

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13467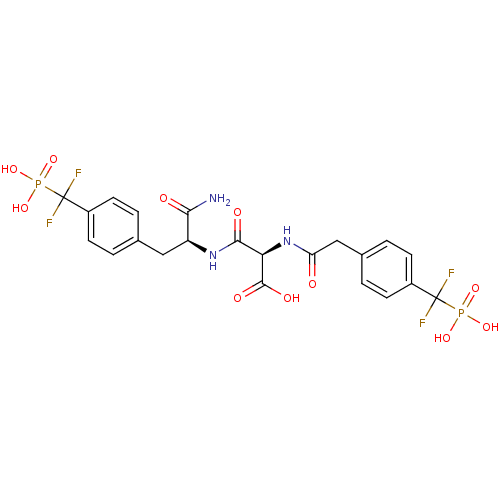 ((2R)-2-{[(1S)-1-carbamoyl-2-{4-[difluoro(phosphono...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 38013-21 (2006) Article DOI: 10.1074/jbc.M607913200 BindingDB Entry DOI: 10.7270/Q2JW8C4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13469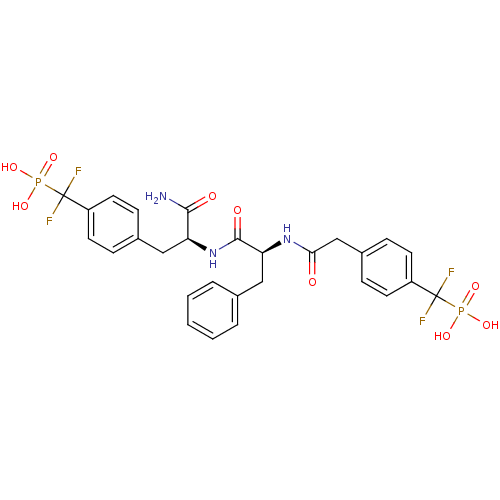 (({4-[(2S)-2-carbamoyl-2-[(2S)-2-(1-{4-[difluoro(ph...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 38013-21 (2006) Article DOI: 10.1074/jbc.M607913200 BindingDB Entry DOI: 10.7270/Q2JW8C4X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13599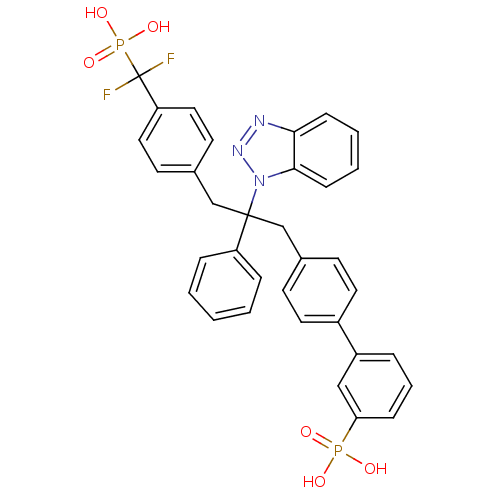 (3-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Research Laboratories | Assay Description Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... | Biochemistry 42: 11451-9 (2003) Article DOI: 10.1021/bi035098j BindingDB Entry DOI: 10.7270/Q2HX19XS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13604 (6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Research Laboratories | Assay Description Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... | Biochemistry 42: 11451-9 (2003) Article DOI: 10.1021/bi035098j BindingDB Entry DOI: 10.7270/Q2HX19XS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13603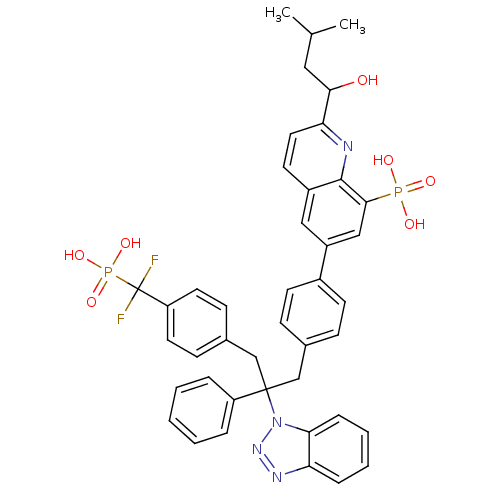 (6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Research Laboratories | Assay Description Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... | Biochemistry 42: 11451-9 (2003) Article DOI: 10.1021/bi035098j BindingDB Entry DOI: 10.7270/Q2HX19XS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13601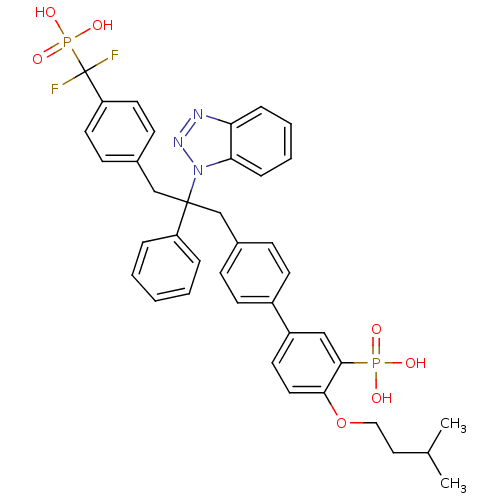 (5-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Research Laboratories | Assay Description Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... | Biochemistry 42: 11451-9 (2003) Article DOI: 10.1021/bi035098j BindingDB Entry DOI: 