

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM602186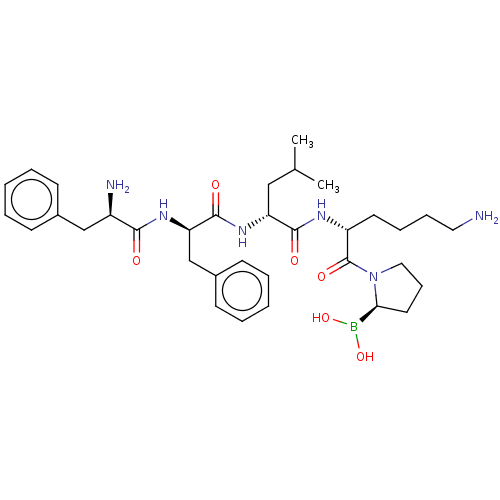 (US11643436, Compound TM-9A) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | n/a | n/a | 0.00100 | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2MC93Z6 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM235813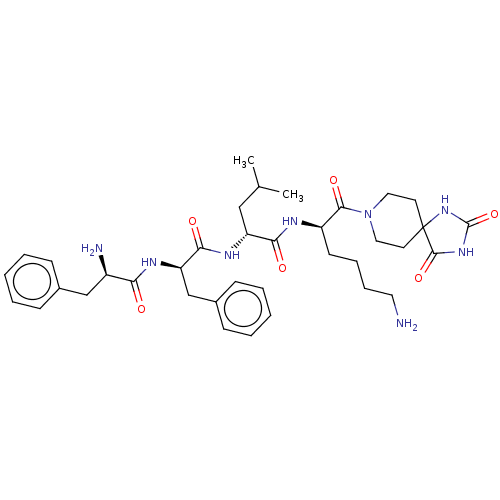 (US9359399, 27) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <0.00100 | n/a | n/a | n/a | 25 |
Cara Therapeutics, Inc. US Patent | Assay Description Mouse R1.G1 cells (ATCC, Manassas, Va.) were grown in suspension in high glucose-DMEM (Dulbecco's Modified Eagle's Medium, Cellgro, Herndon, Va.) con... | US Patent US9359399 (2016) BindingDB Entry DOI: 10.7270/Q23B5Z2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50561764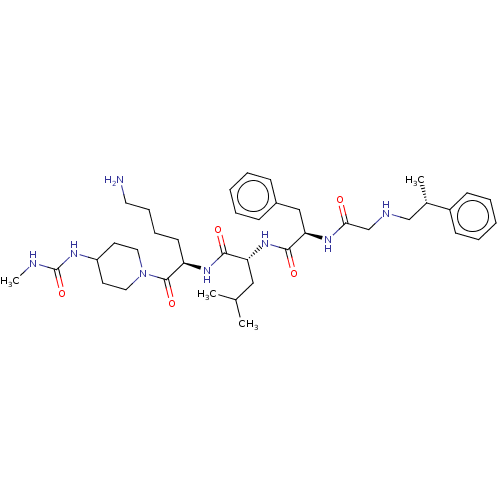 (CHEMBL4743180) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.00100 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human kappa opioid receptor expressed in HEK293 cells assessed as inhibition of forskolin-induced cAMP accumulation measured afte... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00287 BindingDB Entry DOI: 10.7270/Q2J67MN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50561762 (CHEMBL4785966) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.00100 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human kappa opioid receptor expressed in HEK293 cells assessed as inhibition of forskolin-induced cAMP accumulation measured afte... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00287 BindingDB Entry DOI: 10.7270/Q2J67MN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM235811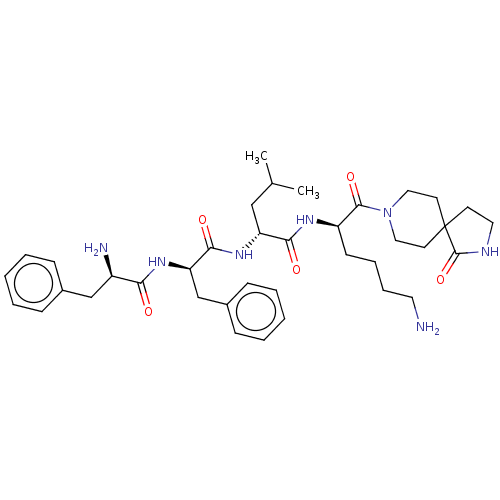 (US9359399, 25) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.00270 | n/a | n/a | n/a | 25 |
Cara Therapeutics, Inc. US Patent | Assay Description Mouse R1.G1 cells (ATCC, Manassas, Va.) were grown in suspension in high glucose-DMEM (Dulbecco's Modified Eagle's Medium, Cellgro, Herndon, Va.) con... | US Patent US9359399 (2016) BindingDB Entry DOI: 10.7270/Q23B5Z2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM393487 (US9963460, 13) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.00280 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description In a [3SS] GTPγS binding test, the opioid receptor agonist activity of test compounds based on a GTP-GDP exchange reaction was evaluated. In the... | Bioorg Med Chem Lett 18: 4232-6 (2008) BindingDB Entry DOI: 10.7270/Q2P55QVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50561760 (CHEMBL4791124) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.00300 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human kappa opioid receptor expressed in HEK293 cells assessed as inhibition of forskolin-induced cAMP accumulation measured afte... