 Found 31 hits of ec50 for UniProtKB: P34975
Found 31 hits of ec50 for UniProtKB: P34975 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50138770
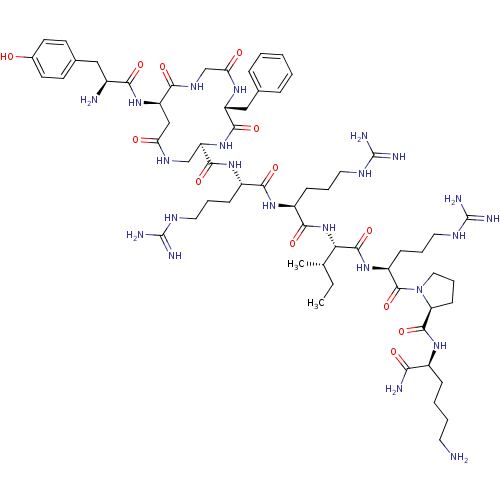
(CHEMBL411282 | cyclo[D-Asp2,Dap5]Dyn A-(1-11)NH2)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H]1CNC(=O)C[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@H](Cc2ccccc2)C(=O)N1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(N)=O Show InChI InChI=1S/C62H99N23O13/c1-3-34(2)49(58(97)81-42(18-11-27-74-62(70)71)59(98)85-28-12-19-46(85)57(96)78-39(50(65)89)15-7-8-24-63)84-54(93)41(17-10-26-73-61(68)69)79-53(92)40(16-9-25-72-60(66)67)80-56(95)45-32-75-47(87)31-44(82-51(90)38(64)29-36-20-22-37(86)23-21-36)52(91)76-33-48(88)77-43(55(94)83-45)30-35-13-5-4-6-14-35/h4-6,13-14,20-23,34,38-46,49,86H,3,7-12,15-19,24-33,63-64H2,1-2H3,(H2,65,89)(H,75,87)(H,76,91)(H,77,88)(H,78,96)(H,79,92)(H,80,95)(H,81,97)(H,82,90)(H,83,94)(H,84,93)(H4,66,67,72)(H4,68,69,73)(H4,70,71,74)/t34-,38-,39-,40-,41-,42-,43+,44+,45+,46-,49-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of forskolin-stimulated adenylyl cyclase activity by compound in CHO cells expressing Opioid receptor kappa 1 |
J Med Chem 47: 446-55 (2004)
Article DOI: 10.1021/jm030298e
BindingDB Entry DOI: 10.7270/Q2Q23ZN2 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50010704

(CHEMBL216640 | Dyn A(1-11)-NH2 | Dynorphin A analo...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(N)=O Show InChI InChI=1S/C63H104N22O12/c1-5-37(4)51(59(96)82-45(20-13-29-75-63(71)72)60(97)85-30-14-21-48(85)58(95)79-42(52(66)89)17-9-10-26-64)84-55(92)44(19-12-28-74-62(69)70)80-54(91)43(18-11-27-73-61(67)68)81-56(93)46(31-36(2)3)83-57(94)47(33-38-15-7-6-8-16-38)78-50(88)35-76-49(87)34-77-53(90)41(65)32-39-22-24-40(86)25-23-39/h6-8,15-16,22-25,36-37,41-48,51,86H,5,9-14,17-21,26-35,64-65H2,1-4H3,(H2,66,89)(H,76,87)(H,77,90)(H,78,88)(H,79,95)(H,80,91)(H,81,93)(H,82,96)(H,83,94)(H,84,92)(H4,67,68,73)(H4,69,70,74)(H4,71,72,75)/t37-,41-,42-,43-,44-,45-,46-,47-,48-,51-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a |
The University of Kansas
Curated by ChEMBL
| Assay Description
Agonist activity at rat cloned kappa opioid receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP production by scintillat... |
J Med Chem 52: 5619-25 (2009)
Article DOI: 10.1021/jm900577k
BindingDB Entry DOI: 10.7270/Q2QN66T6 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50325534
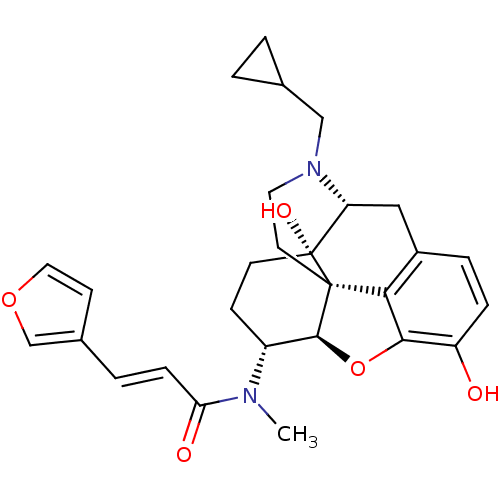
(CHEMBL267495 | nalfurafine)Show SMILES CN([C@@H]1CC[C@@]2(O)[C@H]3Cc4ccc(O)c5O[C@@H]1[C@]2(CCN3CC1CC1)c45)C(=O)\C=C\c1ccoc1 Show InChI InChI=1S/C28H32N2O5/c1-29(23(32)7-4-18-9-13-34-16-18)20-8-10-28(33)22-14-19-5-6-21(31)25-24(19)27(28,26(20)35-25)11-12-30(22)15-17-2-3-17/h4-7,9,13,16-17,20,22,26,31,33H,2-3,8,10-12,14-15H2,1H3/b7-4+/t20-,22-,26+,27+,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
ShanghaiTech University
Curated by ChEMBL
| Assay Description
Agonist activity at GFP-tagged rat kappa opioid receptor expressed in HEK293 cells assessed as increase in ERK1/2 phosphorylation after 5 mins by Wes... |
J Med Chem 61: 9841-9878 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00435
BindingDB Entry DOI: 10.7270/Q2F76GX7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50138766
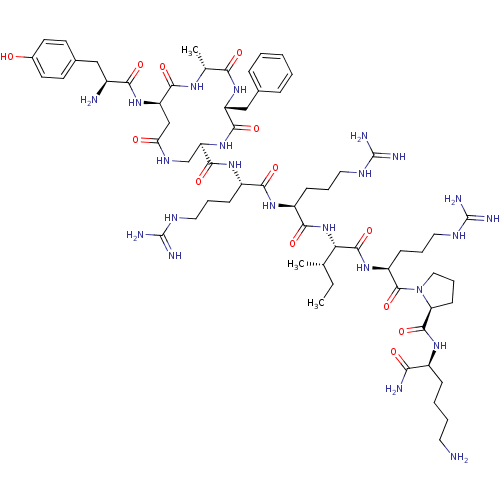
(CHEMBL439272 | cyclo[D-Ala3]Dyn A-(1-11)NH2)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H]1CNC(=O)C[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)N[C@H](C)C(=O)N[C@H](Cc2ccccc2)C(=O)N1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(N)=O Show InChI InChI=1S/C63H101N23O13/c1-4-34(2)49(59(98)81-43(19-12-28-75-63(71)72)60(99)86-29-13-20-47(86)58(97)78-40(50(66)89)16-8-9-25-64)85-54(93)42(18-11-27-74-62(69)70)79-53(92)41(17-10-26-73-61(67)68)80-57(96)46-33-76-48(88)32-45(83-52(91)39(65)30-37-21-23-38(87)24-22-37)55(94)77-35(3)51(90)82-44(56(95)84-46)31-36-14-6-5-7-15-36/h5-7,14-15,21-24,34-35,39-47,49,87H,4,8-13,16-20,25-33,64-65H2,1-3H3,(H2,66,89)(H,76,88)(H,77,94)(H,78,97)(H,79,92)(H,80,96)(H,81,98)(H,82,90)(H,83,91)(H,84,95)(H,85,93)(H4,67,68,73)(H4,69,70,74)(H4,71,72,75)/t34-,35+,39-,40-,41-,42-,43-,44+,45+,46+,47-,49-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of forskolin-stimulated adenylyl cyclase activity by compound in CHO cells expressing Opioid receptor kappa 1 |