10.7270/Q2HX19XS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13600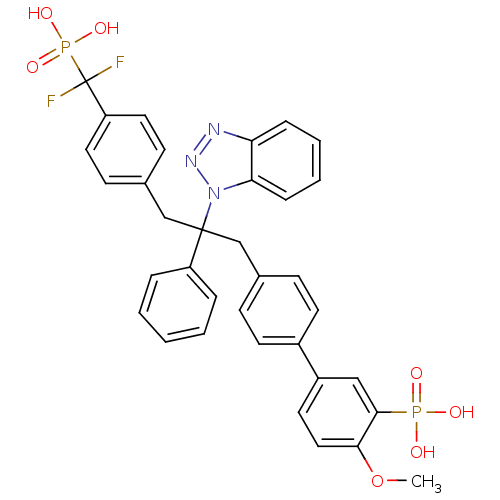 (5-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Research Laboratories | Assay Description Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... | Biochemistry 42: 11451-9 (2003) Article DOI: 10.1021/bi035098j BindingDB Entry DOI: 10.7270/Q2HX19XS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13605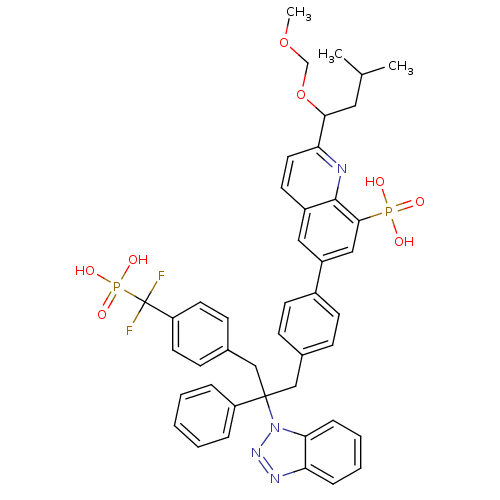 (6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Research Laboratories | Assay Description Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... | Biochemistry 42: 11451-9 (2003) Article DOI: 10.1021/bi035098j BindingDB Entry DOI: 10.7270/Q2HX19XS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13602 (6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Research Laboratories | Assay Description Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... | Biochemistry 42: 11451-9 (2003) Article DOI: 10.1021/bi035098j BindingDB Entry DOI: 10.7270/Q2HX19XS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13596 (({4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluoro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Research Laboratories | Assay Description Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... | Biochemistry 42: 11451-9 (2003) Article DOI: 10.1021/bi035098j BindingDB Entry DOI: 10.7270/Q2HX19XS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13595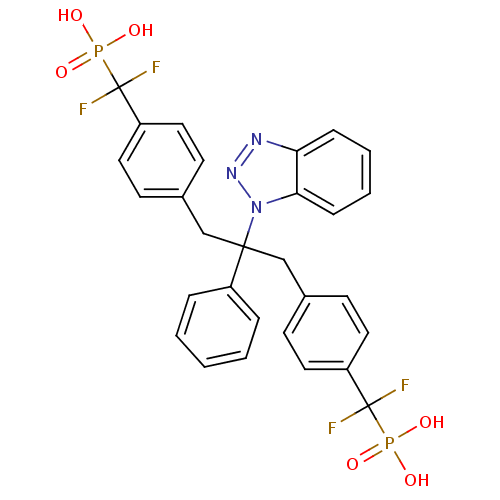 (({4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluoro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Research Laboratories | Assay Description Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... | Biochemistry 42: 11451-9 (2003) Article DOI: 10.1021/bi035098j BindingDB Entry DOI: 10.7270/Q2HX19XS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13597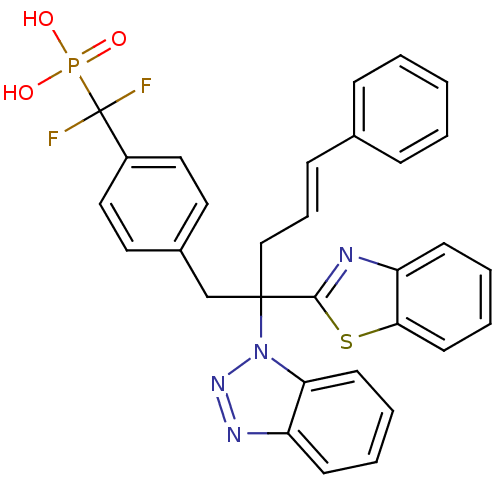 (({4-[(4E)-2-(1,3-benzothiazol-2-yl)-2-(1H-1,2,3-be...