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00287 BindingDB Entry DOI: 10.7270/Q2J67MN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM235785 (CVD-0019439 | US9359399, 2) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.00300 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human kappa opioid receptor expressed in HEK293 cells assessed as inhibition of forskolin-induced cAMP accumulation measured afte... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00287 BindingDB Entry DOI: 10.7270/Q2J67MN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM235815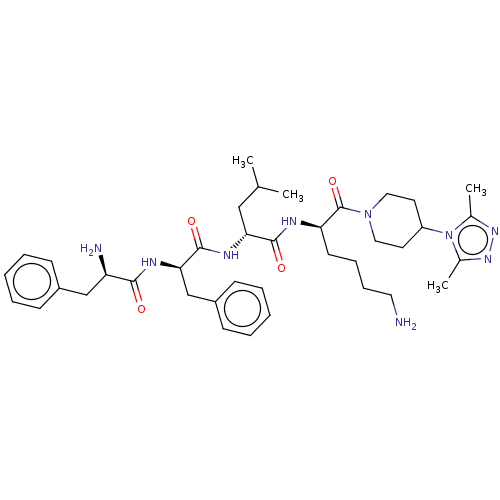 (US9359399, 31) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.00420 | n/a | n/a | n/a | 25 |
Cara Therapeutics, Inc. US Patent | Assay Description Mouse R1.G1 cells (ATCC, Manassas, Va.) were grown in suspension in high glucose-DMEM (Dulbecco's Modified Eagle's Medium, Cellgro, Herndon, Va.) con... | US Patent US9359399 (2016) BindingDB Entry DOI: 10.7270/Q23B5Z2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50269287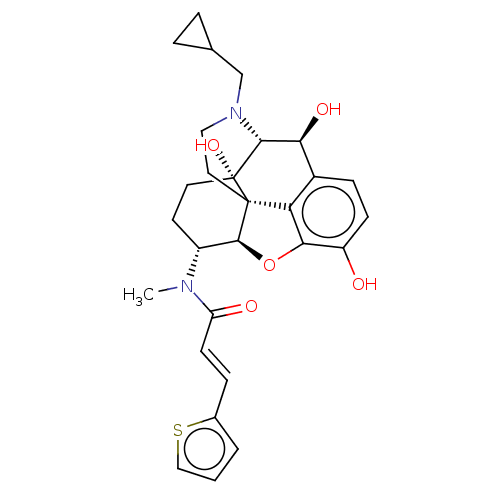 (CHEMBL4087151) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.00466 | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-induced cAMP accumulation by TR-FRET as... | Bioorg Med Chem Lett 27: 3920-3924 (2017) Article DOI: 10.1016/j.bmcl.2017.06.017 BindingDB Entry DOI: 10.7270/Q2ZW1PD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM235816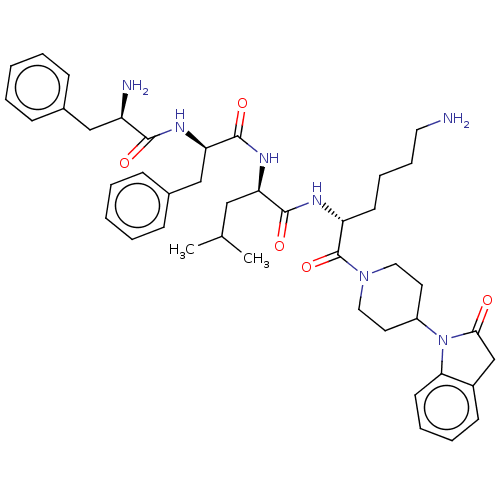 (US9359399, 32) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.00500 | n/a | n/a | n/a | 25 |
Cara Therapeutics, Inc. US Patent | Assay Description Mouse R1.G1 cells (ATCC, Manassas, Va.) were grown in suspension in high glucose-DMEM (Dulbecco's Modified Eagle's Medium, Cellgro, Herndon, Va.) con... | US Patent US9359399 (2016) BindingDB Entry DOI: 10.7270/Q23B5Z2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM235792 (US9359399, 36) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.00520 | n/a | n/a | n/a | 25 |
Cara Therapeutics, Inc. US Patent | Assay Description Mouse R1.G1 cells (ATCC, Manassas, Va.) were grown in suspension in high glucose-DMEM (Dulbecco's Modified Eagle's Medium, Cellgro, Herndon, Va.) con... | US Patent US9359399 (2016) BindingDB Entry DOI: 10.7270/Q23B5Z2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM235813 (US9359399, 27) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.00560 | n/a | n/a | n/a | 25 |
