

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM223316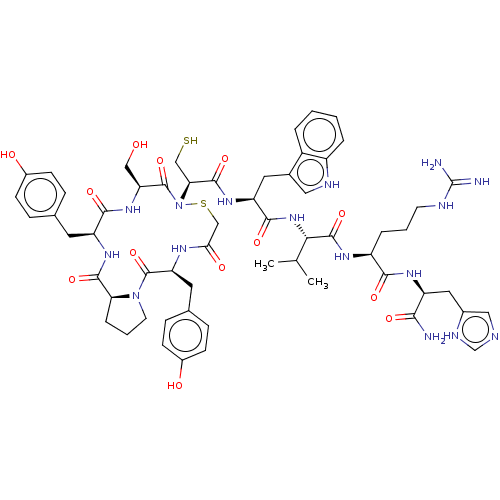 (YPYSCWVRH | piHA-Dm) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | 7.0 | n/a |
The University of Tokyo | Assay Description After kinetic analysis with HPA, piHA-Dm was tested as an inhibitor of ten additional enzymes. The conditions are listed as follows: Agrobacterium fa... | Cell Chem Biol 24: 381-390 (2017) Article DOI: 10.1016/j.chembiol.2017.02.001 BindingDB Entry DOI: 10.7270/Q27D2T0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM50333465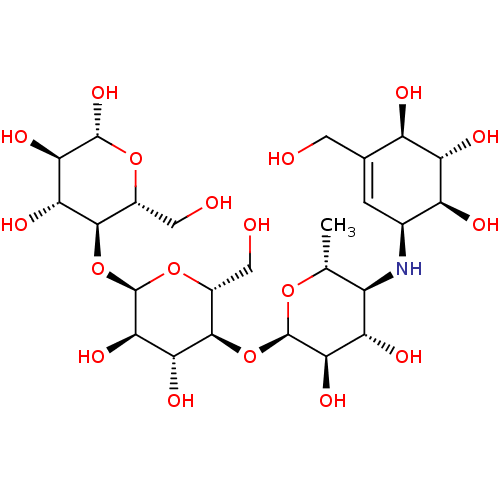 ((2R,3R,4R,5R,6R)-5-((2R,3R,4R,5S,6R)-5-((2R,3R,4S,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human salivary alpha-amylase using GalG2CNP as substrate by UV-Vis spectrophotometric analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM23406 ((3R,4R,5S,6R)-5-{[(2R,3R,4R,5S,6R)-5-{[(2R,3R,4S,5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 996 | n/a | n/a | n/a | n/a | 6.0 | 22 |
Nestle Research Center | Assay Description The assay was carried out at room temperature for 10 min with salivary alpha-amylase, starch, and test compounds. The reducing sugar was determined b... | J Med Chem 51: 3555-61 (2008) Article DOI: 10.1021/jm800115x BindingDB Entry DOI: 10.7270/Q237771Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM50241052 (1,2,3,4,6-Pgg | 1,2,3,4,6-pentakis-O-(3,4,5-trihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human salivary alpha-amylase using GalG2CNP as substrate assessed as CNP liberation by spectrophotometric analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM23411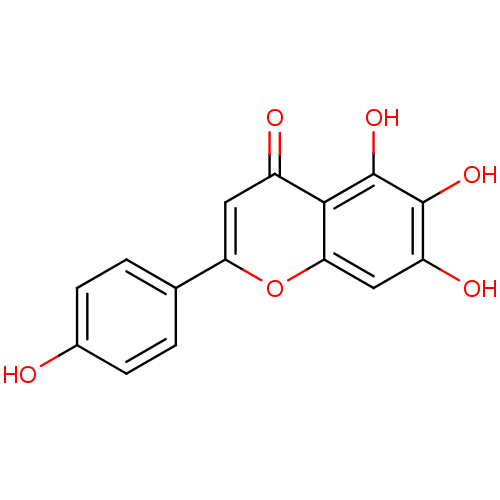 (5,6,7-trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.64E+3 | n/a | n/a | n/a | n/a | 6.0 | 22 |