J Med Chem 47: 446-55 (2004)
Article DOI: 10.1021/jm030298e
BindingDB Entry DOI: 10.7270/Q2Q23ZN2 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50138780

(CHEMBL261992 | cyclo[Ala3]Dyn A-(1-11)NH2)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H]1CNC(=O)C[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)N[C@@H](C)C(=O)N[C@H](Cc2ccccc2)C(=O)N1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(N)=O Show InChI InChI=1S/C63H101N23O13/c1-4-34(2)49(59(98)81-43(19-12-28-75-63(71)72)60(99)86-29-13-20-47(86)58(97)78-40(50(66)89)16-8-9-25-64)85-54(93)42(18-11-27-74-62(69)70)79-53(92)41(17-10-26-73-61(67)68)80-57(96)46-33-76-48(88)32-45(83-52(91)39(65)30-37-21-23-38(87)24-22-37)55(94)77-35(3)51(90)82-44(56(95)84-46)31-36-14-6-5-7-15-36/h5-7,14-15,21-24,34-35,39-47,49,87H,4,8-13,16-20,25-33,64-65H2,1-3H3,(H2,66,89)(H,76,88)(H,77,94)(H,78,97)(H,79,92)(H,80,96)(H,81,98)(H,82,90)(H,83,91)(H,84,95)(H,85,93)(H4,67,68,73)(H4,69,70,74)(H4,71,72,75)/t34-,35-,39-,40-,41-,42-,43-,44+,45+,46+,47-,49-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of forskolin-stimulated adenylyl cyclase activity by compound in CHO cells expressing Opioid receptor kappa 1 |
J Med Chem 47: 446-55 (2004)
Article DOI: 10.1021/jm030298e
BindingDB Entry DOI: 10.7270/Q2Q23ZN2 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50297345
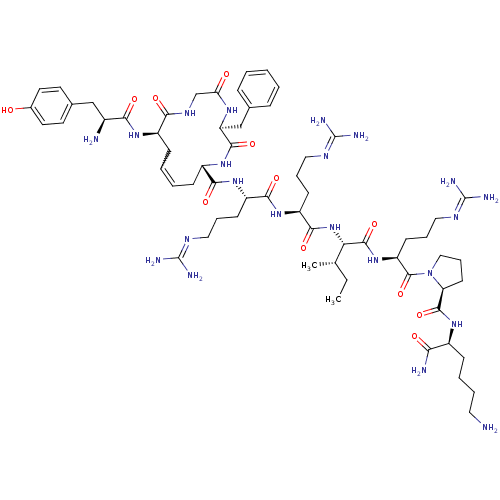
((5S,8S,13R,Z)-13-((S)-2-amino-3-(4-hydroxyphenyl)p...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]=[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c2ccc(-[#8])cc2)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccccc2)-[#6](=O)-[#7]-1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O |r,w:33.33| Show InChI InChI=1S/C63H100N22O12/c1-3-36(2)50(59(96)83-46(22-13-31-75-63(71)72)60(97)85-32-14-23-48(85)58(95)78-41(51(66)88)17-9-10-28-64)84-56(93)45(21-12-30-74-62(69)70)81-55(92)44(20-11-29-73-61(67)68)80-54(91)43-19-8-7-18-42(79-52(89)40(65)33-38-24-26-39(86)27-25-38)53(90)76-35-49(87)77-47(57(94)82-43)34-37-15-5-4-6-16-37/h4-8,15-16,24-27,36,40-48,50,86H,3,9-14,17-23,28-35,64-65H2,1-2H3,(H2,66,88)(H,76,90)(H,77,87)(H,78,95)(H,79,89)(H,80,91)(H,81,92)(H,82,94)(H,83,96)(H,84,93)(H4,67,68,73)(H4,69,70,74)(H4,71,72,75)/t36-,40-,41-,42+,43-,44-,45-,46-,47-,48-,50-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a |
The University of Kansas
Curated by ChEMBL
| Assay Description
Agonist activity at rat cloned kappa opioid receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP production by scintillat... |
J Med Chem 52: 5619-25 (2009)
Article DOI: 10.1021/jm900577k
BindingDB Entry DOI: 10.7270/Q2QN66T6 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50133053
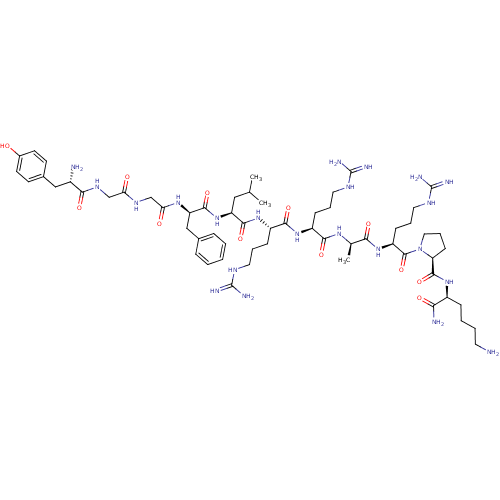
(CHEMBL405057 | Opioid Peptide [d-Ala(8)]Dynorphin ...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(N)=O Show InChI InChI=1S/C60H98N22O12/c1-34(2)29-44(81-55(92)45(31-36-13-5-4-6-14-36)76-48(85)33-73-47(84)32-74-51(88)39(62)30-37-20-22-38(83)23-21-37)54(91)79-42(17-10-26-71-59(66)67)53(90)78-41(16-9-25-70-58(64)65)52(89)75-35(3)50(87)80-43(18-11-27-72-60(68)69)57(94)82-28-12-19-46(82)56(93)77-40(49(63)86)15-7-8-24-61/h4-6,13-14,20-23,34-35,39-46,83H,7-12,15-19,24-33,61-62H2,1-3H3,(H2,63,86)(H,73,84)(H,74,88)(H,75,89)(H,76,85)(H,77,93)(H,78,90)(H,79,91)(H,80,87)(H,81,92)(H4,64,65,70)(H4,66,67,71)(H4,68,69,72)/t35-,39+,40+,41+,42+,43+,44+,45-,46+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.24 | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of Forskolin-Stimulated Adenylyl Cyclase in CHO cells expressing Opioid receptor kappa 1 |
J Med Chem 46: 4002-8 (2003)
Article DOI: 10.1021/jm030075o