Cara Therapeutics, Inc. US Patent | Assay Description Mouse R1.G1 cells (ATCC, Manassas, Va.) were grown in suspension in high glucose-DMEM (Dulbecco's Modified Eagle's Medium, Cellgro, Herndon, Va.) con... | US Patent US9359399 (2016) BindingDB Entry DOI: 10.7270/Q23B5Z2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50430586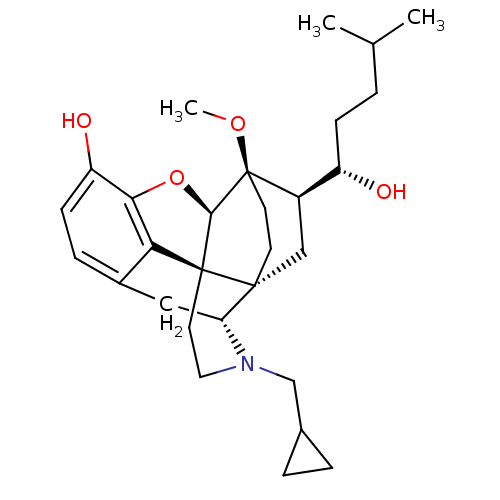 (CHEMBL2338721) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.00610 | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Agonist activity at human KOR expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 56: 3207-16 (2013) Article DOI: 10.1021/jm301543e BindingDB Entry DOI: 10.7270/Q28G8N2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM235818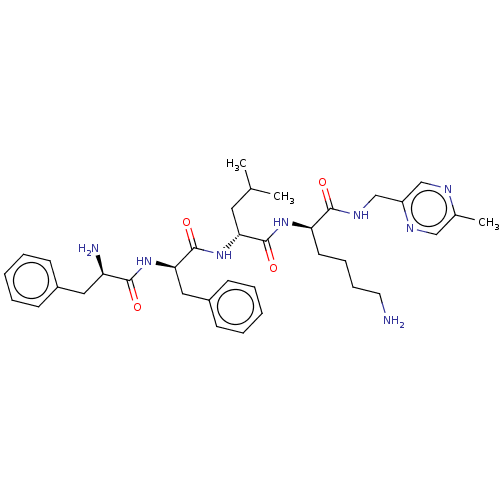 (US9359399, 35) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.00640 | n/a | n/a | n/a | 25 |
Cara Therapeutics, Inc. US Patent | Assay Description Mouse R1.G1 cells (ATCC, Manassas, Va.) were grown in suspension in high glucose-DMEM (Dulbecco's Modified Eagle's Medium, Cellgro, Herndon, Va.) con... | US Patent US9359399 (2016) BindingDB Entry DOI: 10.7270/Q23B5Z2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50430613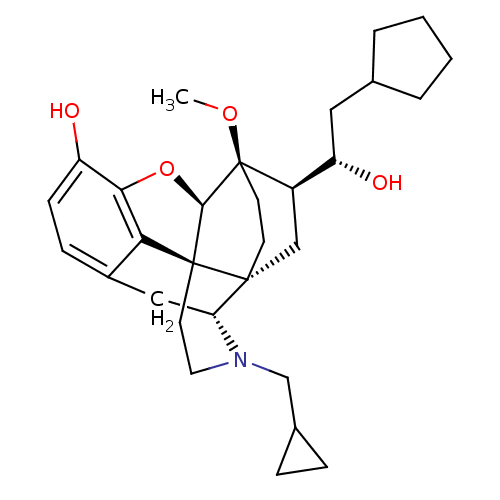 (CHEMBL2338723) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.00700 | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Agonist activity at human KOR expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 56: 3207-16 (2013) Article DOI: 10.1021/jm301543e BindingDB Entry DOI: 10.7270/Q28G8N2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50430612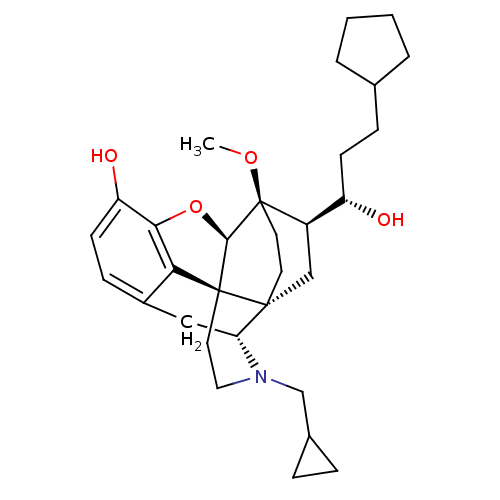 (CHEMBL2338724) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.00770 | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Agonist activity at human KOR expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 56: 3207-16 (2013) Article DOI: 10.1021/jm301543e BindingDB Entry DOI: 10.7270/Q28G8N2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM602189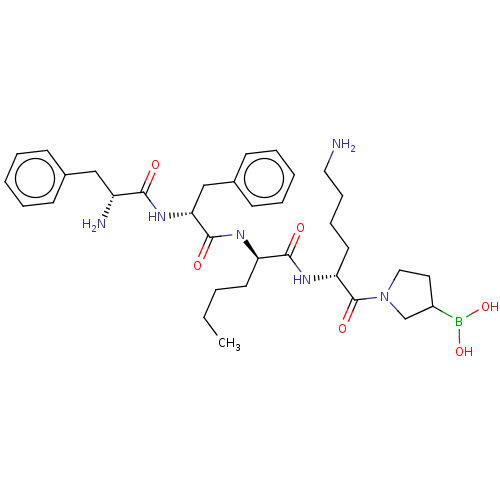 (US11643436, Compound TM-26) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2MC93Z6 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50274347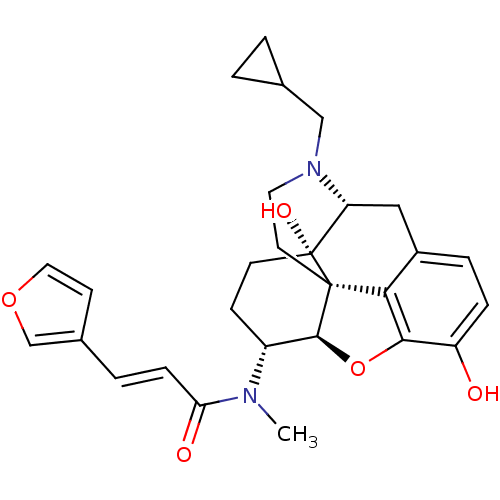 ((2E)-N-[(5R,6R)-17-(cyclopropylmethyl)-4,5-epoxy-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.00820 | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Agonist activity at kappa opioid receptor (unknown origin) | Bioorg Med Chem Lett 27: 3920-3924 (2017) Article DOI: 10.1016/j.bmcl.2017.06.017 BindingDB Entry DOI: 10.7270/Q2ZW1PD9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM235789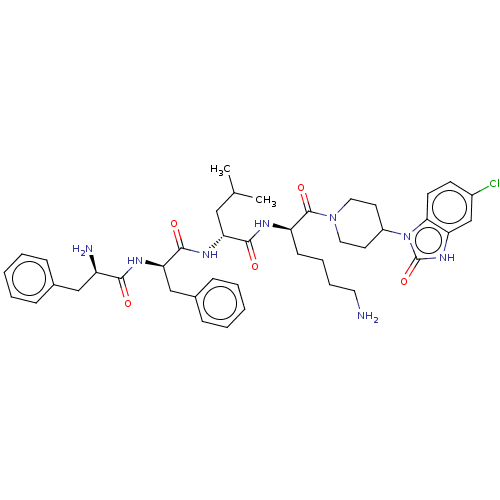 (US9359399, 28) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.00830 | n/a | n/a | n/a | 25 |
Cara Therapeutics, Inc. US Patent | Assay Description Mouse R1.G1 cells (ATCC, Manassas, Va.) were grown in suspension in high glucose-DMEM (Dulbecco's Modified Eagle's Medium, Cellgro, Herndon, Va.) con... | US Patent US9359399 (2016) BindingDB Entry DOI: 10.7270/Q23B5Z2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM235812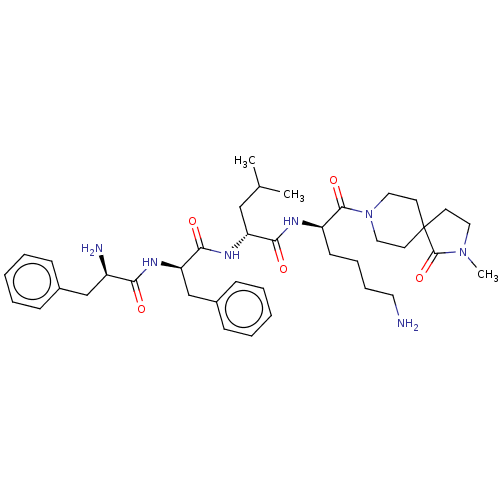 (US9359399, 26) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.00830 | n/a | n/a | n/a | 25 |
Cara Therapeutics, Inc. US Patent | Assay Description Mouse R1.G1 cells (ATCC, Manassas, Va.) were grown in suspension in high glucose-DMEM (Dulbecco's Modified Eagle's Medium, Cellgro, Herndon, Va.) con... | US Patent US9359399 (2016) BindingDB Entry DOI: 10.7270/Q23B5Z2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM602184 (US11643436, Compound TM-7) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | n/a | n/a | 0.00900 | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2MC93Z6 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM602188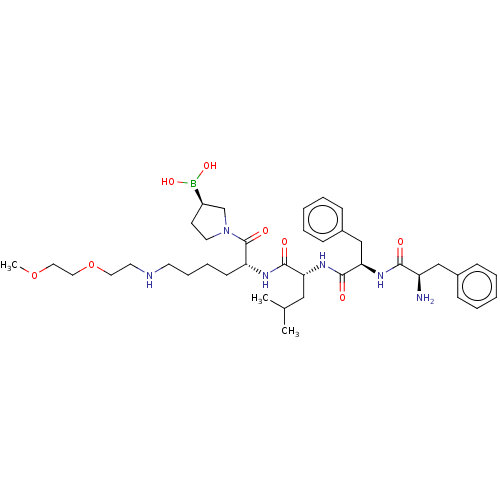 (US11643436, Compound TM-22B) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | n/a | n/a | 0.00900 | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2MC93Z6 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50430611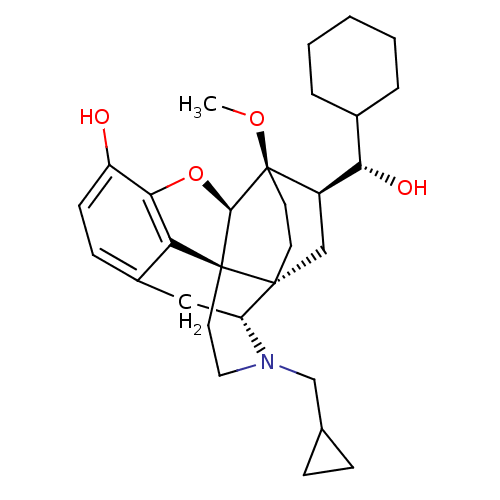 (CHEMBL2338725) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.00980 | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Agonist activity at human KOR expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 56: 3207-16 (2013) Article DOI: 10.1021/jm301543e BindingDB Entry DOI: 10.7270/Q28G8N2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50561763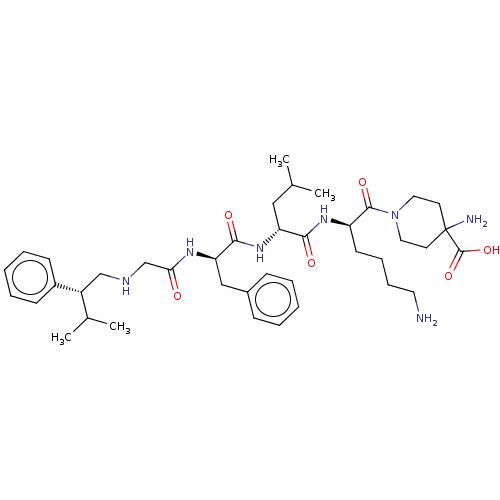 (CHEMBL4756370) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human kappa opioid receptor expressed in HEK293 cells assessed as inhibition of forskolin-induced cAMP accumulation measured afte... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00287 BindingDB Entry DOI: 10.7270/Q2J67MN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50430622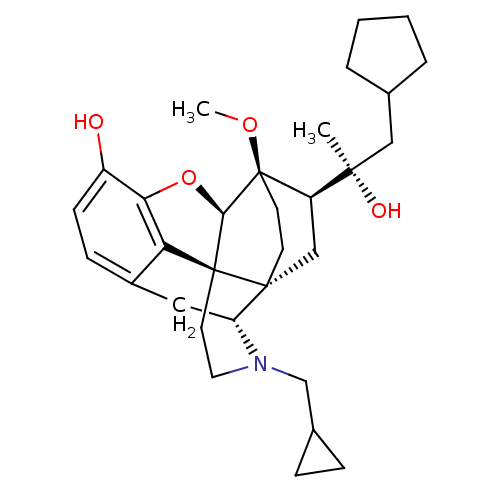 (CHEMBL2338747) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0110 | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Agonist activity at human KOR expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 56: 3207-16 (2013) Article DOI: 10.1021/jm301543e BindingDB Entry DOI: 10.7270/Q28G8N2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM235790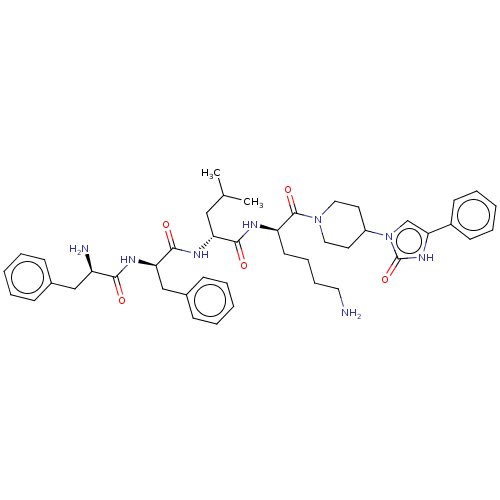 (US9359399, 30) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.0116 | n/a | n/a | n/a | 25 |
Cara Therapeutics, Inc. US Patent | Assay Description Mouse R1.G1 cells (ATCC, Manassas, Va.) were grown in suspension in high glucose-DMEM (Dulbecco's Modified Eagle's Medium, Cellgro, Herndon, Va.) con... | US Patent US9359399 (2016) BindingDB Entry DOI: 10.7270/Q23B5Z2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM235795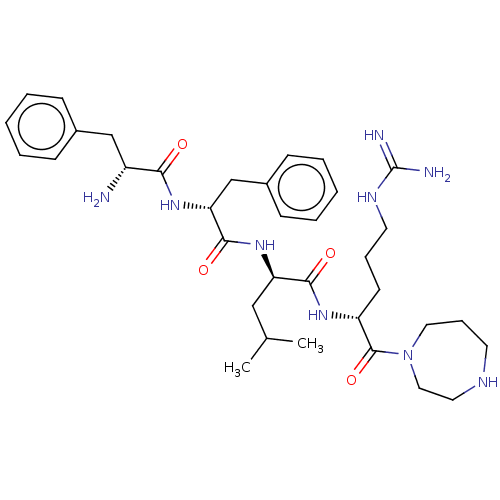 (US9359399, 7) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.0120 | n/a | n/a | n/a | 25 |
Cara Therapeutics, Inc. US Patent | Assay Description Mouse R1.G1 cells (ATCC, Manassas, Va.) were grown in suspension in high glucose-DMEM (Dulbecco's Modified Eagle's Medium, Cellgro, Herndon, Va.) con... | US Patent US9359399 (2016) BindingDB Entry DOI: 10.7270/Q23B5Z2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50430587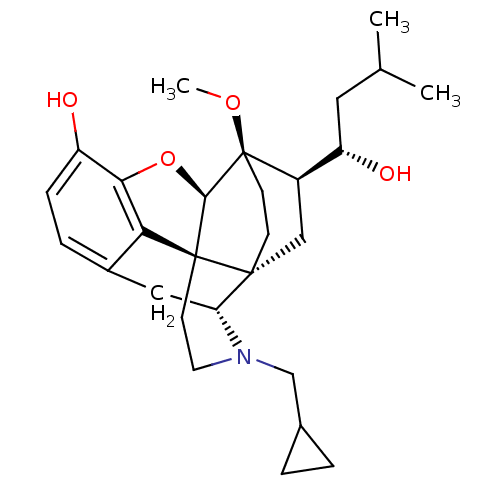 (CHEMBL2338720) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0130 | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Agonist activity at human KOR expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 56: 3207-16 (2013) Article DOI: 10.1021/jm301543e BindingDB Entry DOI: 10.7270/Q28G8N2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM235814 (US9359399, 29) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.0145 | n/a | n/a | n/a | 25 |
Cara Therapeutics, Inc. US Patent | Assay Description Mouse R1.G1 cells (ATCC, Manassas, Va.) were grown in suspension in high glucose-DMEM (Dulbecco's Modified Eagle's Medium, Cellgro, Herndon, Va.) con... | US Patent US9359399 (2016) BindingDB Entry DOI: 10.7270/Q23B5Z2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50159165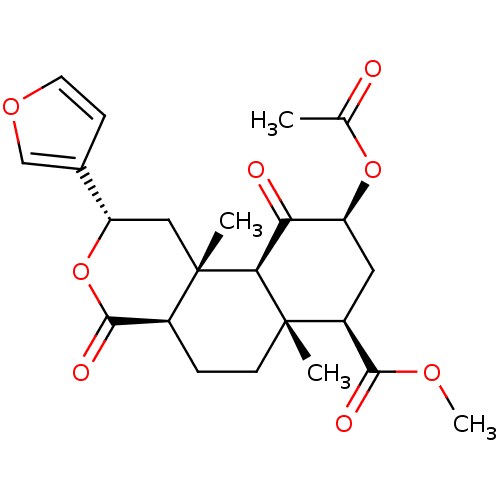 ((2S,4aR,6aR,7R,9S,10aS,10bR)-9-(acetyloxy)-2-(fura...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0150 | n/a | n/a | n/a | n/a |
