 Found 4371 hits of ic50 for UniProtKB: P42338
Found 4371 hits of ic50 for UniProtKB: P42338 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50420316
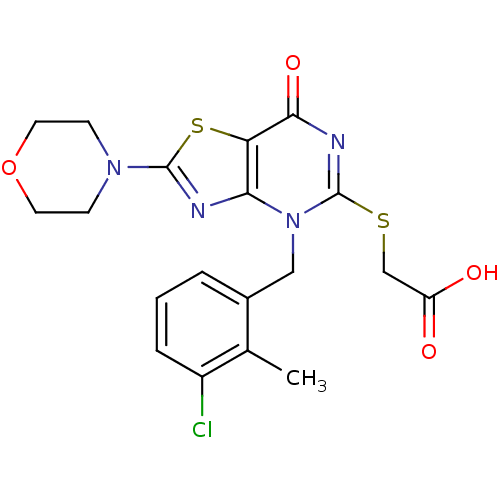
(CHEMBL2089119)Show SMILES Cc1c(Cl)cccc1Cn1c(SCC(O)=O)nc(=O)c2sc(nc12)N1CCOCC1 Show InChI InChI=1S/C19H19ClN4O4S2/c1-11-12(3-2-4-13(11)20)9-24-16-15(17(27)22-19(24)29-10-14(25)26)30-18(21-16)23-5-7-28-8-6-23/h2-4H,5-10H2,1H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3K beta by continuous TR-FRET assay |
ACS Med Chem Lett 3: 524-529 (2012)
Article DOI: 10.1021/ml300045b
BindingDB Entry DOI: 10.7270/Q2J967PT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50420311
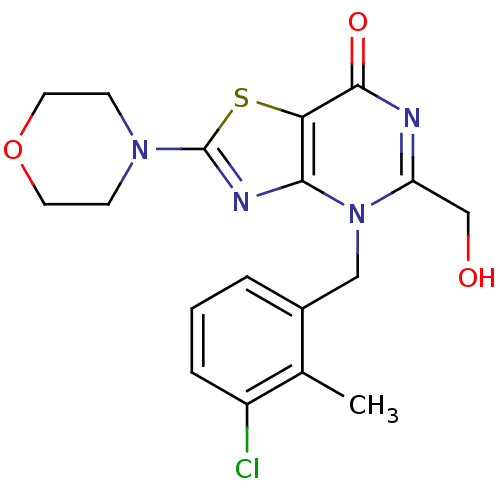
(CHEMBL2089114)Show SMILES Cc1c(Cl)cccc1Cn1c(CO)nc(=O)c2sc(nc12)N1CCOCC1 Show InChI InChI=1S/C18H19ClN4O3S/c1-11-12(3-2-4-13(11)19)9-23-14(10-24)20-17(25)15-16(23)21-18(27-15)22-5-7-26-8-6-22/h2-4,24H,5-10H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3K beta by continuous TR-FRET assay |
ACS Med Chem Lett 3: 524-529 (2012)
Article DOI: 10.1021/ml300045b
BindingDB Entry DOI: 10.7270/Q2J967PT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50145416

(GSK2126458 | Omipalisib)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1ccc2nccc(-c3ccnnc3)c2c1 Show InChI InChI=1S/C25H17F2N5O3S/c1-35-25-23(32-36(33,34)24-5-3-18(26)12-21(24)27)11-17(13-29-25)15-2-4-22-20(10-15)19(7-8-28-22)16-6-9-30-31-14-16/h2-14,32H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kbeta (unknown origin) using PIP2 as substrate measured after 1 hr by ADP-glo assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113249
BindingDB Entry DOI: 10.7270/Q2PC3641 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50420309

(CHEMBL2089120)Show SMILES Cc1c(Cl)cccc1Cn1c(N)nc(=O)c2sc(nc12)N1CCOCC1 Show InChI InChI=1S/C17H18ClN5O2S/c1-10-11(3-2-4-12(10)18)9-23-14-13(15(24)21-16(23)19)26-17(20-14)22-5-7-25-8-6-22/h2-4H,5-9H2,1H3,(H2,19,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3K beta by continuous TR-FRET assay |
ACS Med Chem Lett 3: 524-529 (2012)
Article DOI: 10.1021/ml300045b
BindingDB Entry DOI: 10.7270/Q2J967PT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50341056

(5-(1-(4-amino-6-methyl-1,3,5-triazin-2-yl)-1H-benz...)Show SMILES Cc1nc(N)nc(n1)-n1c(Nc2cc(O)cc(O)c2)nc2ccccc12 Show InChI InChI=1S/C17H15N7O2/c1-9-19-15(18)23-16(20-9)24-14-5-3-2-4-13(14)22-17(24)21-10-6-11(25)8-12(26)7-10/h2-8,25-26H,1H3,(H,21,22)(H2,18,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta |
Bioorg Med Chem Lett 21: 2064-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.007
BindingDB Entry DOI: 10.7270/Q2FT8MBW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50420321

(CHEMBL2089112)Show SMILES Cc1c(Cn2c(CO)nc(=O)c3sc(nc23)N2CCOCC2)cccc1C(F)(F)F Show InChI InChI=1S/C19H19F3N4O3S/c1-11-12(3-2-4-13(11)19(20,21)22)9-26-14(10-27)23-17(28)15-16(26)24-18(30-15)25-5-7-29-8-6-25/h2-4,27H,5-10H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3K beta by continuous TR-FRET assay |
ACS Med Chem Lett 3: 524-529 (2012)
Article DOI: 10.1021/ml300045b
BindingDB Entry DOI: 10.7270/Q2J967PT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50420310
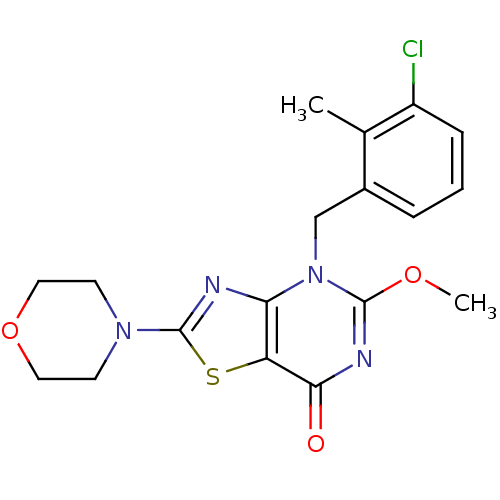
(CHEMBL2089116)Show SMILES COc1nc(=O)c2sc(nc2n1Cc1cccc(Cl)c1C)N1CCOCC1 Show InChI InChI=1S/C18H19ClN4O3S/c1-11-12(4-3-5-13(11)19)10-23-15-14(16(24)21-17(23)25-2)27-18(20-15)22-6-8-26-9-7-22/h3-5H,6-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3K beta by continuous TR-FRET assay |