BindingDB Entry DOI: 10.7270/Q2CN739H |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50133054

(CHEMBL412228 | Opioid Peptide [d-Ala(8)]Dynorphin ...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](Cc1ccccc1)N(C)C(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(N)=O Show InChI InChI=1S/C61H100N22O12/c1-35(2)30-45(81-57(94)47(32-37-14-6-5-7-15-37)82(4)49(86)34-74-48(85)33-75-52(89)40(63)31-38-21-23-39(84)24-22-38)55(92)79-43(18-11-27-72-60(67)68)54(91)78-42(17-10-26-71-59(65)66)53(90)76-36(3)51(88)80-44(19-12-28-73-61(69)70)58(95)83-29-13-20-46(83)56(93)77-41(50(64)87)16-8-9-25-62/h5-7,14-15,21-24,35-36,40-47,84H,8-13,16-20,25-34,62-63H2,1-4H3,(H2,64,87)(H,74,85)(H,75,89)(H,76,90)(H,77,93)(H,78,91)(H,79,92)(H,80,88)(H,81,94)(H4,65,66,71)(H4,67,68,72)(H4,69,70,73)/t36-,40+,41+,42+,43+,44+,45+,46+,47-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.56 | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of Forskolin-Stimulated Adenylyl Cyclase in CHO cells expressing Opioid receptor kappa 1 |
J Med Chem 46: 4002-8 (2003)
Article DOI: 10.1021/jm030075o
BindingDB Entry DOI: 10.7270/Q2CN739H |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50138777

(CHEMBL414904 | cyclo[Trp3]Dyn A-(1-11)NH2)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H]1CNC(=O)C[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@H](Cc2ccccc2)C(=O)N1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(N)=O Show InChI InChI=1S/C71H106N24O13/c1-3-39(2)57(67(107)89-50(22-13-31-83-71(79)80)68(108)95-32-14-23-55(95)66(106)86-47(58(74)98)19-9-10-28-72)94-61(101)49(21-12-30-82-70(77)78)87-60(100)48(20-11-29-81-69(75)76)88-65(105)54-38-85-56(97)36-53(90-59(99)45(73)33-41-24-26-43(96)27-25-41)64(104)92-52(35-42-37-84-46-18-8-7-17-44(42)46)63(103)91-51(62(102)93-54)34-40-15-5-4-6-16-40/h4-8,15-18,24-27,37,39,45,47-55,57,84,96H,3,9-14,19-23,28-36,38,72-73H2,1-2H3,(H2,74,98)(H,85,97)(H,86,106)(H,87,100)(H,88,105)(H,89,107)(H,90,99)(H,91,103)(H,92,104)(H,93,102)(H,94,101)(H4,75,76,81)(H4,77,78,82)(H4,79,80,83)/t39-,45-,47-,48-,49-,50-,51+,52-,53+,54+,55-,57-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.19 | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of forskolin-stimulated adenylyl cyclase activity by compound in CHO cells expressing Opioid receptor kappa 1 |
J Med Chem 47: 446-55 (2004)
Article DOI: 10.1021/jm030298e
BindingDB Entry DOI: 10.7270/Q2Q23ZN2 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50325534

(CHEMBL267495 | nalfurafine)Show SMILES CN([C@@H]1CC[C@@]2(O)[C@H]3Cc4ccc(O)c5O[C@@H]1[C@]2(CCN3CC1CC1)c45)C(=O)\C=C\c1ccoc1 Show InChI InChI=1S/C28H32N2O5/c1-29(23(32)7-4-18-9-13-34-16-18)20-8-10-28(33)22-14-19-5-6-21(31)25-24(19)27(28,26(20)35-25)11-12-30(22)15-17-2-3-17/h4-7,9,13,16-17,20,22,26,31,33H,2-3,8,10-12,14-15H2,1H3/b7-4+/t20-,22-,26+,27+,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a |
ShanghaiTech University
Curated by ChEMBL
| Assay Description
Agonist activity at GFP-tagged rat kappa opioid receptor expressed in HEK293 cells assessed as increase in beta-arrestin mediated p38 phosphorylation... |
J Med Chem 61: 9841-9878 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00435
BindingDB Entry DOI: 10.7270/Q2F76GX7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50133051

(CHEMBL413832 | Opioid Peptide [d-Ala(8)]Dynorphin ...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](CCc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(N)=O Show InChI InChI=1S/C61H100N22O12/c1-35(2)31-46(82-55(92)44(25-22-37-13-5-4-6-14-37)77-49(86)34-74-48(85)33-75-52(89)40(63)32-38-20-23-39(84)24-21-38)56(93)80-43(17-10-28-72-60(67)68)54(91)79-42(16-9-27-71-59(65)66)53(90)76-36(3)51(88)81-45(18-11-29-73-61(69)70)58(95)83-30-12-19-47(83)57(94)78-41(50(64)87)15-7-8-26-62/h4-6,13-14,20-21,23-24,35-36,40-47,84H,7-12,15-19,22,25-34,62-63H2,1-3H3,(H2,64,87)(H,74,85)(H,75,89)(H,76,90)(H,77,86)(H,78,94)(H,79,91)(H,80,93)(H,81,88)(H,82,92)(H4,65,66,71)(H4,67,68,72)(H4,69,70,73)/t36-,40+,41+,42+,43+,44-,45+,46+,47+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of Forskolin-Stimulated Adenylyl Cyclase in CHO cells expressing Opioid receptor kappa 1 |