Nestle Research Center | Assay Description The assay was carried out at room temperature for 10 min with salivary alpha-amylase, starch, and test compounds. The reducing sugar was determined b... | J Med Chem 51: 3555-61 (2008) Article DOI: 10.1021/jm800115x BindingDB Entry DOI: 10.7270/Q237771Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM23408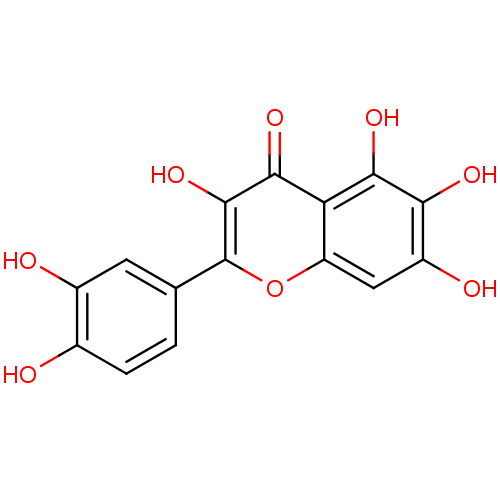 (2-(3,4-dihydroxyphenyl)-3,5,6,7-tetrahydroxy-4H-ch...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | 6.0 | 22 |
Nestle Research Center | Assay Description The assay was carried out at room temperature for 10 min with salivary alpha-amylase, starch, and test compounds. The reducing sugar was determined b... | J Med Chem 51: 3555-61 (2008) Article DOI: 10.1021/jm800115x BindingDB Entry DOI: 10.7270/Q237771Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM7459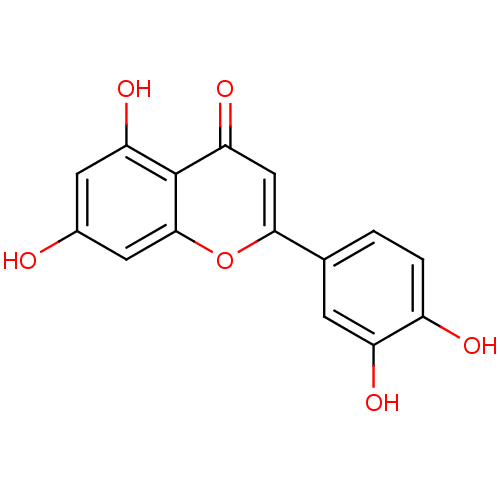 (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.84E+4 | n/a | n/a | n/a | n/a | 6.0 | 22 |
Nestle Research Center | Assay Description The assay was carried out at room temperature for 10 min with salivary alpha-amylase, starch, and test compounds. The reducing sugar was determined b... | J Med Chem 51: 3555-61 (2008) Article DOI: 10.1021/jm800115x BindingDB Entry DOI: 10.7270/Q237771Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM7457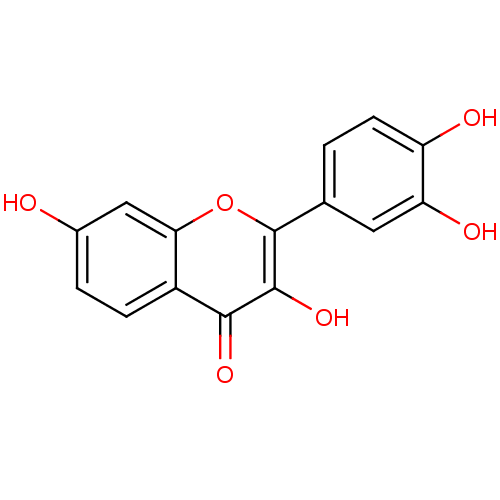 (2-(3,4-dihydroxyphenyl)-3,7-dihydroxy-4H-chromen-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.96E+4 | n/a | n/a | n/a | n/a | 6.0 | 22 |