ACS Med Chem Lett 3: 524-529 (2012)
Article DOI: 10.1021/ml300045b
BindingDB Entry DOI: 10.7270/Q2J967PT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50420317
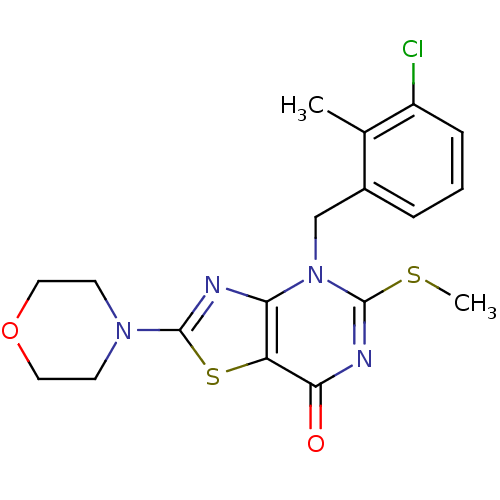
(CHEMBL2089118)Show SMILES CSc1nc(=O)c2sc(nc2n1Cc1cccc(Cl)c1C)N1CCOCC1 Show InChI InChI=1S/C18H19ClN4O2S2/c1-11-12(4-3-5-13(11)19)10-23-15-14(16(24)21-18(23)26-2)27-17(20-15)22-6-8-25-9-7-22/h3-5H,6-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3K beta by continuous TR-FRET assay |
ACS Med Chem Lett 3: 524-529 (2012)
Article DOI: 10.1021/ml300045b
BindingDB Entry DOI: 10.7270/Q2J967PT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50489445
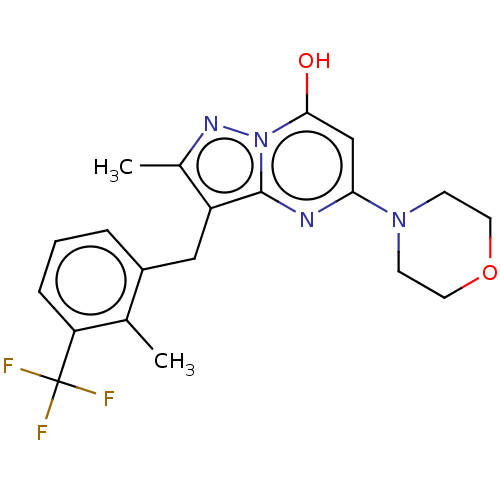
(CHEMBL2322331)Show SMILES Cc1nn2c(O)cc(nc2c1Cc1cccc(c1C)C(F)(F)F)N1CCOCC1 Show InChI InChI=1S/C20H21F3N4O2/c1-12-14(4-3-5-16(12)20(21,22)23)10-15-13(2)25-27-18(28)11-17(24-19(15)27)26-6-8-29-9-7-26/h3-5,11,28H,6-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta (unknown origin) |
ACS Med Chem Lett 4: 230-4 (2013)
Article DOI: 10.1021/ml300330m
BindingDB Entry DOI: 10.7270/Q2HD7ZJT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50489439
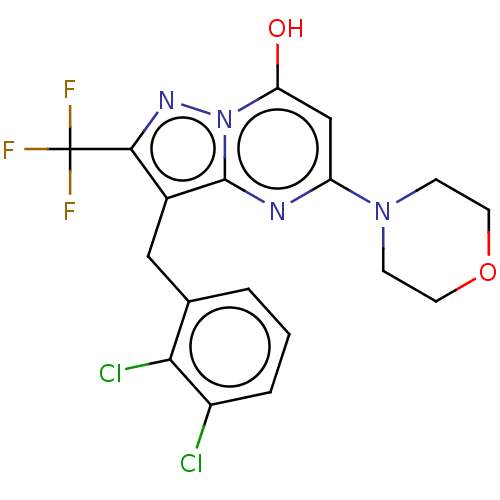
(CHEMBL2322340)Show SMILES Oc1cc(nc2c(Cc3cccc(Cl)c3Cl)c(nn12)C(F)(F)F)N1CCOCC1 Show InChI InChI=1S/C18H15Cl2F3N4O2/c19-12-3-1-2-10(15(12)20)8-11-16(18(21,22)23)25-27-14(28)9-13(24-17(11)27)26-4-6-29-7-5-26/h1-3,9,28H,4-8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta (unknown origin) |
ACS Med Chem Lett 4: 230-4 (2013)
Article DOI: 10.1021/ml300330m
BindingDB Entry DOI: 10.7270/Q2HD7ZJT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50381156

(CHEMBL2018220 | D3RKN_94)Show SMILES CSc1nn2c(nc(cc2=O)N2CCOCC2)n1Cc1cccc(c1C)C(F)(F)F Show InChI InChI=1S/C19H20F3N5O2S/c1-12-13(4-3-5-14(12)19(20,21)22)11-26-17-23-15(25-6-8-29-9-7-25)10-16(28)27(17)24-18(26)30-2/h3-5,10H,6-9,11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta by continuous read time resolved fluorescence resonance energy transfer displacement assay |
Bioorg Med Chem Lett 22: 3198-202 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.039
BindingDB Entry DOI: 10.7270/Q2TD9ZBP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50070262
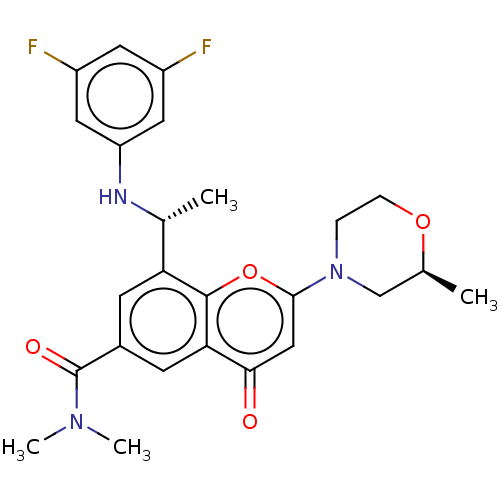
(CHEMBL3408270 | US9718800, 9.02b)Show SMILES C[C@@H](Nc1cc(F)cc(F)c1)c1cc(cc2c1oc(cc2=O)N1CCO[C@@H](C)C1)C(=O)N(C)C |r| Show InChI InChI=1S/C25H27F2N3O4/c1-14-13-30(5-6-33-14)23-12-22(31)21-8-16(25(32)29(3)4)7-20(24(21)34-23)15(2)28-19-10-17(26)9-18(27)11-19/h7-12,14-15,28H,5-6,13H2,1-4H3/t14-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN-null human MDA-MB-468 cells assessed as inhibition of Akt phosphorylation after 2 hrs |
J Med Chem 58: 943-62 (2015)
Article DOI: 10.1021/jm501629p
BindingDB Entry DOI: 10.7270/Q2VT1TS8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50341409
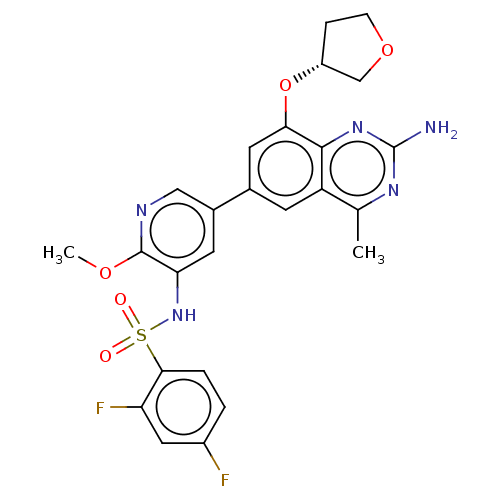
(CHEMBL4176771 | US11534443, Example 9)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1cc(O[C@@H]2CCOC2)c2nc(N)nc(C)c2c1 |r| Show InChI InChI=1S/C25H23F2N5O5S/c1-13-18-7-14(9-21(23(18)31-25(28)30-13)37-17-5-6-36-12-17)15-8-20(24(35-2)29-11-15)32-38(33,34)22-4-3-16(26)10-19(22)27/h3-4,7-11,17,32H,5-6,12H2,1-2H3,(H2,28,30,31)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta (unknown origin) using PIP2 as substrate by ADP-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50420314
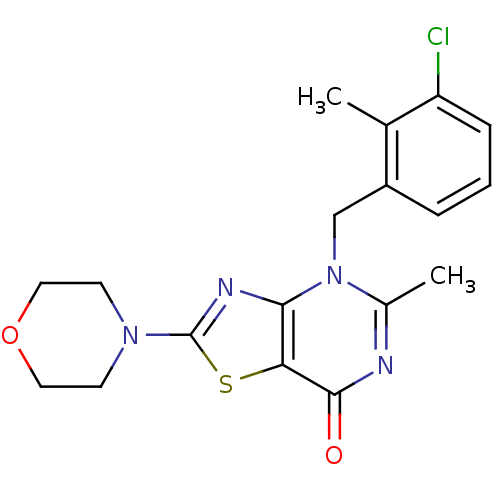