Universit£t M£nster Curated by ChEMBL | Assay Description Agonist activity at human KOR expressed in HEK293T cells assessed as inhibition of Galphai-mediated cAMP accumulation after 15 mins by microbeta coun... | J Med Chem 62: 893-907 (2019) Article DOI: 10.1021/acs.jmedchem.8b01609 BindingDB Entry DOI: 10.7270/Q2F47SHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50598862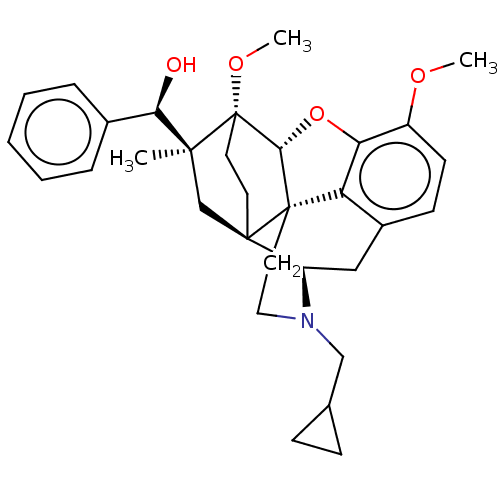 (CHEMBL5178795) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0150 | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00014 BindingDB Entry DOI: 10.7270/Q2QJ7N96 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50561761 (CHEMBL4760663) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0160 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human kappa opioid receptor expressed in HEK293 cells assessed as inhibition of forskolin-induced cAMP accumulation measured afte... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00287 BindingDB Entry DOI: 10.7270/Q2J67MN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50430601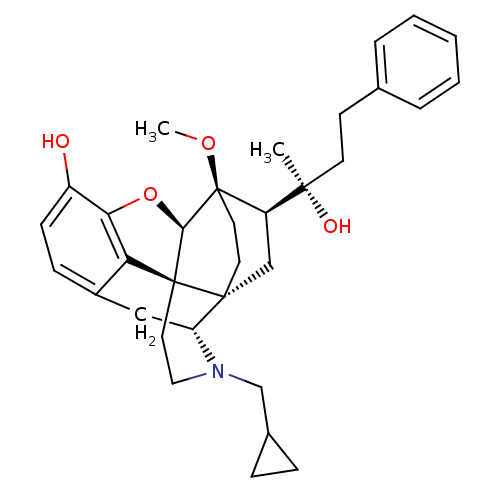 (CHEMBL2338753) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0160 | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Agonist activity at human KOR expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 56: 3207-16 (2013) Article DOI: 10.1021/jm301543e BindingDB Entry DOI: 10.7270/Q28G8N2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM235791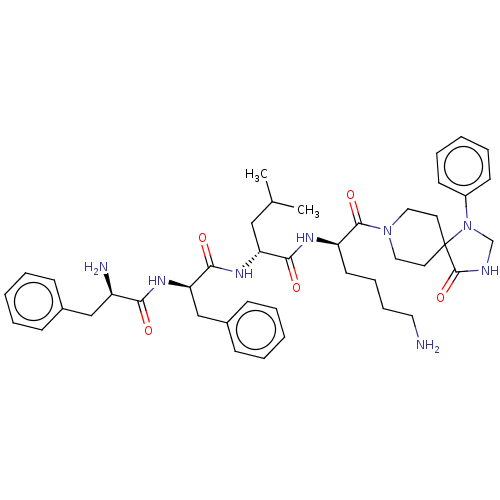 (US9359399, 33) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.0165 | n/a | n/a | n/a | 25 |
Cara Therapeutics, Inc. US Patent | Assay Description Mouse R1.G1 cells (ATCC, Manassas, Va.) were grown in suspension in high glucose-DMEM (Dulbecco's Modified Eagle's Medium, Cellgro, Herndon, Va.) con... | US Patent US9359399 (2016) BindingDB Entry DOI: 10.7270/Q23B5Z2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50430619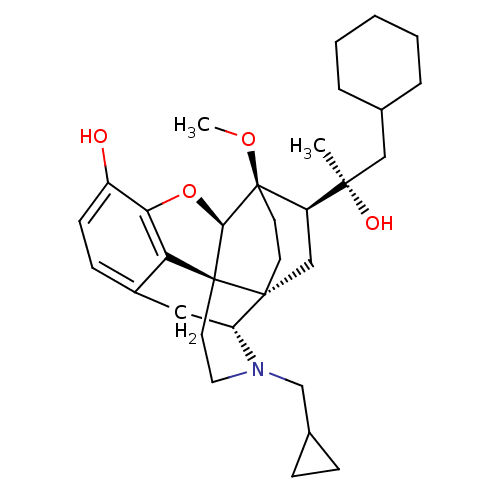 (CHEMBL2338750) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0170 | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Agonist activity at human KOR expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 56: 3207-16 (2013) Article DOI: 10.1021/jm301543e BindingDB Entry DOI: 10.7270/Q28G8N2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50034553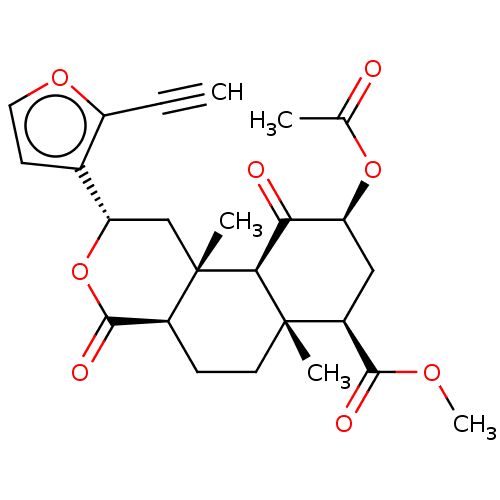 (CHEMBL3359804) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0190 | n/a | n/a | n/a | n/a |
The University of Kansas Curated by ChEMBL | Assay Description Agonist activity at human KOR expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation after 30 mins by luminescence assa... | J Med Chem 57: 10464-75 (2014) Article DOI: 10.1021/jm501521d BindingDB Entry DOI: 10.7270/Q2J38V6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM235812 (US9359399, 26) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.0194 | n/a | n/a | n/a | 25 |