Nestle Research Center | Assay Description The assay was carried out at room temperature for 10 min with salivary alpha-amylase, starch, and test compounds. The reducing sugar was determined b... | J Med Chem 51: 3555-61 (2008) Article DOI: 10.1021/jm800115x BindingDB Entry DOI: 10.7270/Q237771Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM7460 (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.14E+4 | n/a | n/a | n/a | n/a | 6.0 | 22 |
Nestle Research Center | Assay Description The assay was carried out at room temperature for 10 min with salivary alpha-amylase, starch, and test compounds. The reducing sugar was determined b... | J Med Chem 51: 3555-61 (2008) Article DOI: 10.1021/jm800115x BindingDB Entry DOI: 10.7270/Q237771Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM15236 (3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-4H-chr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 3.02E+4 | n/a | n/a | n/a | n/a | 6.0 | 22 |
Nestle Research Center | Assay Description The assay was carried out at room temperature for 10 min with salivary alpha-amylase, starch, and test compounds. The reducing sugar was determined b... | J Med Chem 51: 3555-61 (2008) Article DOI: 10.1021/jm800115x BindingDB Entry DOI: 10.7270/Q237771Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM50120845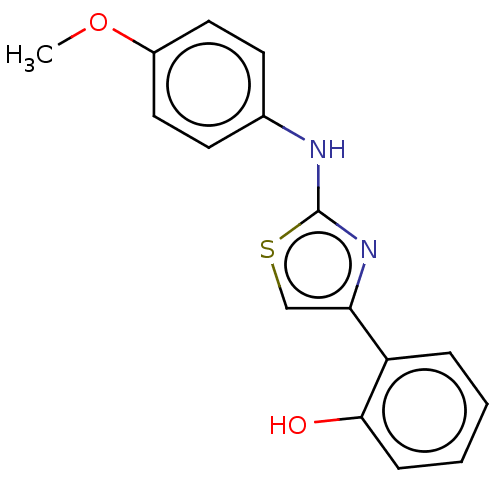 (CHEMBL3618485) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human salivary alpha-amylase using GalG2CNP as substrate by UV-Vis spectrophotometric analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM23412 (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-6-methoxy-4H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | 6.0 | 22 |