(CHEMBL2089107)Show InChI InChI=1S/C18H19ClN4O2S/c1-11-13(4-3-5-14(11)19)10-23-12(2)20-17(24)15-16(23)21-18(26-15)22-6-8-25-9-7-22/h3-5H,6-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3K beta by continuous TR-FRET assay |
ACS Med Chem Lett 3: 524-529 (2012)
Article DOI: 10.1021/ml300045b
BindingDB Entry DOI: 10.7270/Q2J967PT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50420313

(CHEMBL2089108)Show SMILES CCc1nc(=O)c2sc(nc2n1Cc1cccc(c1C)C(F)(F)F)N1CCOCC1 Show InChI InChI=1S/C20H21F3N4O2S/c1-3-15-24-18(28)16-17(25-19(30-16)26-7-9-29-10-8-26)27(15)11-13-5-4-6-14(12(13)2)20(21,22)23/h4-6H,3,7-11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3K beta by continuous TR-FRET assay |
ACS Med Chem Lett 3: 524-529 (2012)
Article DOI: 10.1021/ml300045b
BindingDB Entry DOI: 10.7270/Q2J967PT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM288405

(US10087187, Compound 106)Show SMILES Cc1nc2c(N)cc(nn2c1Cc1cccc(F)c1C1CCNCC1)N1CCOCC1 Show InChI InChI=1S/C23H29FN6O/c1-15-20(13-17-3-2-4-18(24)22(17)16-5-7-26-8-6-16)30-23(27-15)19(25)14-21(28-30)29-9-11-31-12-10-29/h2-4,14,16,26H,5-13,25H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50319926
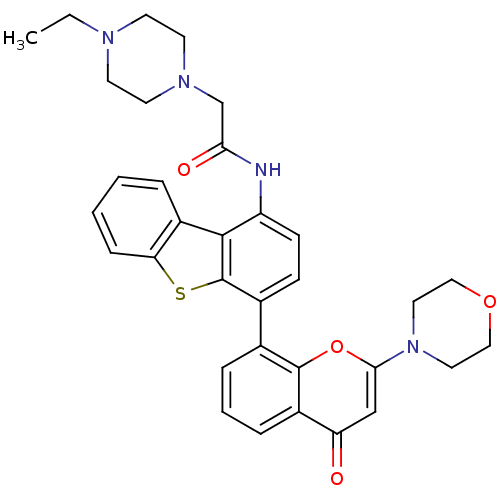
(2-(4-ethylpiperazin-1-yl)-N-(4-(2-morpholino-4-oxo...)Show SMILES CCN1CCN(CC(=O)Nc2ccc(-c3cccc4c3oc(cc4=O)N3CCOCC3)c3sc4ccccc4c23)CC1 Show InChI InChI=1S/C33H34N4O4S/c1-2-35-12-14-36(15-13-35)21-29(39)34-26-11-10-23(33-31(26)25-6-3-4-9-28(25)42-33)22-7-5-8-24-27(38)20-30(41-32(22)24)37-16-18-40-19-17-37/h3-11,20H,2,12-19,21H2,1H3,(H,34,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University
Curated by ChEMBL
| Assay Description
Inhibition of PI-3K beta (unknown origin) |
J Med Chem 56: 6386-401 (2013)
Article DOI: 10.1021/jm400915j
BindingDB Entry DOI: 10.7270/Q2RF5WFD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50420312
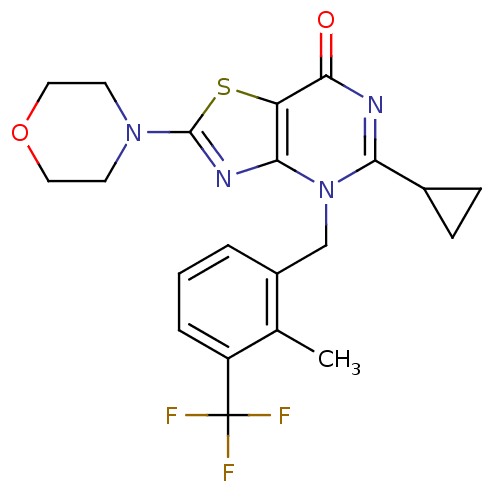
(CHEMBL2089109)Show SMILES Cc1c(Cn2c(nc(=O)c3sc(nc23)N2CCOCC2)C2CC2)cccc1C(F)(F)F Show InChI InChI=1S/C21H21F3N4O2S/c1-12-14(3-2-4-15(12)21(22,23)24)11-28-17(13-5-6-13)25-19(29)16-18(28)26-20(31-16)27-7-9-30-10-8-27/h2-4,13H,5-11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3K beta by continuous TR-FRET assay |
ACS Med Chem Lett 3: 524-529 (2012)
Article DOI: 10.1021/ml300045b
BindingDB Entry DOI: 10.7270/Q2J967PT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50341410
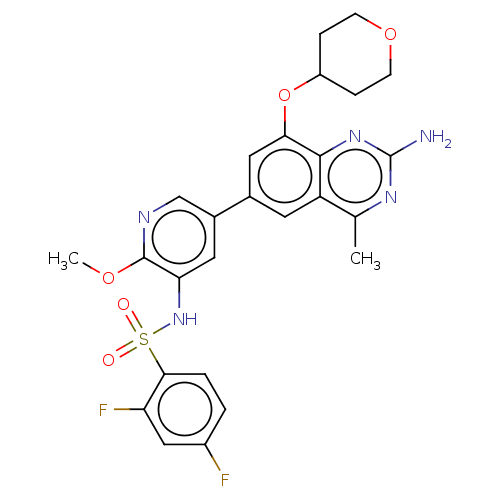
(CHEMBL4166144 | US11534443, Example 1)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1cc(OC2CCOCC2)c2nc(N)nc(C)c2c1 Show InChI InChI=1S/C26H25F2N5O5S/c1-14-19-9-15(11-22(24(19)32-26(29)31-14)38-18-5-7-37-8-6-18)16-10-21(25(36-2)30-13-16)33-39(34,35)23-4-3-17(27)12-20(23)28/h3-4,9-13,18,33H,5-8H2,1-2H3,(H2,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta (unknown origin) using PIP2 as substrate by ADP-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50505302
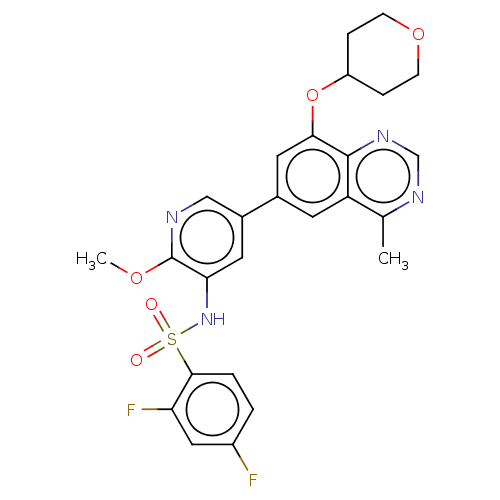
(CHEMBL4527563 | US11534443, Example 44)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1cc(OC2CCOCC2)c2ncnc(C)c2c1 Show InChI InChI=1S/C26H24F2N4O5S/c1-15-20-9-16(11-23(25(20)31-14-30-15)37-19-5-7-36-8-6-19)17-10-22(26(35-2)29-13-17)32-38(33,34)24-4-3-18(27)12-21(24)28/h3-4,9-14,19,32H,5-8H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3K beta (unknown origin) using PIP2 as substrate incubated in dark at room temperature for 1 hr in presence of ATP by ADP-Glo lumines... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02186
BindingDB Entry DOI: 10.7270/Q2833WWP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50420315