J Med Chem 46: 4002-8 (2003)
Article DOI: 10.1021/jm030075o
BindingDB Entry DOI: 10.7270/Q2CN739H |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM224024

(BDBM50241435 | Dynorphin A (1-13) | YGGFLRRXRPKLK)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#8])=O Show InChI InChI=1S/C75H126N24O15/c1-7-45(6)61(70(111)94-53(25-17-35-87-75(83)84)71(112)99-36-18-26-58(99)69(110)93-50(21-11-13-31-76)64(105)96-56(38-44(4)5)67(108)95-54(72(113)114)22-12-14-32-77)98-65(106)52(24-16-34-86-74(81)82)91-63(104)51(23-15-33-85-73(79)80)92-66(107)55(37-43(2)3)97-68(109)57(40-46-19-9-8-10-20-46)90-60(102)42-88-59(101)41-89-62(103)49(78)39-47-27-29-48(100)30-28-47/h8-10,19-20,27-30,43-45,49-58,61,100H,7,11-18,21-26,31-42,76-78H2,1-6H3,(H,88,101)(H,89,103)(H,90,102)(H,91,104)(H,92,107)(H,93,110)(H,94,111)(H,95,108)(H,96,105)(H,97,109)(H,98,106)(H,113,114)(H4,79,80,85)(H4,81,82,86)(H4,83,84,87)/t45-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,61-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Effective concentration to inhibit E203Q,D204N,D206N KL-2 Opioid receptor kappa 1 binding to [35S]GTP-gamma-S, expressed in COS cells |
J Med Chem 43: 1251-2 (2001)
BindingDB Entry DOI: 10.7270/Q270824F |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50381677
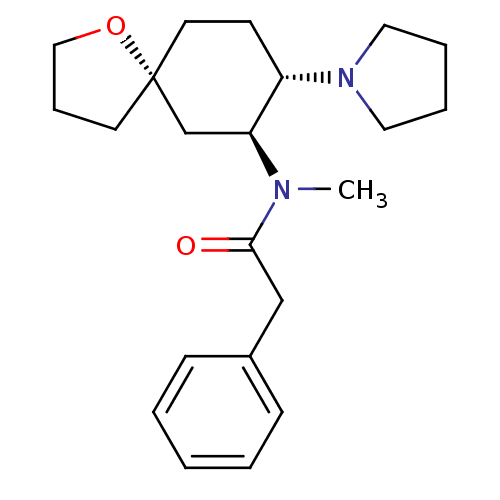
(CHEMBL1256748 | U-69593)Show SMILES CN([C@H]1C[C@@]2(CCCO2)CC[C@@H]1N1CCCC1)C(=O)Cc1ccccc1 |r| Show InChI InChI=1S/C22H32N2O2/c1-23(21(25)16-18-8-3-2-4-9-18)20-17-22(11-7-15-26-22)12-10-19(20)24-13-5-6-14-24/h2-4,8-9,19-20H,5-7,10-17H2,1H3/t19-,20-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara"G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Agonist activity at rat kappa opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay |
ACS Med Chem Lett 5: 1032-6 (2014)
Article DOI: 10.1021/ml500241n
BindingDB Entry DOI: 10.7270/Q23T9JS5 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM224024

(BDBM50241435 | Dynorphin A (1-13) | YGGFLRRXRPKLK)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#8])=O Show InChI InChI=1S/C75H126N24O15/c1-7-45(6)61(70(111)94-53(25-17-35-87-75(83)84)71(112)99-36-18-26-58(99)69(110)93-50(21-11-13-31-76)64(105)96-56(38-44(4)5)67(108)95-54(72(113)114)22-12-14-32-77)98-65(106)52(24-16-34-86-74(81)82)91-63(104)51(23-15-33-85-73(79)80)92-66(107)55(37-43(2)3)97-68(109)57(40-46-19-9-8-10-20-46)90-60(102)42-88-59(101)41-89-62(103)49(78)39-47-27-29-48(100)30-28-47/h8-10,19-20,27-30,43-45,49-58,61,100H,7,11-18,21-26,31-42,76-78H2,1-6H3,(H,88,101)(H,89,103)(H,90,102)(H,91,104)(H,92,107)(H,93,110)(H,94,111)(H,95,108)(H,96,105)(H,97,109)(H,98,106)(H,113,114)(H4,79,80,85)(H4,81,82,86)(H4,83,84,87)/t45-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,61-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Effective concentration to inhibit D216,ND217N,E218Q KL-2 Opioid receptor kappa 1 binding to [35S]GTP-gamma-S, expressed in COS cells |
J Med Chem 43: 1251-2 (2001)
BindingDB Entry DOI: 10.7270/Q270824F |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50297347
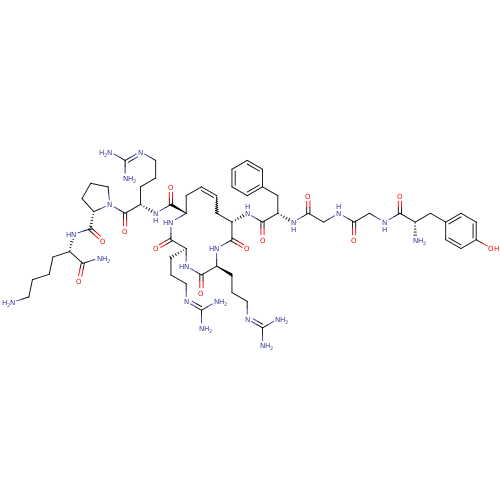
((2S,5S,8S,13S,Z)-13-((S)-2-(2-(2-((S)-2-amino-3-(4...)Show SMILES [#7]-[#6]-[#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]=[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c2ccc(-[#8])cc2)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1)-[#6](-[#7])=O |r,w:30.31| Show InChI InChI=1S/C59H92N22O12/c60-25-7-6-14-38(48(62)85)75-55(92)45-20-11-29-81(45)56(93)43(19-10-28-71-59(67)68)80-53(90)40-16-5-4-15-39(50(87)77-42(18-9-27-70-58(65)66)52(89)78-41(51(88)76-40)17-8-26-69-57(63)64)79-54(91)44(31-34-12-2-1-3-13-34)74-47(84)33-72-46(83)32-73-49(86)37(61)30-35-21-23-36(82)24-22-35/h1-5,12-13,21-24,37-45,82H,6-11,14-20,25-33,60-61H2,(H2,62,85)(H,72,83)(H,73,86)(H,74,84)(H,75,92)(H,76,88)(H,77,87)(H,78,89)(H,79,91)(H,80,90)(H4,63,64,69)(H4,65,66,70)(H4,67,68,71)/t37-,38-,39-,40-,41-,42-,43-,44-,45-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a |