Cara Therapeutics, Inc. US Patent | Assay Description Mouse R1.G1 cells (ATCC, Manassas, Va.) were grown in suspension in high glucose-DMEM (Dulbecco's Modified Eagle's Medium, Cellgro, Herndon, Va.) con... | US Patent US9359399 (2016) BindingDB Entry DOI: 10.7270/Q23B5Z2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50430585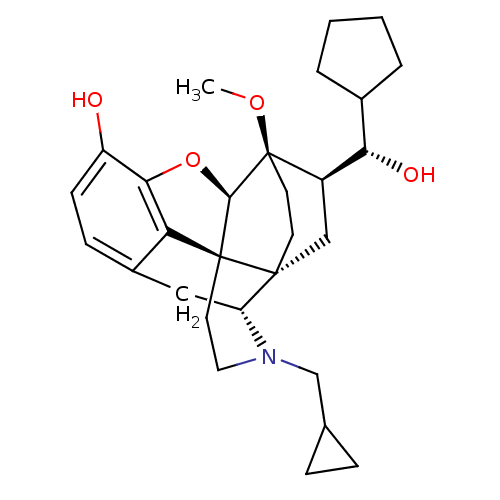 (CHEMBL2338722) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Agonist activity at human KOR expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 56: 3207-16 (2013) Article DOI: 10.1021/jm301543e BindingDB Entry DOI: 10.7270/Q28G8N2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM235817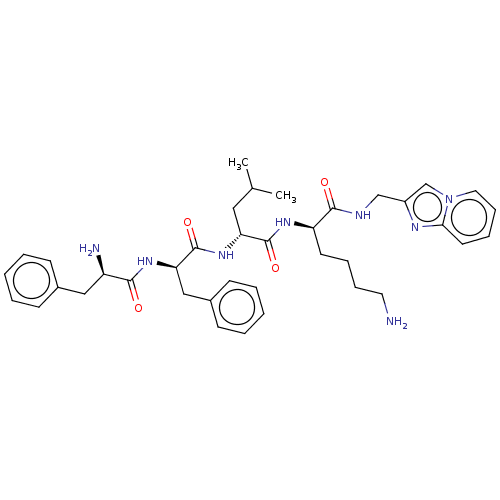 (US9359399, 34) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.0208 | n/a | n/a | n/a | 25 |
Cara Therapeutics, Inc. US Patent | Assay Description Mouse R1.G1 cells (ATCC, Manassas, Va.) were grown in suspension in high glucose-DMEM (Dulbecco's Modified Eagle's Medium, Cellgro, Herndon, Va.) con... | US Patent US9359399 (2016) BindingDB Entry DOI: 10.7270/Q23B5Z2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM235803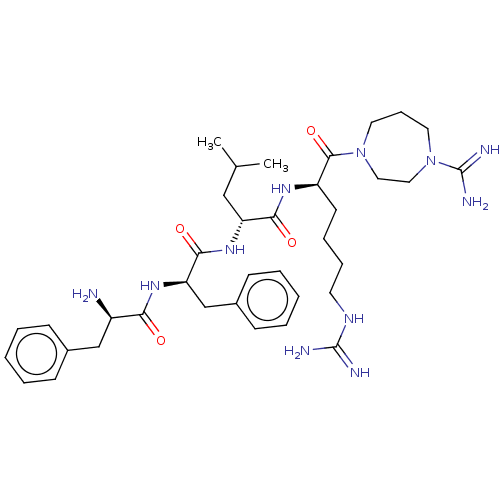 (US9359399, 16) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.0210 | n/a | n/a | n/a | 25 |
Cara Therapeutics, Inc. US Patent | Assay Description Mouse R1.G1 cells (ATCC, Manassas, Va.) were grown in suspension in high glucose-DMEM (Dulbecco's Modified Eagle's Medium, Cellgro, Herndon, Va.) con... | US Patent US9359399 (2016) BindingDB Entry DOI: 10.7270/Q23B5Z2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM235815 (US9359399, 31) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.0216 | n/a | n/a | n/a | 25 |
Cara Therapeutics, Inc. US Patent | Assay Description Mouse R1.G1 cells (ATCC, Manassas, Va.) were grown in suspension in high glucose-DMEM (Dulbecco's Modified Eagle's Medium, Cellgro, Herndon, Va.) con... | US Patent US9359399 (2016) BindingDB Entry DOI: 10.7270/Q23B5Z2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50269282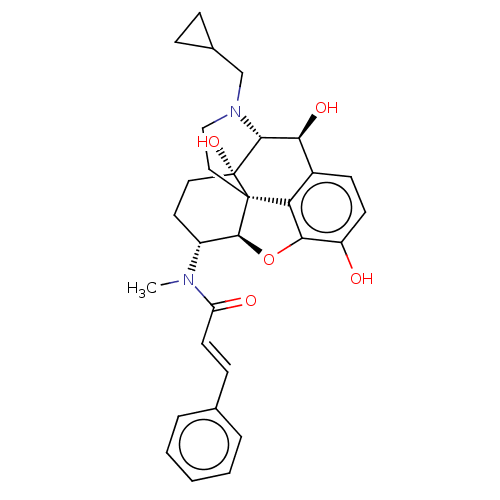 (CHEMBL4082823) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0222 | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-induced cAMP accumulation by TR-FRET as... | Bioorg Med Chem Lett 27: 3920-3924 (2017) Article DOI: 10.1016/j.bmcl.2017.06.017 BindingDB Entry DOI: 10.7270/Q2ZW1PD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM235792 (US9359399, 36) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.0234 | n/a | n/a | n/a | 25 |