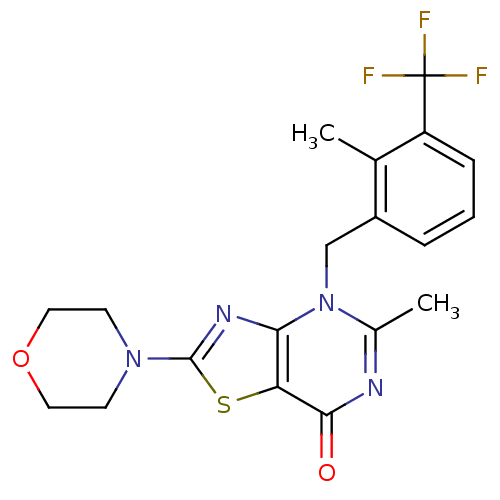
(CHEMBL2089106)Show SMILES Cc1nc(=O)c2sc(nc2n1Cc1cccc(c1C)C(F)(F)F)N1CCOCC1 Show InChI InChI=1S/C19H19F3N4O2S/c1-11-13(4-3-5-14(11)19(20,21)22)10-26-12(2)23-17(27)15-16(26)24-18(29-15)25-6-8-28-9-7-25/h3-5H,6-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3K beta by continuous TR-FRET assay |
ACS Med Chem Lett 3: 524-529 (2012)
Article DOI: 10.1021/ml300045b
BindingDB Entry DOI: 10.7270/Q2J967PT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50381161
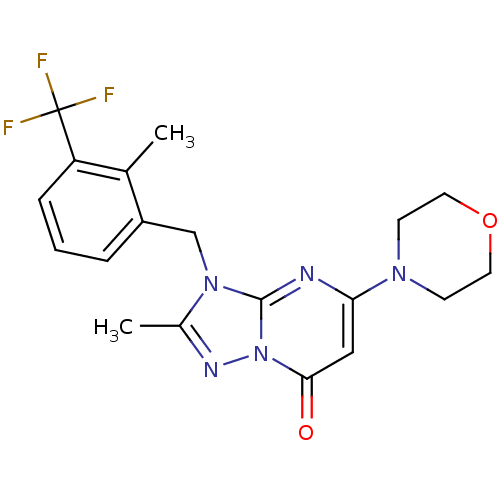
(CHEMBL2018219)Show SMILES Cc1nn2c(nc(cc2=O)N2CCOCC2)n1Cc1cccc(c1C)C(F)(F)F Show InChI InChI=1S/C19H20F3N5O2/c1-12-14(4-3-5-15(12)19(20,21)22)11-26-13(2)24-27-17(28)10-16(23-18(26)27)25-6-8-29-9-7-25/h3-5,10H,6-9,11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta by continuous read time resolved fluorescence resonance energy transfer displacement assay |
Bioorg Med Chem Lett 22: 3198-202 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.039
BindingDB Entry DOI: 10.7270/Q2TD9ZBP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50489454
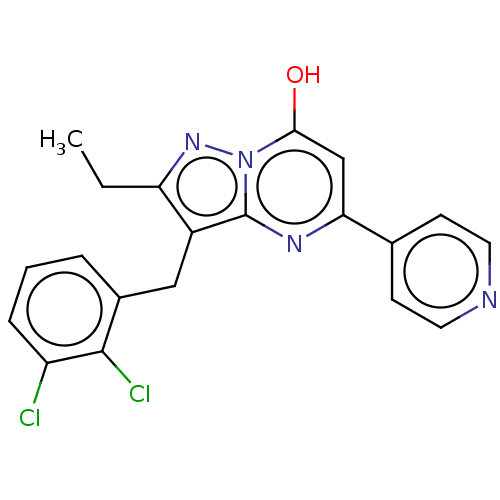
(CHEMBL2322338)Show SMILES CCc1nn2c(O)cc(nc2c1Cc1cccc(Cl)c1Cl)-c1ccncc1 Show InChI InChI=1S/C20H16Cl2N4O/c1-2-16-14(10-13-4-3-5-15(21)19(13)22)20-24-17(11-18(27)26(20)25-16)12-6-8-23-9-7-12/h3-9,11,27H,2,10H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta (unknown origin) |
ACS Med Chem Lett 4: 230-4 (2013)
Article DOI: 10.1021/ml300330m
BindingDB Entry DOI: 10.7270/Q2HD7ZJT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50420315

(CHEMBL2089106)Show SMILES Cc1nc(=O)c2sc(nc2n1Cc1cccc(c1C)C(F)(F)F)N1CCOCC1 Show InChI InChI=1S/C19H19F3N4O2S/c1-11-13(4-3-5-14(11)19(20,21)22)10-26-12(2)23-17(27)15-16(26)24-18(29-15)25-6-8-28-9-7-25/h3-5H,6-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta (unknown origin) |
ACS Med Chem Lett 4: 230-4 (2013)
Article DOI: 10.1021/ml300330m
BindingDB Entry DOI: 10.7270/Q2HD7ZJT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50563252
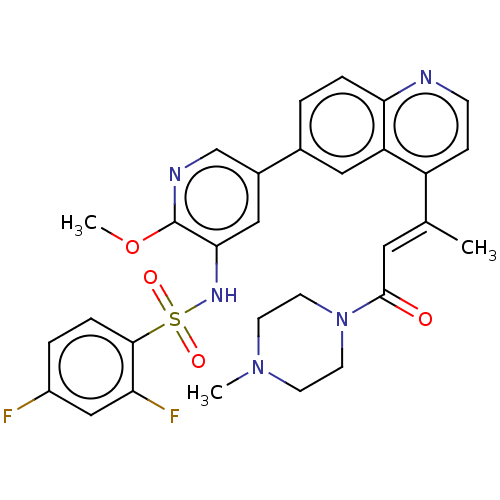
(CHEMBL4739883)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1ccc2nccc(\C(C)=C\C(=O)N3CCN(C)CC3)c2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kbeta (unknown origin) using PIP2 as substrate measured after 1 hr by ADP-glo assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113249
BindingDB Entry DOI: 10.7270/Q2PC3641 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50355691
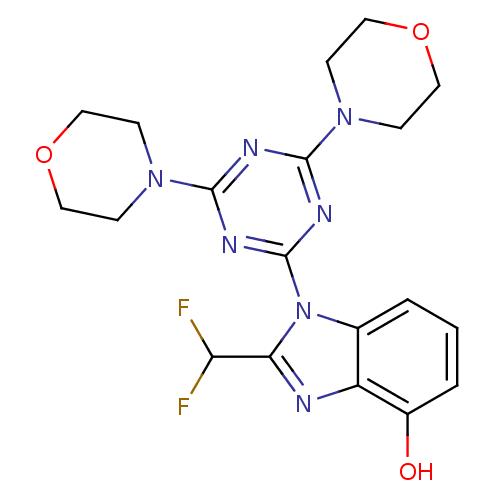
(CHEMBL1910998)Show SMILES Oc1cccc2n(c(nc12)C(F)F)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1 Show InChI InChI=1S/C19H21F2N7O3/c20-15(21)16-22-14-12(2-1-3-13(14)29)28(16)19-24-17(26-4-8-30-9-5-26)23-18(25-19)27-6-10-31-11-7-27/h1-3,15,29H,4-11H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3K p110beta expressed in Sf21 insect cells using phosphatidylinositol as substrate after 1 hr by phosphoimaging |
J Med Chem 54: 7105-26 (2011)
Article DOI: 10.1021/jm200688y
BindingDB Entry DOI: 10.7270/Q20P10FQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM198101
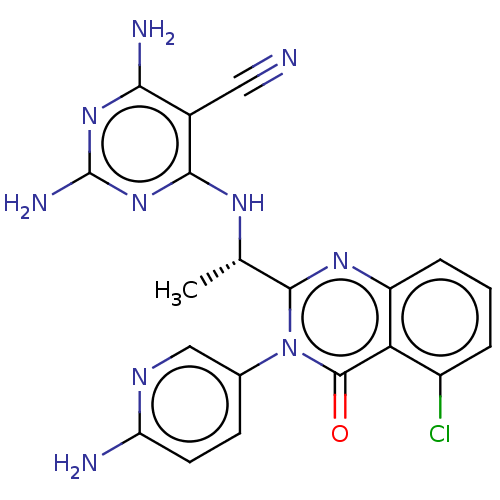
(US9221795, 97)Show SMILES C[C@H](Nc1nc(N)nc(N)c1C#N)c1nc2cccc(Cl)c2c(=O)n1-c1ccc(N)nc1 |r| Show InChI InChI=1S/C20H17ClN10O/c1-9(27-17-11(7-22)16(24)29-20(25)30-17)18-28-13-4-2-3-12(21)15(13)19(32)31(18)10-5-6-14(23)26-8-10/h2-6,8-9H,1H3,(H2,23,26)(H5,24,25,27,29,30)/t9-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Gilead Sciences, Inc.