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Research Laboratories | Assay Description Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... | Biochemistry 42: 11451-9 (2003) Article DOI: 10.1021/bi035098j BindingDB Entry DOI: 10.7270/Q2HX19XS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13507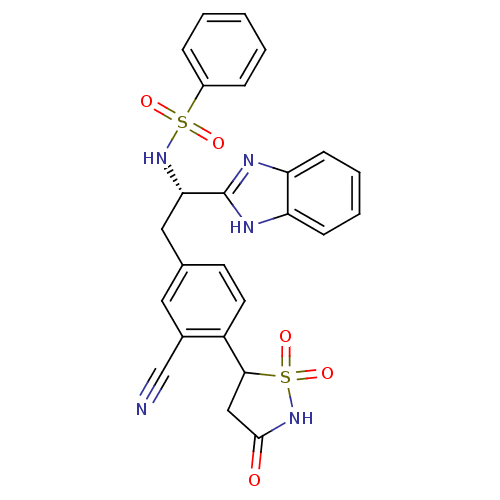 (Isothiazolidinone (IZD) deriv. 43 | N-[(1S)-1-(1H-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 38013-21 (2006) Article DOI: 10.1074/jbc.M607913200 BindingDB Entry DOI: 10.7270/Q2JW8C4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13508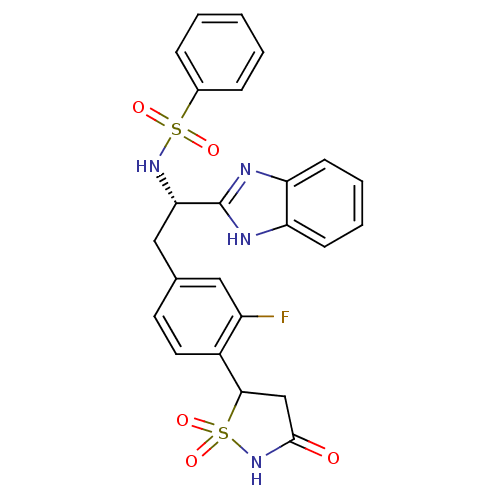 (Isothiazolidinone (IZD) deriv. 44 | N-[(1S)-1-(1H-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 38013-21 (2006) Article DOI: 10.1074/jbc.M607913200 BindingDB Entry DOI: 10.7270/Q2JW8C4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM14269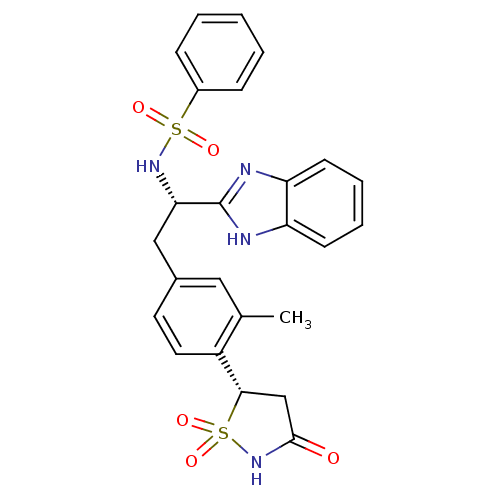 ((S)-isothiazolidinone | IZD deriv. 2 | N-[(1S)-1-(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | Bioorg Med Chem Lett 17: 736-40 (2007) Article DOI: 10.1016/j.bmcl.2006.10.079 BindingDB Entry DOI: 10.7270/Q20R9MM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM14268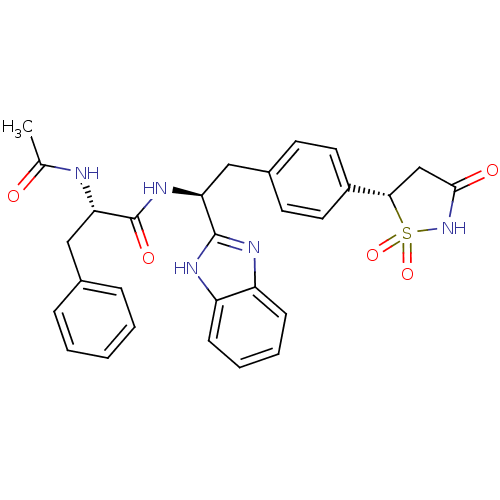 ((2S)-N-[(1S)-1-(1H-1,3-benzodiazol-2-yl)-2-{4-[(5S...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | Bioorg Med Chem Lett 17: 736-40 (2007) Article DOI: 10.1016/j.bmcl.2006.10.079 BindingDB Entry DOI: 10.7270/Q20R9MM4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13598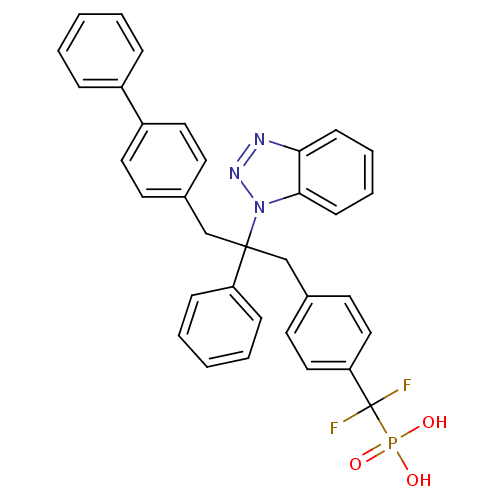 (({4-[2-(1H-1,2,3-benzotriazol-1-yl)-2-phenyl-3-(4-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Research Laboratories | Assay Description Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... | Biochemistry 42: 11451-9 (2003) Article DOI: 10.1021/bi035098j BindingDB Entry DOI: 10.7270/Q2HX19XS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13814 (({4-[(4E)-2-(1H-1,2,3-benzotriazol-1-yl)-2-[4-(met...