Cara Therapeutics, Inc. US Patent | Assay Description Mouse R1.G1 cells (ATCC, Manassas, Va.) were grown in suspension in high glucose-DMEM (Dulbecco's Modified Eagle's Medium, Cellgro, Herndon, Va.) con... | US Patent US9359399 (2016) BindingDB Entry DOI: 10.7270/Q23B5Z2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50269278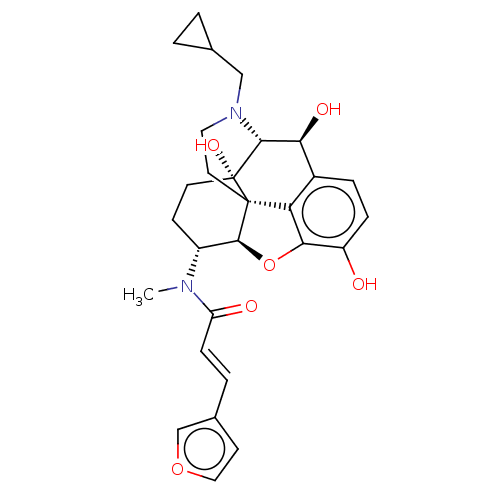 (CHEMBL4103819) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0250 | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-induced cAMP accumulation by TR-FRET as... | Bioorg Med Chem Lett 27: 3920-3924 (2017) Article DOI: 10.1016/j.bmcl.2017.06.017 BindingDB Entry DOI: 10.7270/Q2ZW1PD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50269278 (CHEMBL4103819) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0250 | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor | Bioorg Med Chem Lett 27: 3920-3924 (2017) Article DOI: 10.1016/j.bmcl.2017.06.017 BindingDB Entry DOI: 10.7270/Q2ZW1PD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50325534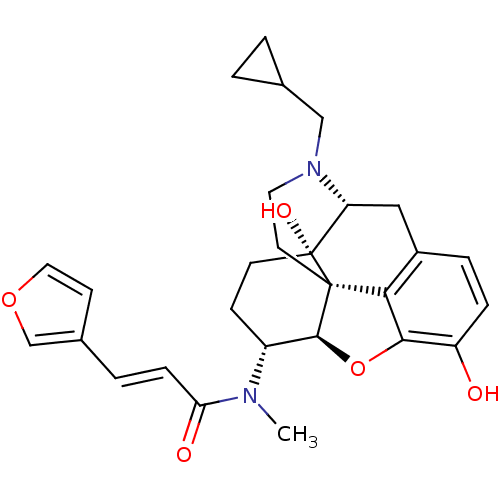 (CHEMBL267495 | nalfurafine) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.0250 | n/a | n/a | n/a | n/a |
McLean Hospital Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | Bioorg Med Chem 16: 1279-86 (2008) Article DOI: 10.1016/j.bmc.2007.10.067 BindingDB Entry DOI: 10.7270/Q2ZK5HJF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50269291 (CHEMBL4064781) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0257 | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-induced cAMP accumulation by TR-FRET as... | Bioorg Med Chem Lett 27: 3920-3924 (2017) Article DOI: 10.1016/j.bmcl.2017.06.017 BindingDB Entry DOI: 10.7270/Q2ZW1PD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50430621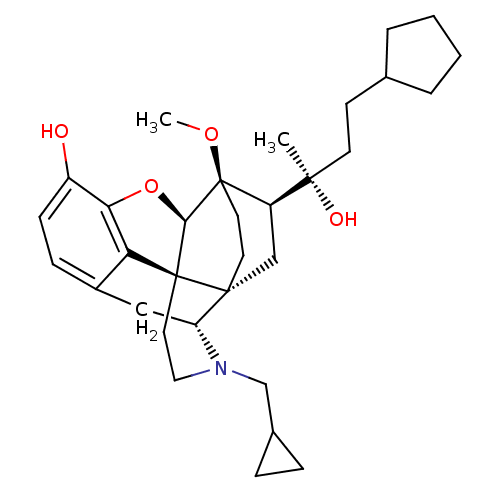 (CHEMBL2338748) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0270 | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Agonist activity at human KOR expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 56: 3207-16 (2013) Article DOI: 10.1021/jm301543e BindingDB Entry DOI: 10.7270/Q28G8N2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM602183 (US11643436, Compound TM-5) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | n/a | n/a | 0.0270 | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2MC93Z6 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2212 total ) | Next | Last >> |