)(pyridin-3-ylamino)met...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix Inc Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum toxin BoNT/A light chain | J Med Chem 53: 2264-76 (2010) Article DOI: 10.1021/jm901852f BindingDB Entry DOI: 10.7270/Q2HQ40V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein (ZEBOV) | BDBM574823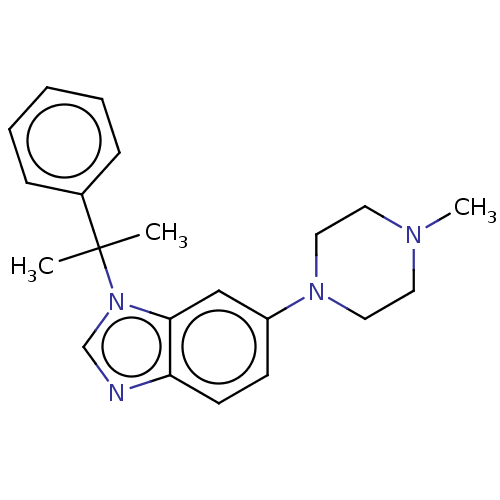 (US11459308, Compound 4212) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The authentic filoviruses Ebola virus/H. sapiens-tc/COD/1995/Kikwit-9510621 (EBOV/Kik-9510621; ‘EBOV-Zaire 1995’) (Jahrling et al., J. Infect. Dis., ... | Citation and Details BindingDB Entry DOI: 10.7270/Q28G8PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein (REBOV) | BDBM574769 (US11459308, Compound 3574) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Recombinant vesicular stomatitis viruses (VSVs) (serotype Indiana) expressing eGFP and EBOV, SUDV, or BDBV GP in place of VSV G, as well as those exp... | Citation and Details BindingDB Entry DOI: 10.7270/Q28G8PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein (ZEBOV) | BDBM574786 (US11459308, Compound 4336) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The authentic filoviruses Ebola virus/H. sapiens-tc/COD/1995/Kikwit-9510621 (EBOV/Kik-9510621; ‘EBOV-Zaire 1995’) (Jahrling et al., J. Infect. Dis., ... | Citation and Details BindingDB Entry DOI: 10.7270/Q28G8PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein (REBOV) | BDBM574778 (US11459308, Compound 3747) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Recombinant vesicular stomatitis viruses (VSVs) (serotype Indiana) expressing eGFP and EBOV, SUDV, or BDBV GP in place of VSV G, as well as those exp... | Citation and Details BindingDB Entry DOI: 10.7270/Q28G8PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein (REBOV) | BDBM574755 (US11459308, Compound 3610) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Recombinant vesicular stomatitis viruses (VSVs) (serotype Indiana) expressing eGFP and EBOV, SUDV, or BDBV GP in place of VSV G, as well as those exp... | Citation and Details BindingDB Entry DOI: 10.7270/Q28G8PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein (ZEBOV) | BDBM574755 (US11459308, Compound 3610) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The authentic filoviruses Ebola virus/H. sapiens-tc/COD/1995/Kikwit-9510621 (EBOV/Kik-9510621; ‘EBOV-Zaire 1995’) (Jahrling et al., J. Infect. Dis., ... | Citation and Details BindingDB Entry DOI: 10.7270/Q28G8PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein (ZEBOV) | BDBM574757 (US11459308, Compound 3612) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The authentic filoviruses Ebola virus/H. sapiens-tc/COD/1995/Kikwit-9510621 (EBOV/Kik-9510621; ‘EBOV-Zaire 1995’) (Jahrling et al., J. Infect. Dis., ... | Citation and Details BindingDB Entry DOI: 10.7270/Q28G8PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein (ZEBOV) | BDBM574777 (US11459308, Compound 3748) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The authentic filoviruses Ebola virus/H. sapiens-tc/COD/1995/Kikwit-9510621 (EBOV/Kik-9510621; ‘EBOV-Zaire 1995’) (Jahrling et al., J. Infect. Dis., ... | Citation and Details BindingDB Entry DOI: 10.7270/Q28G8PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein (REBOV) | BDBM574783 (US11459308, Compound 3749) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Recombinant vesicular stomatitis viruses (VSVs) (serotype Indiana) expressing eGFP and EBOV, SUDV, or BDBV GP in place of VSV G, as well as those exp... | Citation and Details BindingDB Entry DOI: 10.7270/Q28G8PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicative DNA helicase (Staphylococcus aureus) | BDBM50295892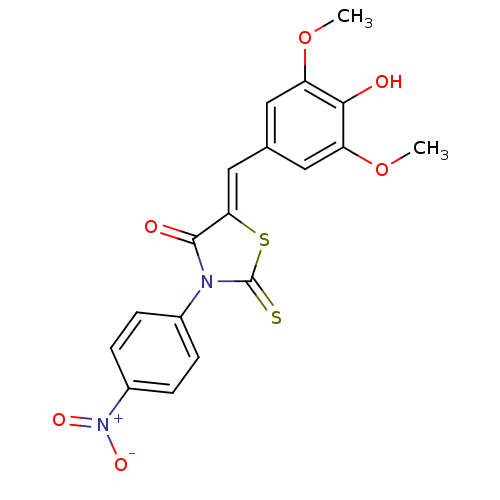 ((Z)-5-(4-hydroxy-3,5-dimethoxybenzylidene)-3-(4-ni...