Nestle Research Center | Assay Description The assay was carried out at room temperature for 10 min with salivary alpha-amylase, starch, and test compounds. The reducing sugar was determined b... | J Med Chem 51: 3555-61 (2008) Article DOI: 10.1021/jm800115x BindingDB Entry DOI: 10.7270/Q237771Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM50120834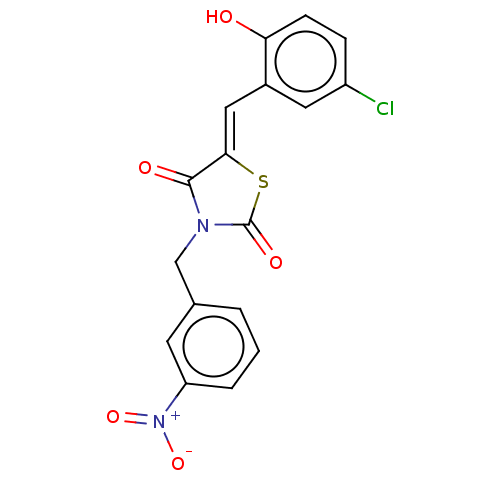 (CHEMBL2011396) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human salivary alpha-amylase using GalG2CNP as substrate by UV-Vis spectrophotometric analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM50120835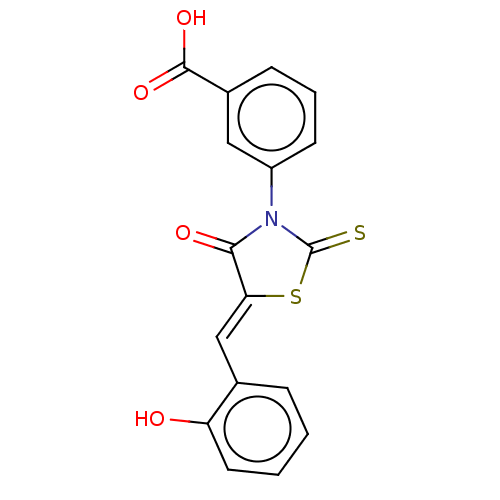 (CHEMBL2011401) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human salivary alpha-amylase using GalG2CNP as substrate by UV-Vis spectrophotometric analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM50120832 (CHEMBL3618483) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human salivary alpha-amylase using GalG2CNP as substrate by UV-Vis spectrophotometric analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM50120831 (CHEMBL3618482) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human salivary alpha-amylase using GalG2CNP as substrate by UV-Vis spectrophotometric analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM50293578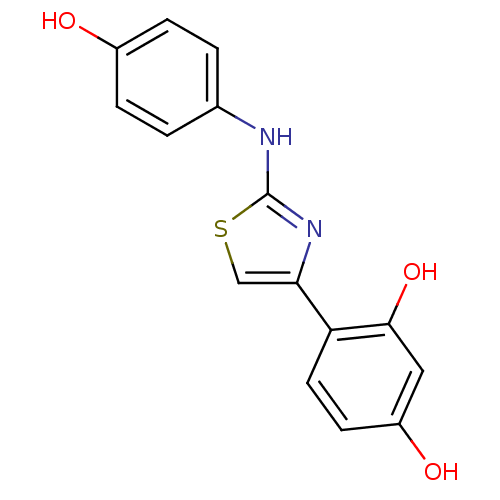 (4-(2-(4-Hydroxyphenylamino)thiazol-4-yl)benzene-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.38E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human salivary alpha-amylase using GalG2CNP as substrate by UV-Vis spectrophotometric analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM50293573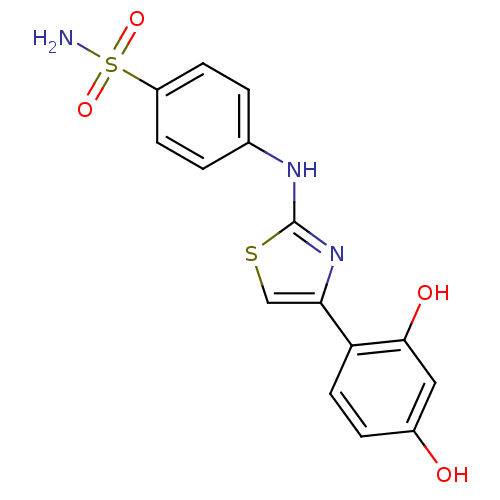 (4-(4-(2,4-dihydroxyphenyl)thiazol-2-ylamino)benzen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.65E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human