US Patent
| Assay Description
Class I PI3K isoforms were expressed and purified as heterodimeric recombinant proteins. All assay reagents and buffers for the TR-FRET assay were pu... |
US Patent US9221795 (2015)
BindingDB Entry DOI: 10.7270/Q29C6W8T |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50320101
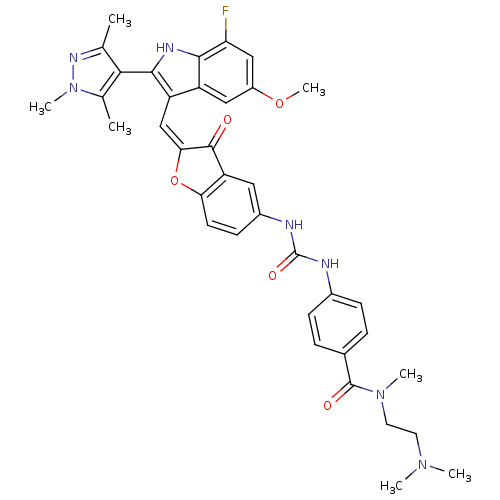
(CHEMBL1082621 | N-(2-(dimethylamino)ethyl)-4-(3-(2...)Show SMILES COc1cc(F)c2[nH]c(c(\C=C3\Oc4ccc(NC(=O)Nc5ccc(cc5)C(=O)N(C)CCN(C)C)cc4C3=O)c2c1)-c1c(C)nn(C)c1C |(16.64,-8.95,;16.64,-10.49,;17.98,-11.27,;17.98,-12.81,;19.32,-13.58,;19.32,-15.12,;20.65,-12.81,;22.12,-13.28,;23.02,-12.03,;22.12,-10.78,;22.71,-9.36,;21.95,-8.02,;22.57,-6.62,;21.42,-5.6,;21.42,-4.06,;20.1,-3.29,;18.76,-4.07,;17.42,-3.3,;17.42,-1.76,;18.75,-.99,;16.08,-1,;16.07,.53,;17.4,1.31,;17.39,2.84,;16.05,3.6,;14.73,2.81,;14.74,1.29,;16.04,5.13,;17.36,5.91,;14.71,5.89,;14.69,7.42,;13.38,5.11,;12.05,5.87,;10.73,5.09,;9.39,5.85,;10.74,3.56,;18.77,-5.6,;20.1,-6.36,;20.42,-7.87,;19.38,-9.02,;20.65,-11.26,;19.31,-10.49,;24.56,-12.03,;25.46,-13.28,;24.99,-14.74,;26.93,-12.8,;26.93,-11.26,;28.18,-10.35,;25.46,-10.78,;24.99,-9.32,)| Show InChI InChI=1S/C37H38FN7O5/c1-20-32(21(2)45(6)42-20)34-27(26-17-25(49-7)18-29(38)33(26)41-34)19-31-35(46)28-16-24(12-13-30(28)50-31)40-37(48)39-23-10-8-22(9-11-23)36(47)44(5)15-14-43(3)4/h8-13,16-19,41H,14-15H2,1-7H3,(H2,39,40,48)/b31-19+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta |
Bioorg Med Chem Lett 20: 3526-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.139
BindingDB Entry DOI: 10.7270/Q2FQ9WSC |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM198091
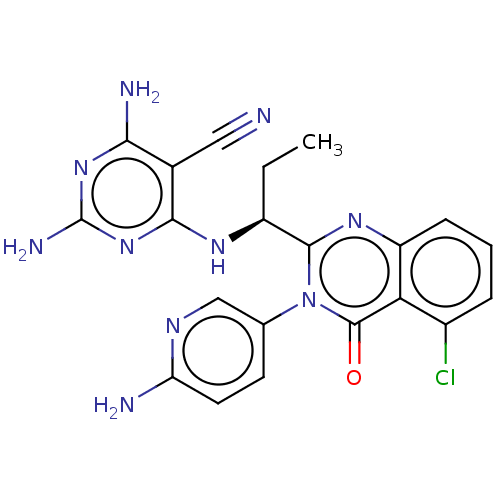
(US9221795, 87)Show SMILES CC[C@H](Nc1nc(N)nc(N)c1C#N)c1nc2cccc(Cl)c2c(=O)n1-c1ccc(N)nc1 |r| Show InChI InChI=1S/C21H19ClN10O/c1-2-13(28-18-11(8-23)17(25)30-21(26)31-18)19-29-14-5-3-4-12(22)16(14)20(33)32(19)10-6-7-15(24)27-9-10/h3-7,9,13H,2H2,1H3,(H2,24,27)(H5,25,26,28,30,31)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Gilead Sciences, Inc.
US Patent
| Assay Description
Class I PI3K isoforms were expressed and purified as heterodimeric recombinant proteins. All assay reagents and buffers for the TR-FRET assay were pu... |
US Patent US9221795 (2015)
BindingDB Entry DOI: 10.7270/Q29C6W8T |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50355687
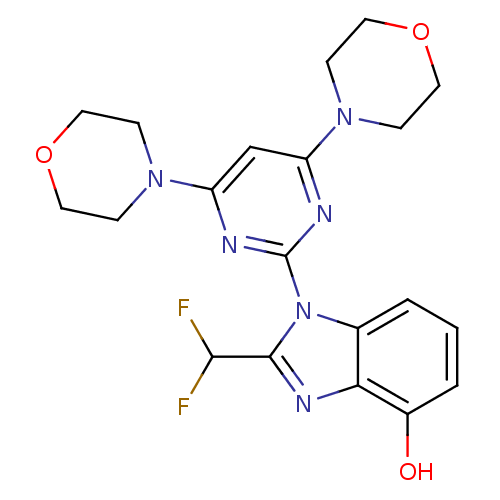
(CHEMBL1911122)Show SMILES Oc1cccc2n(c(nc12)C(F)F)-c1nc(cc(n1)N1CCOCC1)N1CCOCC1 Show InChI InChI=1S/C20H22F2N6O3/c21-18(22)19-25-17-13(2-1-3-14(17)29)28(19)20-23-15(26-4-8-30-9-5-26)12-16(24-20)27-6-10-31-11-7-27/h1-3,12,18,29H,4-11H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3K p110beta expressed in Sf21 insect cells using phosphatidylinositol as substrate after 1 hr by phosphoimaging |
J Med Chem 54: 7105-26 (2011)
Article DOI: 10.1021/jm200688y
BindingDB Entry DOI: 10.7270/Q20P10FQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50059637
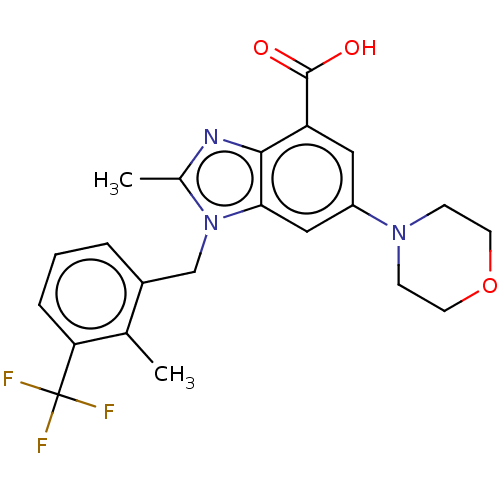
(GSK-2636771 | GSK2636771 | US10660898, Example 31)Show SMILES Cc1nc2c(cc(cc2n1Cc1cccc(c1C)C(F)(F)F)N1CCOCC1)C(O)=O Show InChI InChI=1S/C22H22F3N3O3/c1-13-15(4-3-5-18(13)22(23,24)25)12-28-14(2)26-20-17(21(29)30)10-16(11-19(20)28)27-6-8-31-9-7-27/h3-5,10-11H,6-9,12H2,1-2H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110beta (unknown origin) |
J Med Chem 58: 41-71 (2015)
Article DOI: 10.1021/jm501026z
BindingDB Entry DOI: 10.7270/Q2P84DJ7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50433521
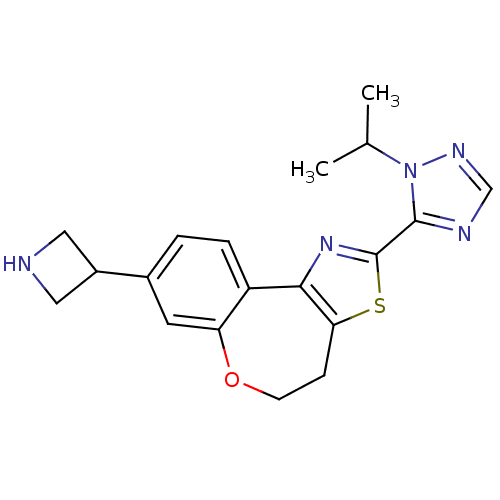
(CHEMBL2381265)Show SMILES CC(C)n1ncnc1-c1nc-2c(CCOc3cc(ccc-23)C2CNC2)s1 Show InChI InChI=1S/C19H21N5OS/c1-11(2)24-18(21-10-22-24)19-23-17-14-4-3-12(13-8-20-9-13)7-15(14)25-6-5-16(17)26-19/h3-4,7,10-11,13,20H,5-6,8-9H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant PI3Kbeta expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presenc... |
Bioorg Med Chem Lett 23: 2606-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.102