The University of Kansas
Curated by ChEMBL
| Assay Description
Agonist activity at rat cloned kappa opioid receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP production by scintillat... |
J Med Chem 52: 5619-25 (2009)
Article DOI: 10.1021/jm900577k
BindingDB Entry DOI: 10.7270/Q2QN66T6 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50138765
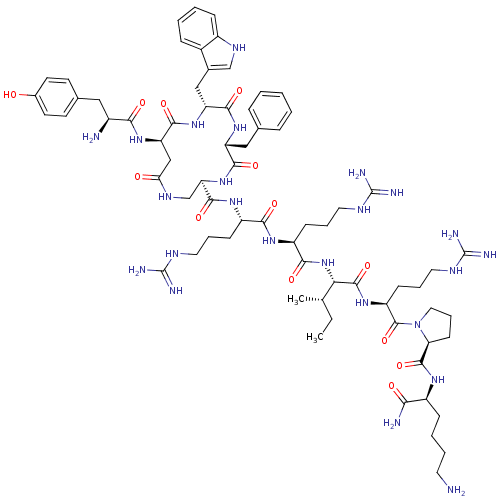
(CHEMBL405307 | cyclo[D-Trp3]Dyn A-(1-11)NH2)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H]1CNC(=O)C[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@H](Cc2ccccc2)C(=O)N1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(N)=O Show InChI InChI=1S/C71H106N24O13/c1-3-39(2)57(67(107)89-50(22-13-31-83-71(79)80)68(108)95-32-14-23-55(95)66(106)86-47(58(74)98)19-9-10-28-72)94-61(101)49(21-12-30-82-70(77)78)87-60(100)48(20-11-29-81-69(75)76)88-65(105)54-38-85-56(97)36-53(90-59(99)45(73)33-41-24-26-43(96)27-25-41)64(104)92-52(35-42-37-84-46-18-8-7-17-44(42)46)63(103)91-51(62(102)93-54)34-40-15-5-4-6-16-40/h4-8,15-18,24-27,37,39,45,47-55,57,84,96H,3,9-14,19-23,28-36,38,72-73H2,1-2H3,(H2,74,98)(H,85,97)(H,86,106)(H,87,100)(H,88,105)(H,89,107)(H,90,99)(H,91,103)(H,92,104)(H,93,102)(H,94,101)(H4,75,76,81)(H4,77,78,82)(H4,79,80,83)/t39-,45-,47-,48-,49-,50-,51+,52+,53+,54+,55-,57-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of forskolin-stimulated adenylyl cyclase activity by compound in CHO cells expressing Opioid receptor kappa 1 |
J Med Chem 47: 446-55 (2004)
Article DOI: 10.1021/jm030298e
BindingDB Entry DOI: 10.7270/Q2Q23ZN2 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM224024

(BDBM50241435 | Dynorphin A (1-13) | YGGFLRRXRPKLK)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#8])=O Show InChI InChI=1S/C75H126N24O15/c1-7-45(6)61(70(111)94-53(25-17-35-87-75(83)84)71(112)99-36-18-26-58(99)69(110)93-50(21-11-13-31-76)64(105)96-56(38-44(4)5)67(108)95-54(72(113)114)22-12-14-32-77)98-65(106)52(24-16-34-86-74(81)82)91-63(104)51(23-15-33-85-73(79)80)92-66(107)55(37-43(2)3)97-68(109)57(40-46-19-9-8-10-20-46)90-60(102)42-88-59(101)41-89-62(103)49(78)39-47-27-29-48(100)30-28-47/h8-10,19-20,27-30,43-45,49-58,61,100H,7,11-18,21-26,31-42,76-78H2,1-6H3,(H,88,101)(H,89,103)(H,90,102)(H,91,104)(H,92,107)(H,93,110)(H,94,111)(H,95,108)(H,96,105)(H,97,109)(H,98,106)(H,113,114)(H4,79,80,85)(H4,81,82,86)(H4,83,84,87)/t45-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,61-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | n/a | n/a | 18 | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Effective concentration to inhibit wild type KL-2 Opioid receptor kappa 1 binding to [35S]GTP-gamma-S, expressed in COS cells |
J Med Chem 43: 1251-2 (2001)
BindingDB Entry DOI: 10.7270/Q270824F |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50297347

((2S,5S,8S,13S,Z)-13-((S)-2-(2-(2-((S)-2-amino-3-(4...)Show SMILES [#7]-[#6]-[#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]=[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c2ccc(-[#8])cc2)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1)-[#6](-[#7])=O |r,w:30.31| Show InChI InChI=1S/C59H92N22O12/c60-25-7-6-14-38(48(62)85)75-55(92)45-20-11-29-81(45)56(93)43(19-10-28-71-59(67)68)80-53(90)40-16-5-4-15-39(50(87)77-42(18-9-27-70-58(65)66)52(89)78-41(51(88)76-40)17-8-26-69-57(63)64)79-54(91)44(31-34-12-2-1-3-13-34)74-47(84)33-72-46(83)32-73-49(86)37(61)30-35-21-23-36(82)24-22-35/h1-5,12-13,21-24,37-45,82H,6-11,14-20,25-33,60-61H2,(H2,62,85)(H,72,83)(H,73,86)(H,74,84)(H,75,92)(H,76,88)(H,77,87)(H,78,89)(H,79,91)(H,80,90)(H4,63,64,69)(H4,65,66,70)(H4,67,68,71)/t37-,38-,39-,40-,41-,42-,43-,44-,45-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a |
The University of Kansas
Curated by ChEMBL
| Assay Description
Agonist activity at rat cloned kappa opioid receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP production by scintillat... |
J Med Chem 52: 5619-25 (2009)