salivary alpha-amylase using GalG2CNP as substrate by UV-Vis spectrophotometric analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM50120823 (CHEMBL1904421) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human salivary alpha-amylase using GalG2CNP as substrate by UV-Vis spectrophotometric analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM50120836 (CHEMBL572150) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human salivary alpha-amylase using GalG2CNP as substrate by UV-Vis spectrophotometric analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM50120846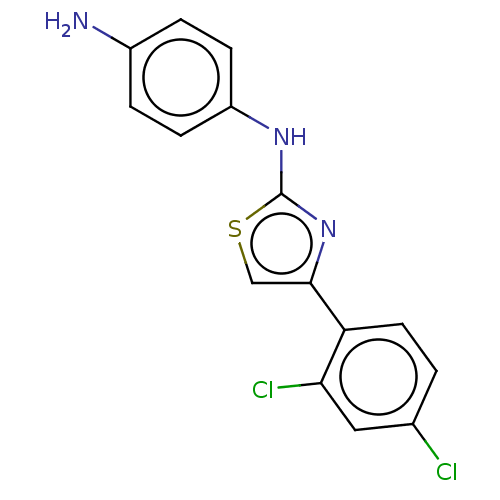 (CHEMBL3618486) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human salivary alpha-amylase using GalG2CNP as substrate by UV-Vis spectrophotometric analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM50120824 (CHEMBL3618478) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human salivary alpha-amylase using GalG2CNP as substrate by UV-Vis spectrophotometric analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM50120826 (CHEMBL1510984) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human salivary alpha-amylase using GalG2CNP as substrate by UV-Vis spectrophotometric analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM50120830 (CHEMBL599385) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.23E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human salivary alpha-amylase using GalG2CNP as substrate by UV-Vis spectrophotometric analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM50293577 (4-(4-(4-Hydroxyphenyl)thiazol-2-ylamino)phenol | C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.92E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human