BindingDB Entry DOI: 10.7270/Q2W95BKF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50355683
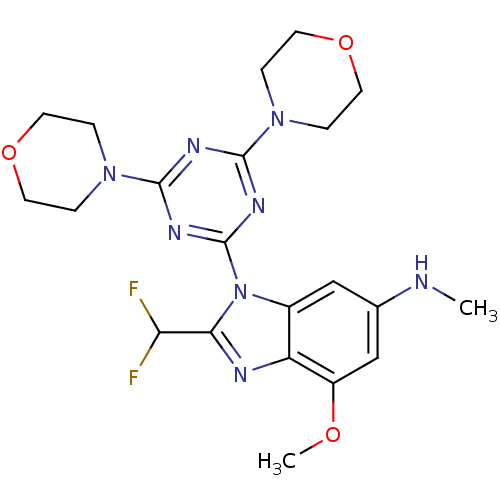
(CHEMBL1911118)Show SMILES CNc1cc(OC)c2nc(C(F)F)n(-c3nc(nc(n3)N3CCOCC3)N3CCOCC3)c2c1 Show InChI InChI=1S/C21H26F2N8O3/c1-24-13-11-14-16(15(12-13)32-2)25-18(17(22)23)31(14)21-27-19(29-3-7-33-8-4-29)26-20(28-21)30-5-9-34-10-6-30/h11-12,17,24H,3-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3K p110beta expressed in Sf21 insect cells using phosphatidylinositol as substrate after 1 hr by phosphoimaging |
J Med Chem 54: 7105-26 (2011)
Article DOI: 10.1021/jm200688y
BindingDB Entry DOI: 10.7270/Q20P10FQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM198097
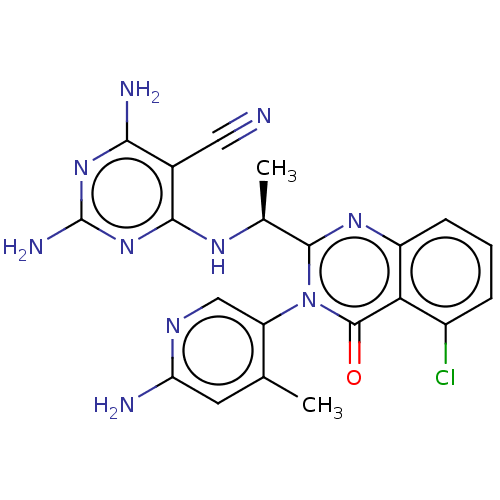
(BDBM198098 | US9221795, 93)Show SMILES C[C@H](Nc1nc(N)nc(N)c1C#N)c1nc2cccc(Cl)c2c(=O)n1-c1cnc(N)cc1C |r,wD:1.0,(2,1.15,;.67,.38,;.67,-1.16,;2,-1.93,;3.33,-1.16,;4.67,-1.93,;6,-1.15,;4.67,-3.47,;3.33,-4.23,;3.33,-5.78,;2,-3.47,;.67,-4.24,;-.67,-5.01,;-.67,1.15,;-2,.38,;-3.33,1.15,;-4.67,.38,;-6,1.15,;-6,2.69,;-4.67,3.46,;-4.67,5,;-3.33,2.69,;-2,3.46,;-2,5,;-.67,2.69,;.67,3.46,;2,2.69,;3.33,3.46,;3.33,5,;4.67,5.77,;2,5.78,;.67,5,;-.67,5.77,)| Show InChI InChI=1S/C21H19ClN10O/c1-9-6-15(24)27-8-14(9)32-19(29-13-5-3-4-12(22)16(13)20(32)33)10(2)28-18-11(7-23)17(25)30-21(26)31-18/h3-6,8,10H,1-2H3,(H2,24,27)(H5,25,26,28,30,31)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Gilead Sciences, Inc.
US Patent
| Assay Description
Class I PI3K isoforms were expressed and purified as heterodimeric recombinant proteins. All assay reagents and buffers for the TR-FRET assay were pu... |
US Patent US9221795 (2015)
BindingDB Entry DOI: 10.7270/Q29C6W8T |
More data for this
Ligand-Target Pair | |
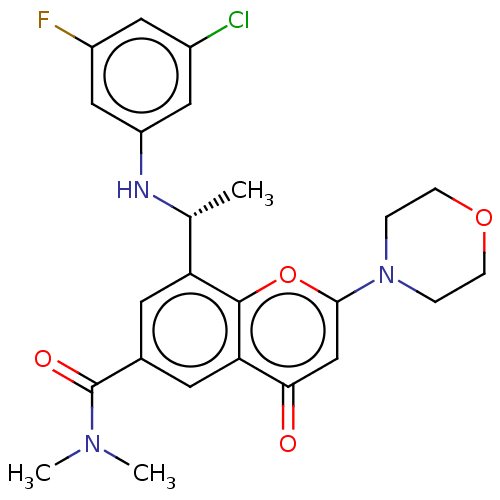
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50070324
(CHEMBL3408252)Show SMILES C[C@@H](Nc1cc(F)cc(Cl)c1)c1cc(cc2c1oc(cc2=O)N1CCOCC1)C(=O)N(C)C |r| Show InChI InChI=1S/C24H25ClFN3O4/c1-14(27-18-11-16(25)10-17(26)12-18)19-8-15(24(31)28(2)3)9-20-21(30)13-22(33-23(19)20)29-4-6-32-7-5-29/h8-14,27H,4-7H2,1-3H3/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN-null human MDA-MB-468 cells assessed as inhibition of Akt phosphorylation after 2 hrs |
J Med Chem 58: 943-62 (2015)
Article DOI: 10.1021/jm501629p
BindingDB Entry DOI: 10.7270/Q2VT1TS8 |
More data for this
Ligand-Target Pair | |
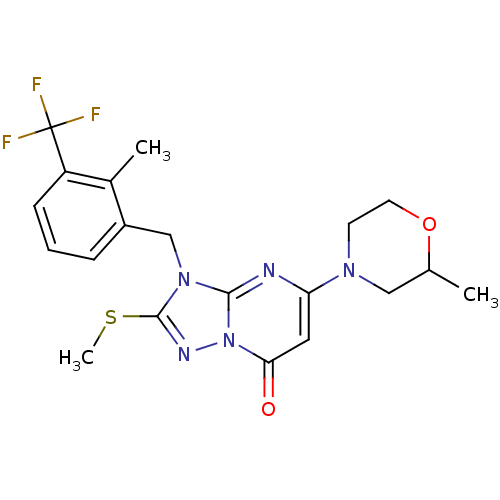
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50381157
(CHEMBL2018360)Show SMILES CSc1nn2c(nc(cc2=O)N2CCOC(C)C2)n1Cc1cccc(c1C)C(F)(F)F Show InChI InChI=1S/C20H22F3N5O2S/c1-12-10-26(7-8-30-12)16-9-17(29)28-18(24-16)27(19(25-28)31-3)11-14-5-4-6-15(13(14)2)20(21,22)23/h4-6,9,12H,7-8,10-11H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta by continuous read time resolved fluorescence resonance energy transfer displacement assay |
Bioorg Med Chem Lett 22: 3198-202 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.039
BindingDB Entry DOI: 10.7270/Q2TD9ZBP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50447089
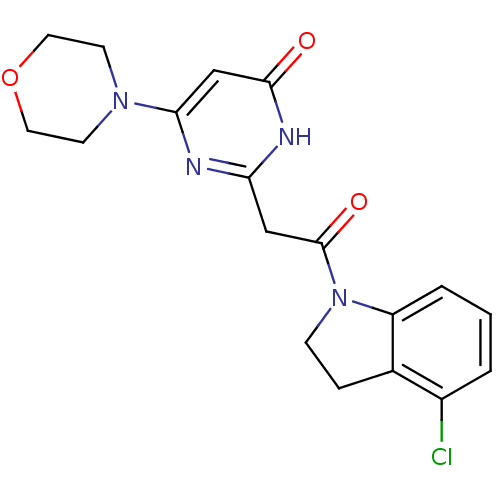
(CHEMBL3112849)Show SMILES Clc1cccc2N(CCc12)C(=O)Cc1nc(cc(=O)[nH]1)N1CCOCC1 Show InChI InChI=1S/C18H19ClN4O3/c19-13-2-1-3-14-12(13)4-5-23(14)18(25)10-15-20-16(11-17(24)21-15)22-6-8-26-9-7-22/h1-3,11H,4-10H2,(H,20,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal ploy-His-tagged human PI3Kbeta expressed in baculovirus-infected sf21 cells using PI(4,5)P2 as substrate after 15 mins by HT... |
J Med Chem 57: 903-20 (2014)
Article DOI: 10.1021/jm401642q
BindingDB Entry DOI: 10.7270/Q2R212VJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50524095
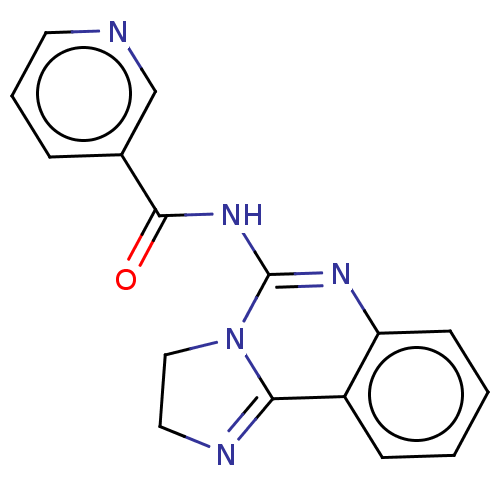
(CHEMBL4532985)Show SMILES O=C(NC1=Nc2ccccc2C2=NCCN12)c1cccnc1 |t:3,12| Show InChI InChI=1S/C16H13N5O/c22-15(11-4-3-7-17-10-11)20-16-19-13-6-2-1-5-12(13)14-18-8-9-21(14)16/h1-7,10H,8-9H2,(H,19,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged truncated human PI3Kbeta (deltaN 1 to 108 residues) after 2 hrs in presence of [33P]ATP by BetaPlate liquid scint... |