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Frosst Center for Therapeutic Research | Assay Description Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... | J Biol Chem 281: 8010-5 (2006) Article DOI: 10.1074/jbc.M511827200 BindingDB Entry DOI: 10.7270/Q2C53J3V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13493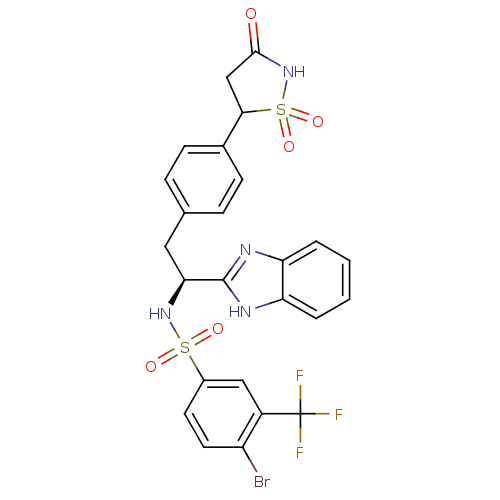 (Isothiazolidinone (IZD) deriv. 29 | N-[(1S)-1-(1H-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 38013-21 (2006) Article DOI: 10.1074/jbc.M607913200 BindingDB Entry DOI: 10.7270/Q2JW8C4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13471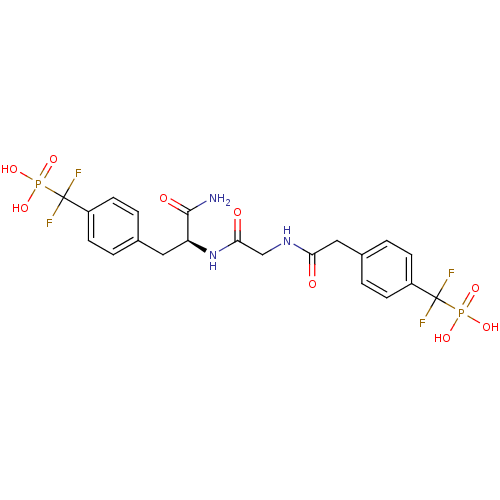 (({4-[(2S)-2-carbamoyl-2-[2-(1-{4-[difluoro(phospho...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 38013-21 (2006) Article DOI: 10.1074/jbc.M607913200 BindingDB Entry DOI: 10.7270/Q2JW8C4X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13470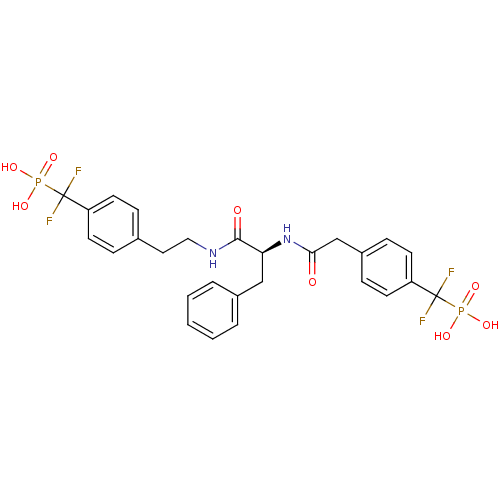 (Difluoromethylphosphonic acid (DFMP) deriv. 9 | [(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 38013-21 (2006) Article DOI: 10.1074/jbc.M607913200 BindingDB Entry DOI: 10.7270/Q2JW8C4X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13494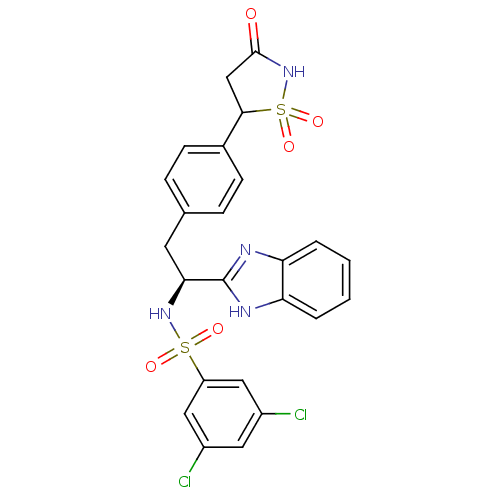 (Isothiazolidinone (IZD) deriv. 30 | N-[(1S)-1-(1H-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 38013-21 (2006) Article DOI: 10.1074/jbc.M607913200 BindingDB Entry DOI: 10.7270/Q2JW8C4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13475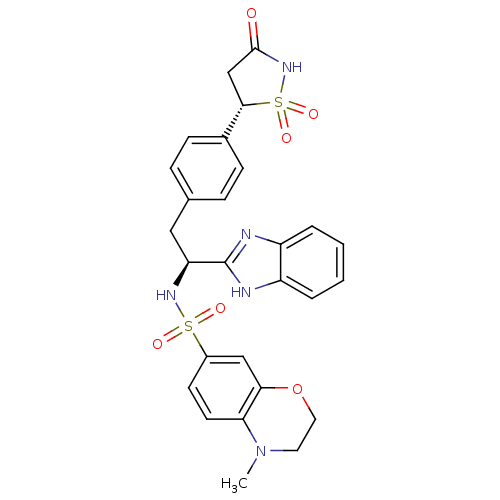 (Isothiazolidinone (IZD) deriv. 4 | N-[(1S)-1-(1H-1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 38013-21 (2006) Article DOI: 10.1074/jbc.M607913200 BindingDB Entry DOI: 10.7270/Q2JW8C4X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13509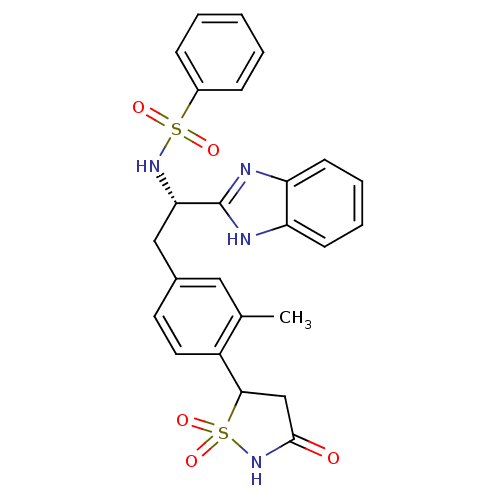 (Isothiazolidinone (IZD) deriv. 45 | N-[(1S)-1-(1H-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 38013-21 (2006) Article DOI: 10.1074/jbc.M607913200 BindingDB Entry DOI: 10.7270/Q2JW8C4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13809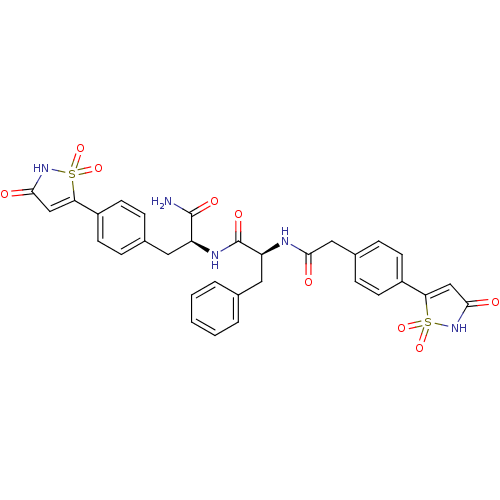 ((2S)-N-[(1S)-1-carbamoyl-2-[4-(1,1,3-trioxo-2,3-di...