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus Smith DNA helicase DnaC assessed as strand unwinding after 30 mins by FRET assay | Bioorg Med Chem 17: 4466-76 (2009) Article DOI: 10.1016/j.bmc.2009.05.014 BindingDB Entry DOI: 10.7270/Q22F7NGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50444728 (CHEMBL3098990) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 729 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc. Curated by ChEMBL | Assay Description Non-competitive inhibition of Bacillus anthracis lethal factor using 6.25 uM MCA-KKVYPYPME[dnp]K amide as substrate after 30 mins by Eadie-Hofstee pl... | Bioorg Med Chem 22: 419-34 (2013) Article DOI: 10.1016/j.bmc.2013.11.009 BindingDB Entry DOI: 10.7270/Q2QV3NZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein (ZEBOV) | BDBM574754 (US11459308, Compound 3588) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The authentic filoviruses Ebola virus/H. sapiens-tc/COD/1995/Kikwit-9510621 (EBOV/Kik-9510621; ‘EBOV-Zaire 1995’) (Jahrling et al., J. Infect. Dis., ... | Citation and Details BindingDB Entry DOI: 10.7270/Q28G8PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein (ZEBOV) | BDBM574751 (US11459308, Compound 3625) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The authentic filoviruses Ebola virus/H. sapiens-tc/COD/1995/Kikwit-9510621 (EBOV/Kik-9510621; ‘EBOV-Zaire 1995’) (Jahrling et al., J. Infect. Dis., ... | Citation and Details BindingDB Entry DOI: 10.7270/Q28G8PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein (REBOV) | BDBM574785 (US11459308, Compound 4335) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Recombinant vesicular stomatitis viruses (VSVs) (serotype Indiana) expressing eGFP and EBOV, SUDV, or BDBV GP in place of VSV G, as well as those exp... | Citation and Details BindingDB Entry DOI: 10.7270/Q28G8PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein (REBOV) | BDBM574795 (US11459308, Compound 3540) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Recombinant vesicular stomatitis viruses (VSVs) (serotype Indiana) expressing eGFP and EBOV, SUDV, or BDBV GP in place of VSV G, as well as those exp... | Citation and Details BindingDB Entry DOI: 10.7270/Q28G8PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein (REBOV) | BDBM574757 (US11459308, Compound 3612) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Recombinant vesicular stomatitis viruses (VSVs) (serotype Indiana) expressing eGFP and EBOV, SUDV, or BDBV GP in place of VSV G, as well as those exp... | Citation and Details BindingDB Entry DOI: 10.7270/Q28G8PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein (ZEBOV) | BDBM574784 (US11459308, Compound 4329 | US11459308, Compound 4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The authentic filoviruses Ebola virus/H. sapiens-tc/COD/1995/Kikwit-9510621 (EBOV/Kik-9510621; ‘EBOV-Zaire 1995’) (Jahrling et al., J. Infect. Dis., ... | Citation and Details BindingDB Entry DOI: 10.7270/Q28G8PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein (REBOV) | BDBM574752 (US11459308, Compound 3589) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Recombinant vesicular stomatitis viruses (VSVs) (serotype Indiana) expressing eGFP and EBOV, SUDV, or BDBV GP in place of VSV G, as well as those exp... | Citation and Details BindingDB Entry DOI: 10.7270/Q28G8PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50444728 (CHEMBL3098990) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 841 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc. Curated by ChEMBL | Assay Description Non-competitive inhibition of Bacillus anthracis lethal factor using 12.5 uM MCA-KKVYPYPME[dnp]K amide as substrate after 30 mins by Eadie-Hofstee pl... | Bioorg Med Chem 22: 419-34 (2013) Article DOI: 10.1016/j.bmc.2013.11.009 BindingDB Entry DOI: 10.7270/Q2QV3NZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein (REBOV) | BDBM574807 (US11459308, Compound 3643) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Recombinant vesicular stomatitis viruses (VSVs) (serotype Indiana) expressing eGFP and EBOV, SUDV, or BDBV GP in place of VSV G, as well as those exp... | Citation and Details BindingDB Entry DOI: 10.7270/Q28G8PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein (REBOV) | BDBM574751 (US11459308, Compound 3625) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Recombinant vesicular stomatitis viruses (VSVs) (serotype Indiana) expressing eGFP and EBOV, SUDV, or BDBV GP in place of VSV G, as well as those exp... | Citation and Details BindingDB Entry DOI: 10.7270/Q28G8PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein (ZEBOV) | BDBM574795 (US11459308, Compound 3540) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The authentic filoviruses Ebola virus/H. sapiens-tc/COD/1995/Kikwit-9510621 (EBOV/Kik-9510621; ‘EBOV-Zaire 1995’) (Jahrling et al., J. Infect. Dis., ... | Citation and Details BindingDB Entry DOI: 10.7270/Q28G8PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 492 total ) | Next | Last >> |