Article DOI: 10.1021/jm900577k
BindingDB Entry DOI: 10.7270/Q2QN66T6 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50133049
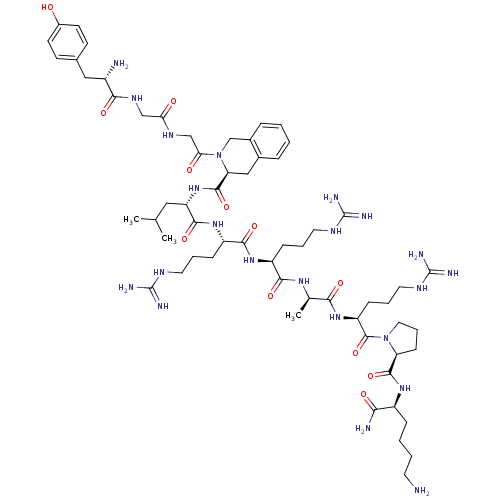
(CHEMBL262310 | Opioid Peptide [d-Ala(8)]Dynorphin ...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H]1Cc2ccccc2CN1C(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(N)=O Show InChI InChI=1S/C61H98N22O12/c1-34(2)28-45(81-57(94)47-30-37-12-4-5-13-38(37)33-83(47)49(86)32-74-48(85)31-75-52(89)40(63)29-36-19-21-39(84)22-20-36)55(92)79-43(16-9-25-72-60(67)68)54(91)78-42(15-8-24-71-59(65)66)53(90)76-35(3)51(88)80-44(17-10-26-73-61(69)70)58(95)82-27-11-18-46(82)56(93)77-41(50(64)87)14-6-7-23-62/h4-5,12-13,19-22,34-35,40-47,84H,6-11,14-18,23-33,62-63H2,1-3H3,(H2,64,87)(H,74,85)(H,75,89)(H,76,90)(H,77,93)(H,78,91)(H,79,92)(H,80,88)(H,81,94)(H4,65,66,71)(H4,67,68,72)(H4,69,70,73)/t35-,40+,41+,42+,43+,44+,45+,46+,47+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 29.4 | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of Forskolin-Stimulated Adenylyl Cyclase in CHO cells expressing Opioid receptor kappa 1 |
J Med Chem 46: 4002-8 (2003)
Article DOI: 10.1021/jm030075o
BindingDB Entry DOI: 10.7270/Q2CN739H |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50133055

(CHEMBL441198 | Opioid Peptide [d-Ala(8)]Dynorphin ...)Show SMILES CC(C)C[C@H](NC(=O)[C@@](C)(Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(N)=O Show InChI InChI=1S/C61H100N22O12/c1-35(2)30-45(81-57(95)61(4,32-38-14-6-5-7-15-38)82-48(86)34-74-47(85)33-75-51(89)40(63)31-37-21-23-39(84)24-22-37)54(92)79-43(18-11-27-72-59(67)68)53(91)78-42(17-10-26-71-58(65)66)52(90)76-36(3)50(88)80-44(19-12-28-73-60(69)70)56(94)83-29-13-20-46(83)55(93)77-41(49(64)87)16-8-9-25-62/h5-7,14-15,21-24,35-36,40-46,84H,8-13,16-20,25-34,62-63H2,1-4H3,(H2,64,87)(H,74,85)(H,75,89)(H,76,90)(H,77,93)(H,78,91)(H,79,92)(H,80,88)(H,81,95)(H,82,86)(H4,65,66,71)(H4,67,68,72)(H4,69,70,73)/t36-,40+,41+,42+,43+,44+,45+,46+,61-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 36.5 | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of Forskolin-Stimulated Adenylyl Cyclase in CHO cells expressing Opioid receptor kappa 1 |
J Med Chem 46: 4002-8 (2003)
Article DOI: 10.1021/jm030075o
BindingDB Entry DOI: 10.7270/Q2CN739H |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50133052
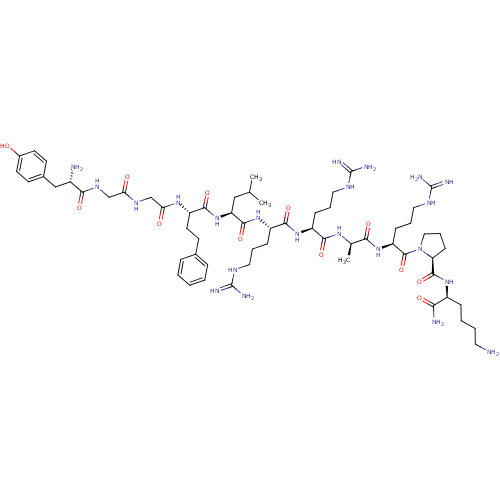
(CHEMBL430081 | Opioid Peptide [d-Ala(8)]Dynorphin ...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(N)=O Show InChI InChI=1S/C61H100N22O12/c1-35(2)31-46(82-55(92)44(25-22-37-13-5-4-6-14-37)77-49(86)34-74-48(85)33-75-52(89)40(63)32-38-20-23-39(84)24-21-38)56(93)80-43(17-10-28-72-60(67)68)54(91)79-42(16-9-27-71-59(65)66)53(90)76-36(3)51(88)81-45(18-11-29-73-61(69)70)58(95)83-30-12-19-47(83)57(94)78-41(50(64)87)15-7-8-26-62/h4-6,13-14,20-21,23-24,35-36,40-47,84H,7-12,15-19,22,25-34,62-63H2,1-3H3,(H2,64,87)(H,74,85)(H,75,89)(H,76,90)(H,77,86)(H,78,94)(H,79,91)(H,80,93)(H,81,88)(H,82,92)(H4,65,66,71)(H4,67,68,72)(H4,69,70,73)/t36-,40+,41+,42+,43+,44+,45+,46+,47+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 46.9 | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of Forskolin-Stimulated Adenylyl Cyclase in CHO cells expressing Opioid receptor kappa 1 |
J Med Chem 46: 4002-8 (2003)
Article DOI: 10.1021/jm030075o
BindingDB Entry DOI: 10.7270/Q2CN739H |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50133050
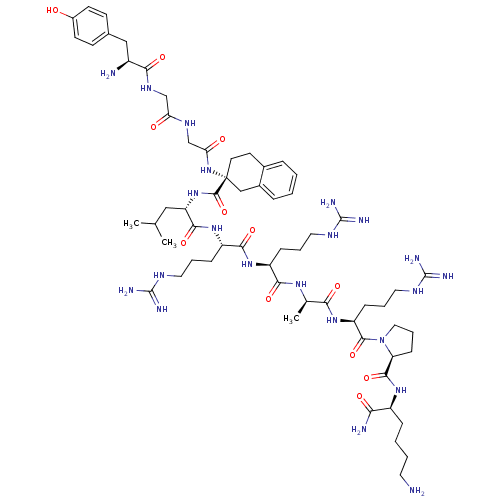
(CHEMBL385696 | Opioid Peptide [d-Ala(8)]Dynorphin ...)Show SMILES CC(C)C[C@H](NC(=O)[C@]1(CCc2ccccc2C1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(N)=O Show InChI InChI=1S/C62H100N22O12/c1-35(2)30-46(82-58(96)62(24-23-38-12-4-5-13-39(38)32-62)83-49(87)34-75-48(86)33-76-52(90)41(64)31-37-19-21-40(85)22-20-37)55(93)80-44(16-9-27-73-60(68)69)54(92)79-43(15-8-26-72-59(66)67)53(91)77-36(3)51(89)81-45(17-10-28-74-61(70)71)57(95)84-29-11-18-47(84)56(94)78-42(50(65)88)14-6-7-25-63/h4-5,12-13,19-22,35-36,41-47,85H,6-11,14-18,23-34,63-64H2,1-3H3,(H2,65,88)(H,75,86)(H,76,90)(H,77,91)(H,78,94)(H,79,92)(H,80,93)(H,81,89)(H,82,96)(H,83,87)(H4,66,67,72)(H4,68,69,73)(H4,70,71,74)/t36-,41+,42+,43+,44+,45+,46+,47+,62-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 72.4 | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of Forskolin-Stimulated Adenylyl Cyclase in CHO cells expressing Opioid receptor kappa 1 |