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 32784-95 (2006) Article DOI: 10.1074/jbc.M606873200 BindingDB Entry DOI: 10.7270/Q2GX48SD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13495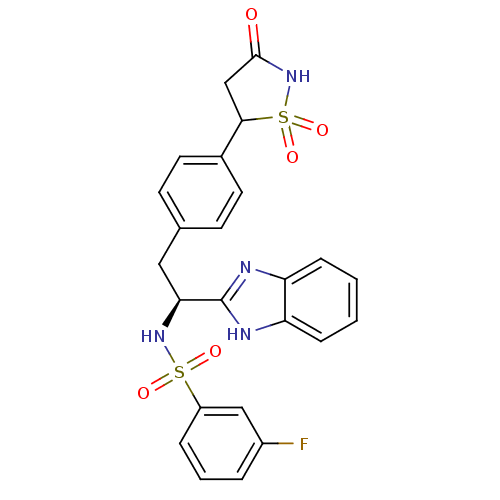 (Isothiazolidinone (IZD) deriv. 31 | N-[(1S)-1-(1H-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 38013-21 (2006) Article DOI: 10.1074/jbc.M607913200 BindingDB Entry DOI: 10.7270/Q2JW8C4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13510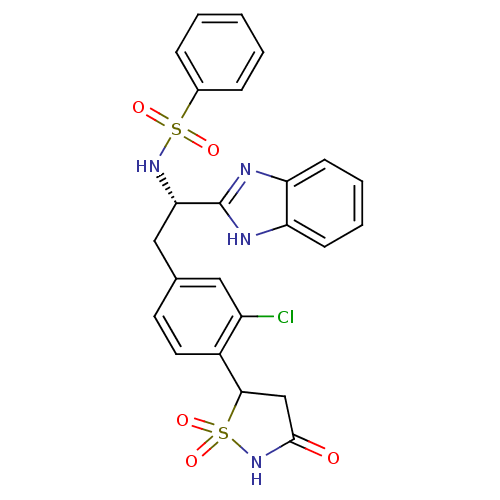 (Isothiazolidinone (IZD) deriv. 46 | N-[(1S)-1-(1H-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 38013-21 (2006) Article DOI: 10.1074/jbc.M607913200 BindingDB Entry DOI: 10.7270/Q2JW8C4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13496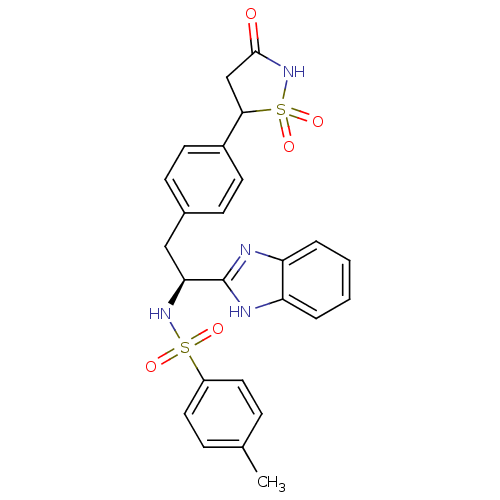 (Isothiazolidinone (IZD) deriv. 32 | N-[(1S)-1-(1H-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 38013-21 (2006) Article DOI: 10.1074/jbc.M607913200 BindingDB Entry DOI: 10.7270/Q2JW8C4X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13497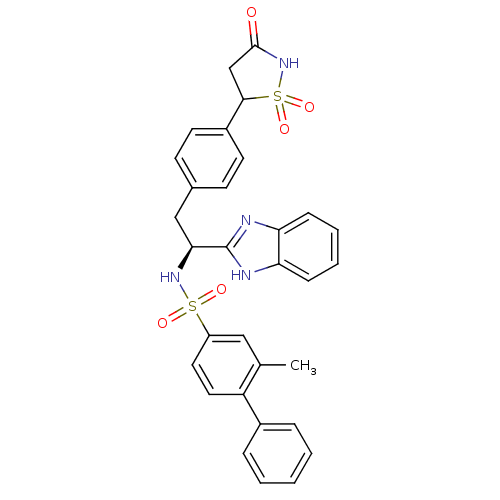 (Isothiazolidinone (IZD) deriv. 33 | N-[(1S)-1-(1H-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 38013-21 (2006) Article DOI: 10.1074/jbc.M607913200 BindingDB Entry DOI: 10.7270/Q2JW8C4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13498 (Isothiazolidinone (IZD) deriv. 34 | N-[(1S)-1-(1H-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 38013-21 (2006) Article DOI: 10.1074/jbc.M607913200 BindingDB Entry DOI: 10.7270/Q2JW8C4X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13815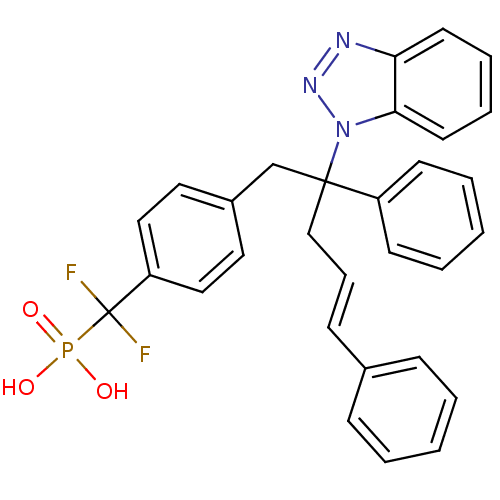 (({4-[(4E)-2-(1H-1,2,3-benzotriazol-1-yl)-2,5-diphe...