salivary alpha-amylase using GalG2CNP as substrate by UV-Vis spectrophotometric analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM50120829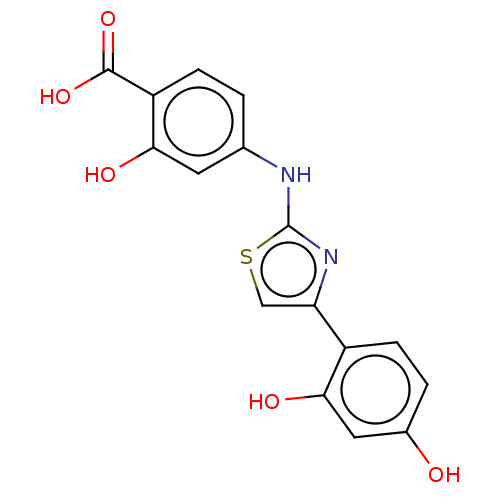 (CHEMBL596726) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.42E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human salivary alpha-amylase using GalG2CNP as substrate by UV-Vis spectrophotometric analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM50120828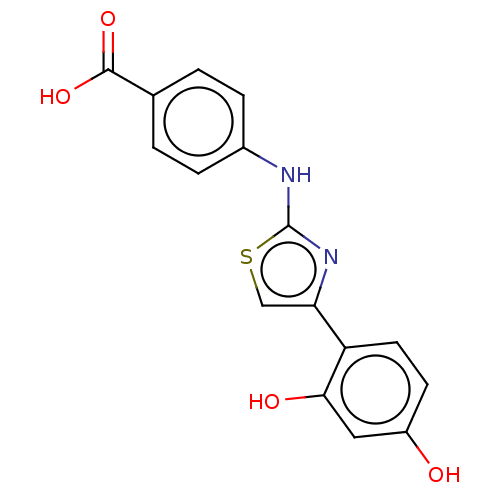 (CHEMBL590969) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human salivary alpha-amylase using GalG2CNP as substrate by UV-Vis spectrophotometric analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM50120837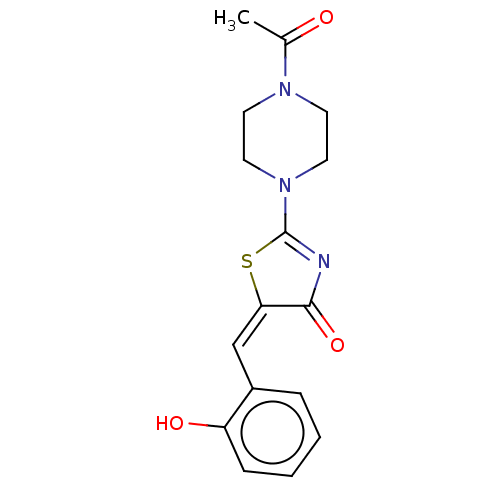 (CHEMBL3618484) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human salivary alpha-amylase using GalG2CNP as substrate by UV-Vis spectrophotometric analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM50051863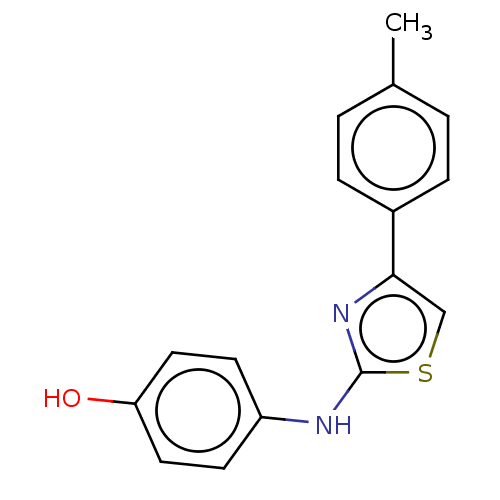 (CHEMBL1081479) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.35E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human salivary alpha-amylase using GalG2CNP as substrate by UV-Vis spectrophotometric analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM50120827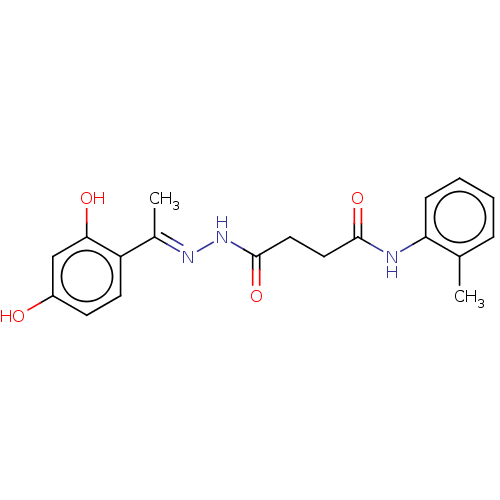 (CHEMBL3618481) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human salivary alpha-amylase using GalG2CNP as substrate by UV-Vis spectrophotometric analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM50120825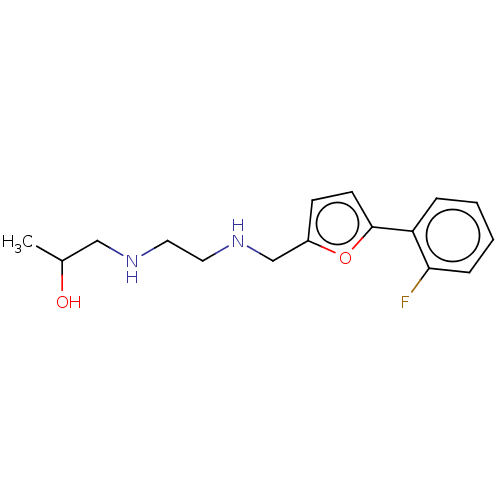 (CHEMBL3618480) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human salivary alpha-amylase using GalG2CNP