J Med Chem 46: 4002-8 (2003)
Article DOI: 10.1021/jm030075o
BindingDB Entry DOI: 10.7270/Q2CN739H |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50138771
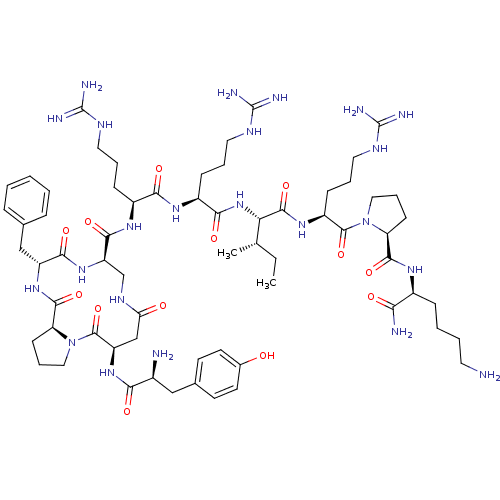
(CHEMBL438044 | cyclo[Pro3]Dyn A-(1-11)NH2)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H]1CNC(=O)C[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)N2CCC[C@H]2C(=O)N[C@H](Cc2ccccc2)C(=O)N1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(N)=O Show InChI InChI=1S/C65H103N23O13/c1-3-36(2)51(60(99)82-44(19-11-29-77-65(73)74)61(100)87-30-12-20-48(87)58(97)79-41(52(68)91)16-7-8-26-66)86-55(94)43(18-10-28-76-64(71)72)80-54(93)42(17-9-27-75-63(69)70)81-57(96)47-35-78-50(90)34-46(84-53(92)40(67)32-38-22-24-39(89)25-23-38)62(101)88-31-13-21-49(88)59(98)83-45(56(95)85-47)33-37-14-5-4-6-15-37/h4-6,14-15,22-25,36,40-49,51,89H,3,7-13,16-21,26-35,66-67H2,1-2H3,(H2,68,91)(H,78,90)(H,79,97)(H,80,93)(H,81,96)(H,82,99)(H,83,98)(H,84,92)(H,85,95)(H,86,94)(H4,69,70,75)(H4,71,72,76)(H4,73,74,77)/t36-,40-,41-,42-,43-,44-,45+,46+,47+,48-,49-,51-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 153 | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of forskolin-stimulated adenylyl cyclase activity by compound in CHO cells expressing Opioid receptor kappa 1 |
J Med Chem 47: 446-55 (2004)
Article DOI: 10.1021/jm030298e
BindingDB Entry DOI: 10.7270/Q2Q23ZN2 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50297343
((5S,8S,13S,Z)-13-((S)-2-amino-3-(4-hydroxyphenyl)p...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CC=CC[C@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)N1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(N)=O |r,w:33.33| Show InChI InChI=1S/C63H100N22O12/c1-3-36(2)50(59(96)83-46(22-13-31-75-63(71)72)60(97)85-32-14-23-48(85)58(95)78-41(51(66)88)17-9-10-28-64)84-56(93)45(21-12-30-74-62(69)70)81-55(92)44(20-11-29-73-61(67)68)80-54(91)43-19-8-7-18-42(79-52(89)40(65)33-38-24-26-39(86)27-25-38)53(90)76-35-49(87)77-47(57(94)82-43)34-37-15-5-4-6-16-37/h4-8,15-16,24-27,36,40-48,50,86H,3,9-14,17-23,28-35,64-65H2,1-2H3,(H2,66,88)(H,76,90)(H,77,87)(H,78,95)(H,79,89)(H,80,91)(H,81,92)(H,82,94)(H,83,96)(H,84,93)(H4,67,68,73)(H4,69,70,74)(H4,71,72,75)/t36-,40-,41-,42-,43-,44-,45-,46-,47-,48-,50-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 190 | n/a | n/a | n/a | n/a |
The University of Kansas
Curated by ChEMBL
| Assay Description
Agonist activity at rat cloned kappa opioid receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP production by scintillat... |
J Med Chem 52: 5619-25 (2009)
Article DOI: 10.1021/jm900577k
BindingDB Entry DOI: 10.7270/Q2QN66T6 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50026763
(CHEMBL3331511)Show SMILES CC1(C)SSC(C)(C)[C@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)NNC(=O)[C@H](Cc2ccccc2)NC(=O)CNC(=O)[C@H]1NC(=O)[C@@H](N)Cc1ccc(O)cc1 |r| Show InChI InChI=1S/C50H62N10O10S2/c1-49(2)41(57-43(65)35(51)23-31-15-19-33(61)20-16-31)47(69)53-27-39(63)55-37(25-29-11-7-5-8-12-29)45(67)59-60-46(68)38(26-30-13-9-6-10-14-30)56-40(64)28-54-48(70)42(50(3,4)72-71-49)58-44(66)36(52)24-32-17-21-34(62)22-18-32/h5-22,35-38,41-42,61-62H,23-28,51-52H2,1-4H3,(H,53,69)(H,54,70)(H,55,63)(H,56,64)(H,57,65)(H,58,66)(H,59,67)(H,60,68)/t35-,36-,37-,38-,41+,42+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 205 | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara"G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Agonist activity at rat kappa opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay |
ACS Med Chem Lett 5: 1032-6 (2014)
Article DOI: 10.1021/ml500241n
BindingDB Entry DOI: 10.7270/Q23T9JS5 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50026762