J Med Chem 62: 4815-4850 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01492
BindingDB Entry DOI: 10.7270/Q29K4FNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM208929
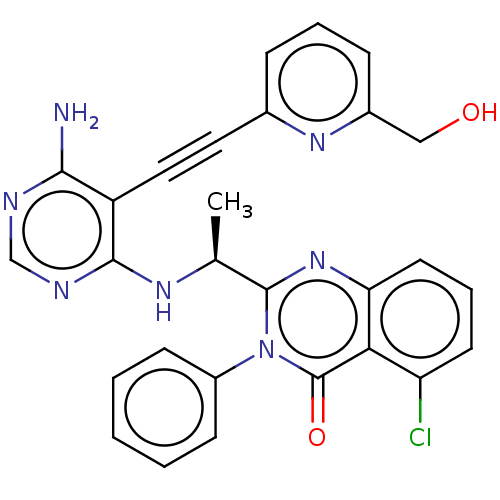
(US9266878, 155a)Show SMILES C[C@H](Nc1ncnc(N)c1C#Cc1cccc(CO)n1)c1nc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C28H22ClN7O2/c1-17(33-26-21(25(30)31-16-32-26)14-13-18-7-5-8-19(15-37)34-18)27-35-23-12-6-11-22(29)24(23)28(38)36(27)20-9-3-2-4-10-20/h2-12,16-17,37H,15H2,1H3,(H3,30,31,32,33)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | >1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Gilead Calistoga LLC
US Patent
| Assay Description
PI3K isoforms were assayed under initial rate conditions in the presence of 25 mM Hepes (pH 7.4), and 2xKm ATP (100-300 uM), 10 uM PIP2, 5% glycerol,... |
US Patent US9266878 (2016)
BindingDB Entry DOI: 10.7270/Q28K77W5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM119230
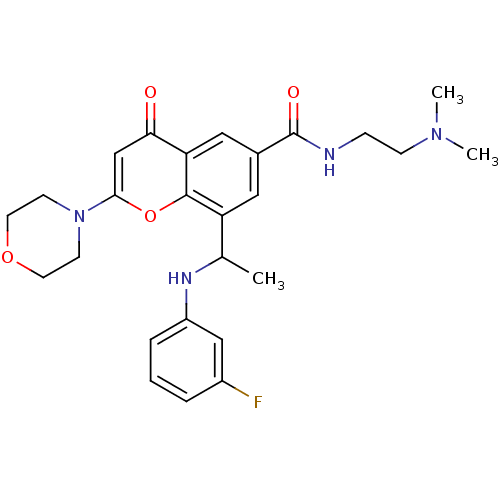
(US8673906, 1.01 | US9718800, 1.01)Show SMILES CC(Nc1cccc(F)c1)c1cc(cc2c1oc(cc2=O)N1CCOCC1)C(=O)NCCN(C)C Show InChI InChI=1S/C26H31FN4O4/c1-17(29-20-6-4-5-19(27)15-20)21-13-18(26(33)28-7-8-30(2)3)14-22-23(32)16-24(35-25(21)22)31-9-11-34-12-10-31/h4-6,13-17,29H,7-12H2,1-3H3,(H,28,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 25 |
ASTRAZENECA AB
US Patent
| Assay Description
Compounds in 100% DMSO were added to assay plates by acoustic dispensing. PI3Kβ was added in a Tris buffer (50 mM Tris pH7.4, 0.05% CHAPS, 2.1 m... |
US Patent US9718800 (2017)
BindingDB Entry DOI: 10.7270/Q2P2714C |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
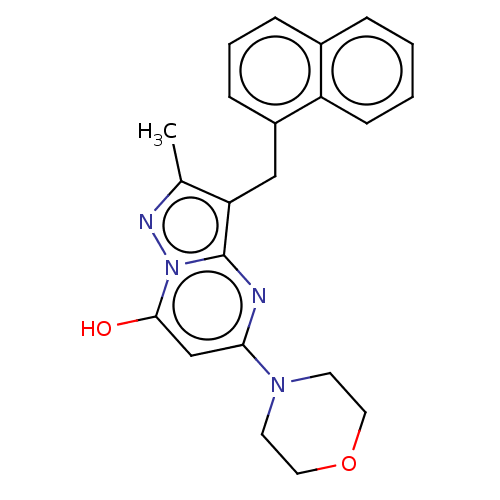
(Homo sapiens (Human)) | BDBM50489460
(CHEMBL2322332)Show InChI InChI=1S/C22H22N4O2/c1-15-19(13-17-7-4-6-16-5-2-3-8-18(16)17)22-23-20(14-21(27)26(22)24-15)25-9-11-28-12-10-25/h2-8,14,27H,9-13H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta (unknown origin) |
ACS Med Chem Lett 4: 230-4 (2013)
Article DOI: 10.1021/ml300330m
BindingDB Entry DOI: 10.7270/Q2HD7ZJT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM119230

(US8673906, 1.01 | US9718800, 1.01)Show SMILES CC(Nc1cccc(F)c1)c1cc(cc2c1oc(cc2=O)N1CCOCC1)C(=O)NCCN(C)C Show InChI InChI=1S/C26H31FN4O4/c1-17(29-20-6-4-5-19(27)15-20)21-13-18(26(33)28-7-8-30(2)3)14-22-23(32)16-24(35-25(21)22)31-9-11-34-12-10-31/h4-6,13-17,29H,7-12H2,1-3H3,(H,28,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The inhibition of PI3Kβ, PI3Kα, PI3Kγ and PI3Kδ was evaluated in a Kinase Glo based enzyme activity assay using human recombinant... |
US Patent US8673906 (2014)
BindingDB Entry DOI: 10.7270/Q2280682 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM350389
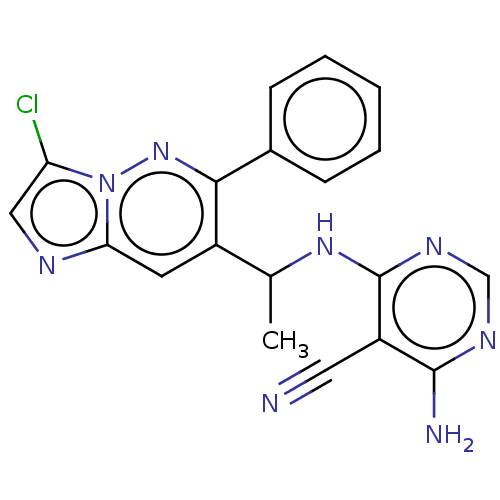
(4-amino-6-((1-(3-chloro-6-phenylimidazo[1,2-b]pyri...)Show SMILES CC(Nc1ncnc(N)c1C#N)c1cc2ncc(Cl)n2nc1-c1ccccc1 Show InChI InChI=1S/C19H15ClN8/c1-11(26-19-14(8-21)18(22)24-10-25-19)13-7-16-23-9-15(20)28(16)27-17(13)12-5-3-2-4-6-12/h2-7,9-11H,1H3,(H3,22,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM198095
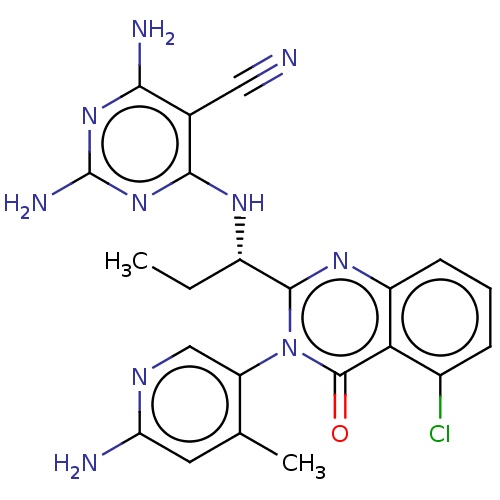
(BDBM198096 | US9221795, 91)Show SMILES CC[C@H](Nc1nc(N)nc(N)c1C#N)c1nc2cccc(Cl)c2c(=O)n1-c1cnc(N)cc1C |r,wD:2.2,(3.33,.38,;2,1.15,;.67,.38,;.67,-1.16,;2,-1.93,;3.33,-1.16,;4.67,-1.93,;6,-1.15,;4.67,-3.47,;3.33,-4.23,;3.33,-5.78,;2,-3.47,;.67,-4.24,;-.67,-5.01,;-.67,1.15,;-2,.38,;-3.33,1.15,;-4.67,.38,;-6,1.15,;-6,2.69,;-4.67,3.46,;-4.67,5,;-3.33,2.69,;-2,3.46,;-2,5,;-.67,2.69,;.67,3.46,;2,2.69,;3.33,3.46,;3.33,5,;4.67,5.77,;2,5.78,;.67,5,;-.67,5.77,)| Show InChI InChI=1S/C22H21ClN10O/c1-3-13(29-19-11(8-24)18(26)31-22(27)32-19)20-30-14-6-4-5-12(23)17(14)21(34)33(20)15-9-28-16(25)7-10(15)2/h4-7,9,13H,3H2,1-2H3,(H2,25,28)(H5,26,27,29,31,32)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Gilead Sciences, Inc.