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 109 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Frosst Center for Therapeutic Research | Assay Description Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... | J Biol Chem 281: 8010-5 (2006) Article DOI: 10.1074/jbc.M511827200 BindingDB Entry DOI: 10.7270/Q2C53J3V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13499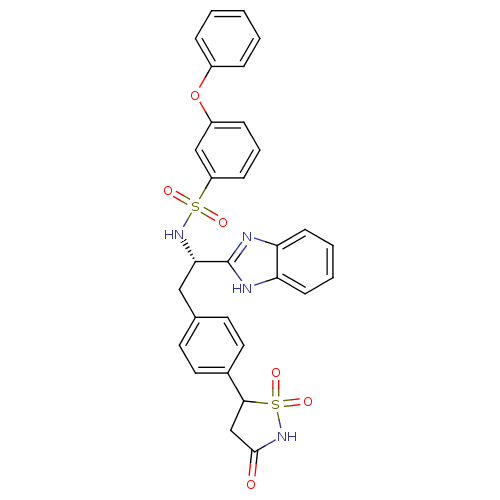 (Isothiazolidinone (IZD) deriv. 35 | N-[(1S)-1-(1H-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 38013-21 (2006) Article DOI: 10.1074/jbc.M607913200 BindingDB Entry DOI: 10.7270/Q2JW8C4X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13474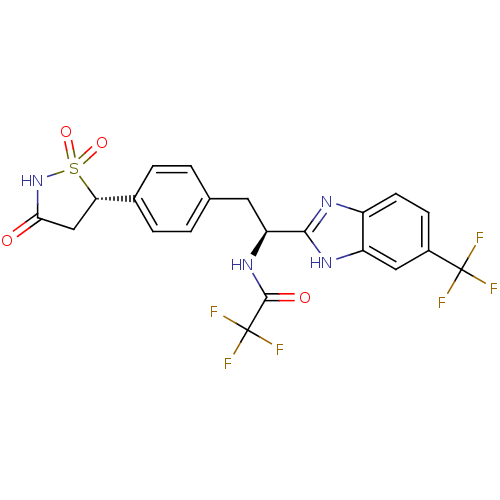 (2,2,2-trifluoro-N-[(1S)-1-[5-(trifluoromethyl)-1H-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 38013-21 (2006) Article DOI: 10.1074/jbc.M607913200 BindingDB Entry DOI: 10.7270/Q2JW8C4X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13511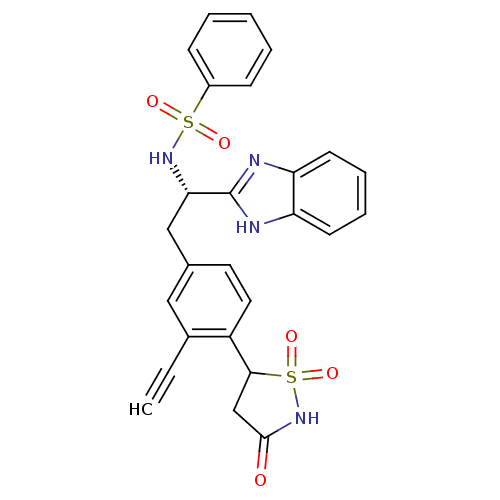 (Isothiazolidinone (IZD) deriv. 47 | N-[(1S)-1-(1H-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 38013-21 (2006) Article DOI: 10.1074/jbc.M607913200 BindingDB Entry DOI: 10.7270/Q2JW8C4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13501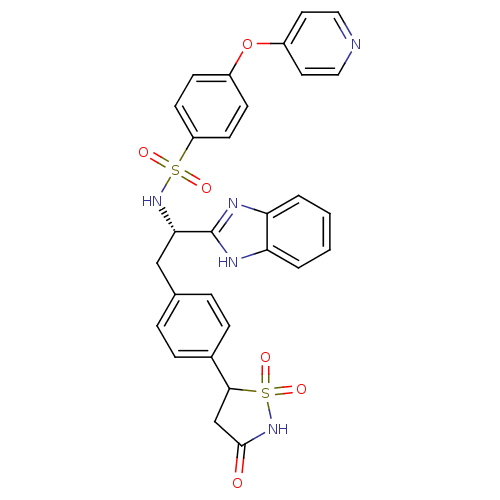 (Isothiazolidinone (IZD) deriv. 37 | N-[(1S)-1-(1H-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 38013-21 (2006) Article DOI: 10.1074/jbc.M607913200 BindingDB Entry DOI: 10.7270/Q2JW8C4X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13500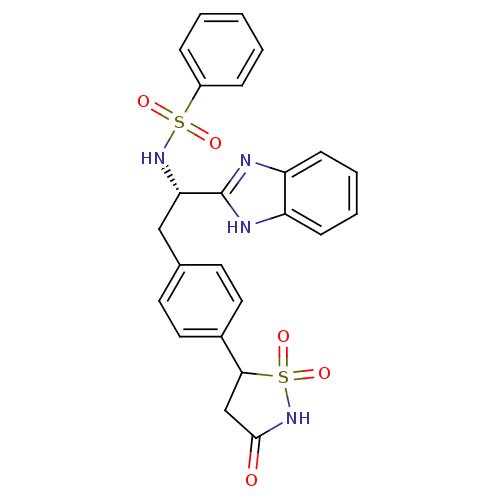 (Isothiazolidinone (IZD) deriv. 36 | N-[(1S)-1-(1H-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 38013-21 (2006) Article DOI: 10.1074/jbc.M607913200 BindingDB Entry DOI: 10.7270/Q2JW8C4X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM14289 (5-{4-[(2S)-2-(1,3-benzothiazol-2-ylamino)-2-[5-(tr...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | Bioorg Med Chem Lett 17: 736-40 (2007) Article DOI: 10.1016/j.bmcl.2006.10.079 BindingDB Entry DOI: 10.7270/Q20R9MM4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13502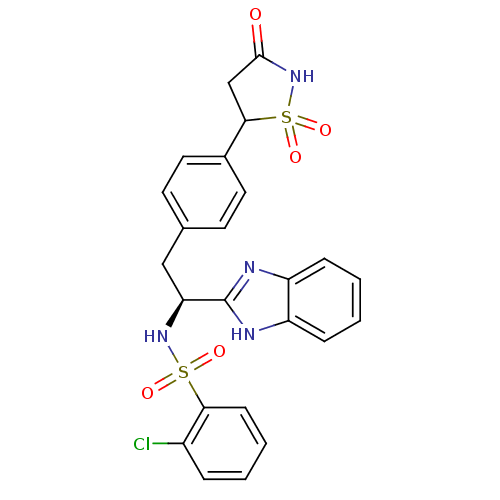 (Isothiazolidinone (IZD) deriv. 