as substrate by UV-Vis spectrophotometric analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM50312869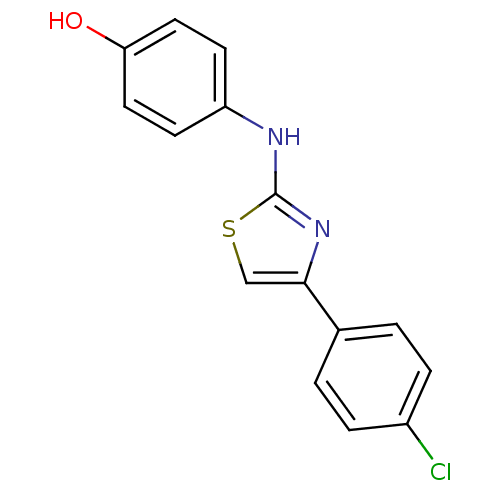 (4-(4-(4-chlorophenyl)thiazol-2-ylamino)phenol | CH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.04E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human salivary alpha-amylase using GalG2CNP as substrate by UV-Vis spectrophotometric analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM50293576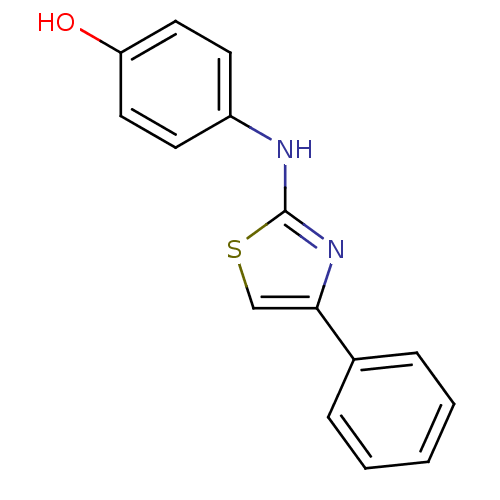 (4-(4-phenylthiazol-2-ylamino)phenol | 4131JH0380 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.27E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human salivary alpha-amylase using GalG2CNP as substrate by UV-Vis spectrophotometric analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM50070942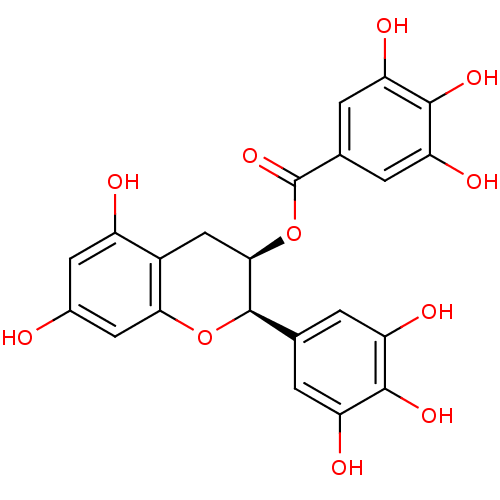 ((-)-Epigallocatechin gallate | (-)-Epigallocatechi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human salivary alpha-amylase using rice starch as substrate after 12 mins by microplate reader analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM50120833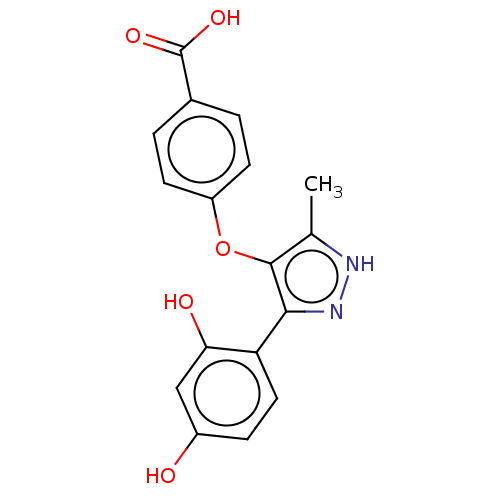 (CHEMBL600247) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human salivary alpha-amylase using GalG2CNP as substrate by UV-Vis spectrophotometric analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM50161650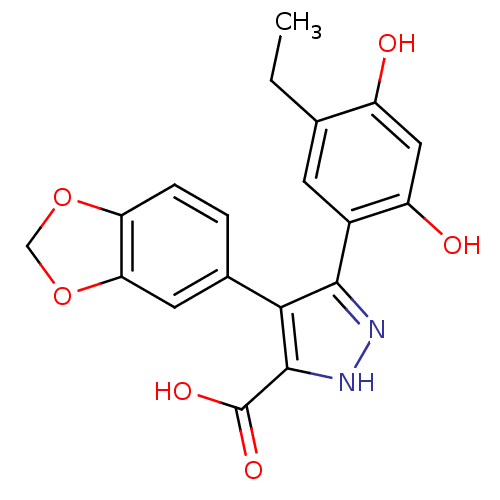 (4-(1,3-BENZODIOXOL-5-YL)-5-(5-ETHYL-2,4-DIHYDROXYP...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 4.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human salivary alpha-amylase using GalG2CNP as substrate by UV-Vis spectrophotometric analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||