US Patent
| Assay Description
Class I PI3K isoforms were expressed and purified as heterodimeric recombinant proteins. All assay reagents and buffers for the TR-FRET assay were pu... |
US Patent US9221795 (2015)
BindingDB Entry DOI: 10.7270/Q29C6W8T |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50447088
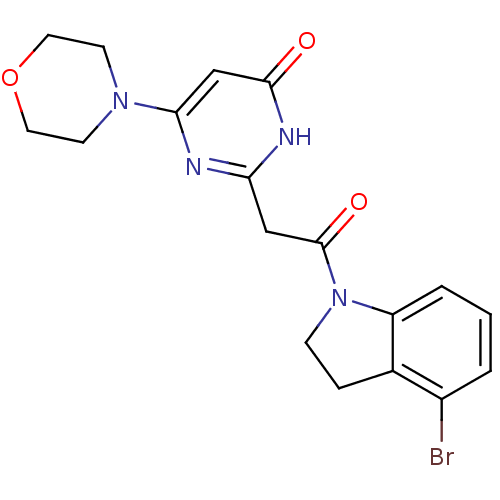
(CHEMBL3112850)Show SMILES Brc1cccc2N(CCc12)C(=O)Cc1nc(cc(=O)[nH]1)N1CCOCC1 Show InChI InChI=1S/C18H19BrN4O3/c19-13-2-1-3-14-12(13)4-5-23(14)18(25)10-15-20-16(11-17(24)21-15)22-6-8-26-9-7-22/h1-3,11H,4-10H2,(H,20,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal ploy-His-tagged human PI3Kbeta expressed in baculovirus-infected sf21 cells using PI(4,5)P2 as substrate after 15 mins by HT... |
J Med Chem 57: 903-20 (2014)
Article DOI: 10.1021/jm401642q
BindingDB Entry DOI: 10.7270/Q2R212VJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50365868

(CHEMBL1957857)Show SMILES Cc1cn2c(nc(cc2=O)N2CCOCC2)n1Cc1cccc(c1C)C(F)(F)F Show InChI InChI=1S/C20H21F3N4O2/c1-13-11-27-18(28)10-17(25-6-8-29-9-7-25)24-19(27)26(13)12-15-4-3-5-16(14(15)2)20(21,22)23/h3-5,10-11H,6-9,12H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta by time-resolved FRET displacement assay |
Bioorg Med Chem Lett 22: 2230-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.092
BindingDB Entry DOI: 10.7270/Q2GH9JF0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM198088

(US9221795, 84)Show SMILES C[C@H](Nc1nc(N)nc(N)c1C#N)c1nc2c(Cl)ccc(Cl)c2c(=O)n1-c1ccc(N)nc1 |r| Show InChI InChI=1S/C20H16Cl2N10O/c1-8(28-17-10(6-23)16(25)30-20(26)31-17)18-29-15-12(22)4-3-11(21)14(15)19(33)32(18)9-2-5-13(24)27-7-9/h2-5,7-8H,1H3,(H2,24,27)(H5,25,26,28,30,31)/t8-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Gilead Sciences, Inc.
US Patent
| Assay Description
Class I PI3K isoforms were expressed and purified as heterodimeric recombinant proteins. All assay reagents and buffers for the TR-FRET assay were pu... |
US Patent US9221795 (2015)
BindingDB Entry DOI: 10.7270/Q29C6W8T |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50365882

(CHEMBL1957870)Show SMILES C[C@@H]1CN(CCO1)c1cc(=O)n2ccn(Cc3cccc(c3C)C(F)(F)F)c2n1 |r| Show InChI InChI=1S/C20H21F3N4O2/c1-13-11-25(8-9-29-13)17-10-18(28)27-7-6-26(19(27)24-17)12-15-4-3-5-16(14(15)2)20(21,22)23/h3-7,10,13H,8-9,11-12H2,1-2H3/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta by time-resolved FRET displacement assay |
Bioorg Med Chem Lett 22: 2230-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.092
BindingDB Entry DOI: 10.7270/Q2GH9JF0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50239117

(CHEMBL4060768)Show SMILES Fc1cc(F)cc(c1)N1CCC[C@@H]1c1cc(cc2c1oc(cc2=O)N1CCOCC1)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C28H29F2N3O5/c29-19-14-20(30)16-21(15-19)33-3-1-2-24(33)22-12-18(28(35)32-6-10-37-11-7-32)13-23-25(34)17-26(38-27(22)23)31-4-8-36-9-5-31/h12-17,24H,1-11H2/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN-deficient human MDA-MB-468 cells assessed as decrease in AKT phosphorylation at Ser473 measured after 2 hrs |
Bioorg Med Chem Lett 27: 1949-1954 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.027
BindingDB Entry DOI: 10.7270/Q2SB47W8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50341416
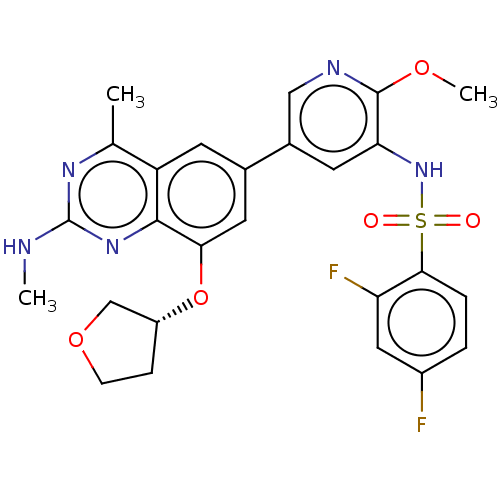
(CHEMBL4174909 | US11534443, Example 32)Show SMILES CNc1nc(C)c2cc(cc(O[C@@H]3CCOC3)c2n1)-c1cnc(OC)c(NS(=O)(=O)c2ccc(F)cc2F)c1 |r| Show InChI InChI=1S/C26H25F2N5O5S/c1-14-19-8-15(10-22(38-18-6-7-37-13-18)24(19)32-26(29-2)31-14)16-9-21(25(36-3)30-12-16)33-39(34,35)23-5-4-17(27)11-20(23)28/h4-5,8-12,18,33H,6-7,13H2,1-3H3,(H,29,31,32)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta (unknown origin) using PIP2 as substrate by ADP-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data