38 | N-[(1S)-1-(1H-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 38013-21 (2006) Article DOI: 10.1074/jbc.M607913200 BindingDB Entry DOI: 10.7270/Q2JW8C4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13816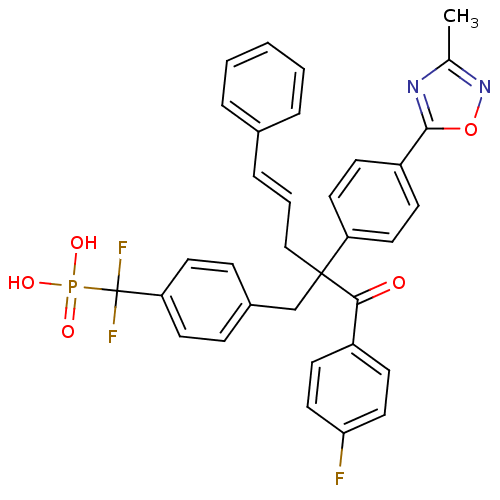 ([difluoro({4-[3-(4-fluorophenyl)-2-[4-(3-methyl-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 163 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Frosst Center for Therapeutic Research | Assay Description Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... | J Biol Chem 281: 8010-5 (2006) Article DOI: 10.1074/jbc.M511827200 BindingDB Entry DOI: 10.7270/Q2C53J3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13503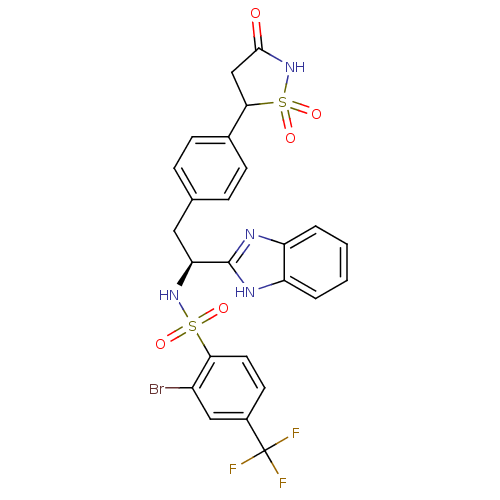 (Isothiazolidinone (IZD) deriv. 39 | N-[(1S)-1-(1H-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 38013-21 (2006) Article DOI: 10.1074/jbc.M607913200 BindingDB Entry DOI: 10.7270/Q2JW8C4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13512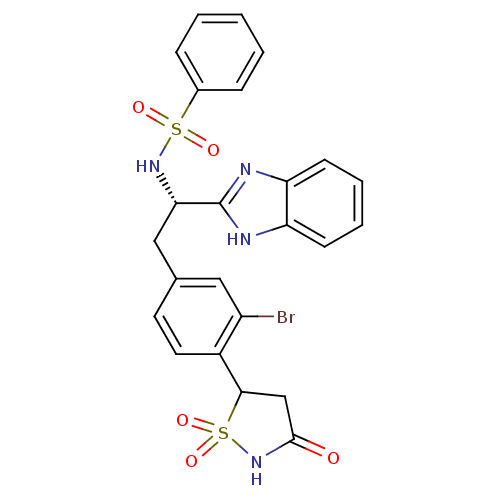 (Isothiazolidinone (IZD) deriv. 48 | N-[(1S)-1-(1H-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 38013-21 (2006) Article DOI: 10.1074/jbc.M607913200 BindingDB Entry DOI: 10.7270/Q2JW8C4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13808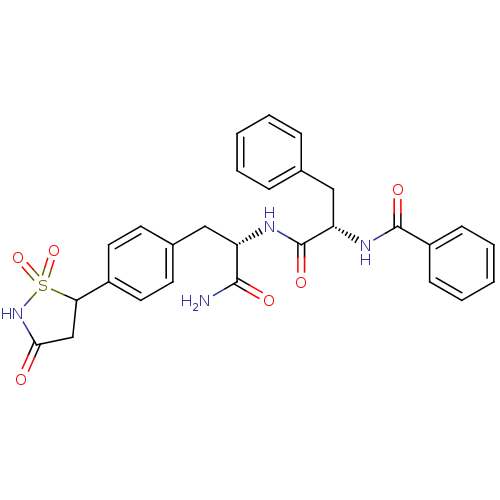 ((2S)-N-[(1S)-1-carbamoyl-2-[4-(1,1,3-trioxo-1,2-th...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 185 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 32784-95 (2006) Article DOI: 10.1074/jbc.M606873200 BindingDB Entry DOI: 10.7270/Q2GX48SD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13465 ((2S)-N-[(1S)-1-carbamoyl-2-{4-[(5S)-1,1,3-trioxo-1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 38013-21 (2006) Article DOI: 10.1074/jbc.M607913200 BindingDB Entry DOI: 10.7270/Q2JW8C4X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13465 ((2S)-N-[(1S)-1-carbamoyl-2-{4-[(5S)-1,1,3-trioxo-1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 32784-95 (2006) Article DOI: 10.1074/jbc.M606873200 BindingDB Entry DOI: 10.7270/Q2GX48SD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13490 (Isothiazolidinone (IZD) deriv. 26 | N-[(1S)-1-(1H-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 38013-21 (2006) Article DOI: 10.1074/jbc.M607913200 BindingDB Entry DOI: 10.7270/Q2JW8C4X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13504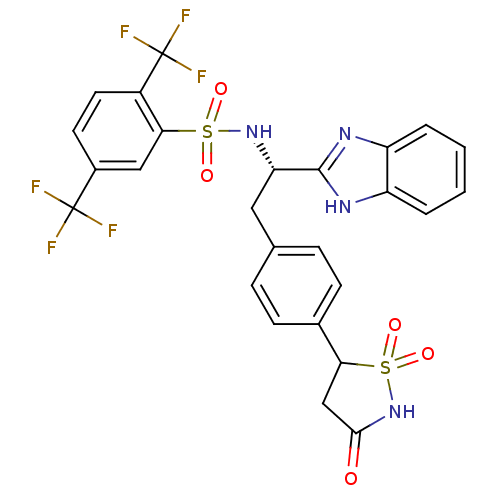 (Isothiazolidinone (IZD) deriv. 40 | N-[(1S)-1-(1H-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 38013-21 (2006) Article DOI: 10.1074/jbc.M607913200 BindingDB Entry DOI: 10.7270/Q2JW8C4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13807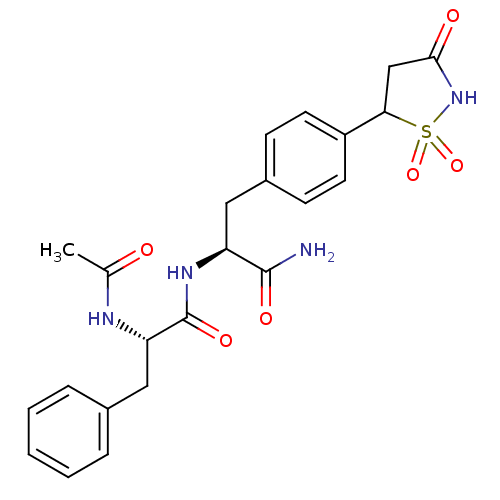 ((2S)-N-[(1S)-1-carbamoyl-2-[4-(1,1,3-trioxo-1,2-th...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 32784-95 (2006) Article DOI: 10.1074/jbc.M606873200 BindingDB Entry DOI: 10.7270/Q2GX48SD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13505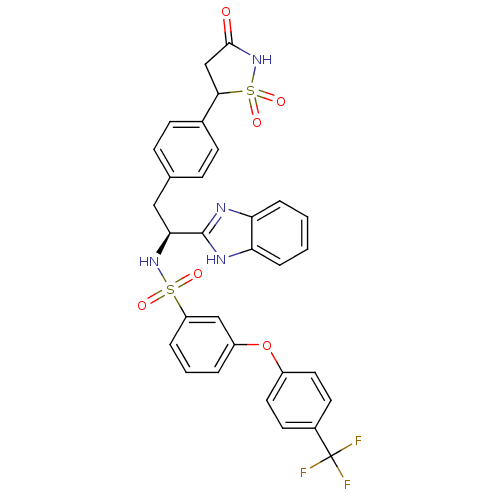 (Isothiazolidinone (IZD) deriv. 41 | N-[(1S)-1-(1H-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 38013-21 (2006) Article DOI: 10.1074/jbc.M607913200 BindingDB Entry DOI: 10.7270/Q2JW8C4X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM14292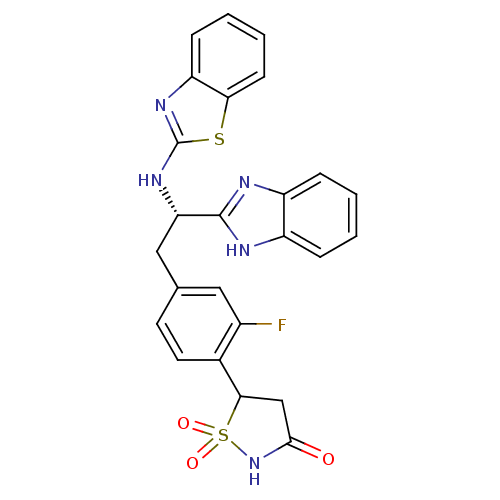 (5-{4-[(2S)-2-(1H-1,3-benzodiazol-2-yl)-2-(1,3-benz...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | Bioorg Med Chem Lett 17: 736-40 (2007) Article DOI: 10.1016/j.bmcl.2006.10.079 BindingDB Entry DOI: 10.7270/Q20R9MM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13473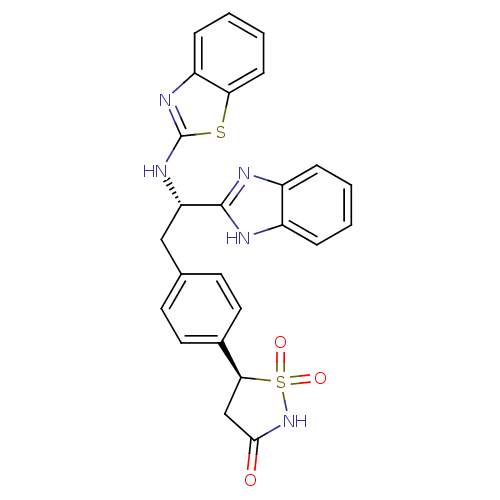 ((5S)-5-{4-[(2S)-2-(1H-1,3-benzodiazol-2-yl)-2-(1,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 38013-21 (2006) Article DOI: 10.1074/jbc.M607913200 BindingDB Entry DOI: 10.7270/Q2JW8C4X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 132 total ) | Next | Last >> |