(CHEMBL3331510)Show SMILES CC1(C)SSC(C)(C)[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)NNC(=O)[C@H](Cc2ccccc2)NC(=O)CNC(=O)[C@@H]1NC(=O)[C@@H](N)Cc1ccc(O)cc1 |r| Show InChI InChI=1S/C50H62N10O10S2/c1-49(2)41(57-43(65)35(51)23-31-15-19-33(61)20-16-31)47(69)53-27-39(63)55-37(25-29-11-7-5-8-12-29)45(67)59-60-46(68)38(26-30-13-9-6-10-14-30)56-40(64)28-54-48(70)42(50(3,4)72-71-49)58-44(66)36(52)24-32-17-21-34(62)22-18-32/h5-22,35-38,41-42,61-62H,23-28,51-52H2,1-4H3,(H,53,69)(H,54,70)(H,55,63)(H,56,64)(H,57,65)(H,58,66)(H,59,67)(H,60,68)/t35-,36-,37-,38-,41-,42-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 480 | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara"G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Agonist activity at rat kappa opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay |
ACS Med Chem Lett 5: 1032-6 (2014)
Article DOI: 10.1021/ml500241n
BindingDB Entry DOI: 10.7270/Q23T9JS5 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50079413
(CHEMBL407084 | Opioid Peptide [d-Ala(8)]Dynorphin ...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@H](C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(N)=O Show InChI InChI=1S/C60H98N22O12/c1-34(2)29-44(81-55(92)45(31-36-13-5-4-6-14-36)76-48(85)33-73-47(84)32-74-51(88)39(62)30-37-20-22-38(83)23-21-37)54(91)79-42(17-10-26-71-59(66)67)53(90)78-41(16-9-25-70-58(64)65)52(89)75-35(3)50(87)80-43(18-11-27-72-60(68)69)57(94)82-28-12-19-46(82)56(93)77-40(49(63)86)15-7-8-24-61/h4-6,13-14,20-23,34-35,39-46,83H,7-12,15-19,24-33,61-62H2,1-3H3,(H2,63,86)(H,73,84)(H,74,88)(H,75,89)(H,76,85)(H,77,93)(H,78,90)(H,79,91)(H,80,87)(H,81,92)(H4,64,65,70)(H4,66,67,71)(H4,68,69,72)/t35-,39+,40+,41+,42+,43+,44+,45+,46+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 515 | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of Forskolin-Stimulated Adenylyl Cyclase in CHO cells expressing Opioid receptor kappa 1 |
J Med Chem 46: 4002-8 (2003)
Article DOI: 10.1021/jm030075o
BindingDB Entry DOI: 10.7270/Q2CN739H |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50133056
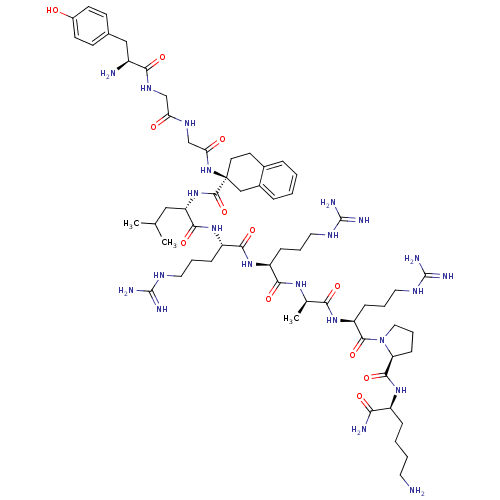
(CHEMBL262838 | Opioid Peptide [d-Ala(8)]Dynorphin ...)Show SMILES CC(C)C[C@H](NC(=O)[C@@]1(CCc2ccccc2C1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(N)=O Show InChI InChI=1S/C62H100N22O12/c1-35(2)30-46(82-58(96)62(24-23-38-12-4-5-13-39(38)32-62)83-49(87)34-75-48(86)33-76-52(90)41(64)31-37-19-21-40(85)22-20-37)55(93)80-44(16-9-27-73-60(68)69)54(92)79-43(15-8-26-72-59(66)67)53(91)77-36(3)51(89)81-45(17-10-28-74-61(70)71)57(95)84-29-11-18-47(84)56(94)78-42(50(65)88)14-6-7-25-63/h4-5,12-13,19-22,35-36,41-47,85H,6-11,14-18,23-34,63-64H2,1-3H3,(H2,65,88)(H,75,86)(H,76,90)(H,77,91)(H,78,94)(H,79,92)(H,80,93)(H,81,89)(H,82,96)(H,83,87)(H4,66,67,72)(H4,68,69,73)(H4,70,71,74)/t36-,41+,42+,43+,44+,45+,46+,47+,62+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 573 | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of Forskolin-Stimulated Adenylyl Cyclase in CHO cells expressing Opioid receptor kappa 1 |
J Med Chem 46: 4002-8 (2003)
Article DOI: 10.1021/jm030075o
BindingDB Entry DOI: 10.7270/Q2CN739H |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50544339
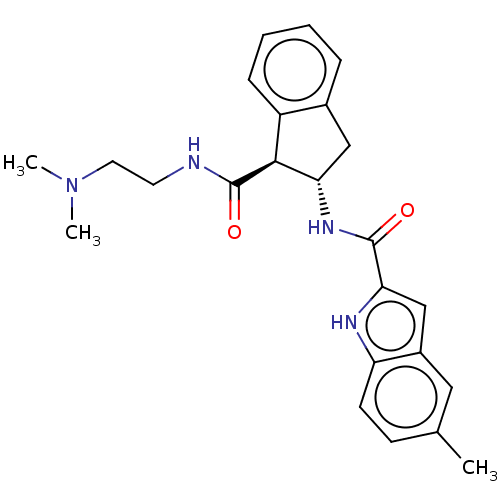
(CHEMBL4639379)Show SMILES CN(C)CCNC(=O)[C@@H]1[C@H](Cc2ccccc12)NC(=O)c1cc2cc(C)ccc2[nH]1 |r| Show InChI InChI=1S/C24H28N4O2/c1-15-8-9-19-17(12-15)14-21(26-19)23(29)27-20-13-16-6-4-5-7-18(16)22(20)24(30)25-10-11-28(2)3/h4-9,12,14,20,22,26H,10-11,13H2,1-3H3,(H,25,30)(H,27,29)/t20-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | <7.94E+3 | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Agonist activity at rat KOR expressed in CHOK1 cells assessed as increase in cAMP accumulation measured after 10 mins by HTRF assay |
J Med Chem 63: 9705-9730 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00828
BindingDB Entry DOI: 10.7270/Q2445R3K |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50544378
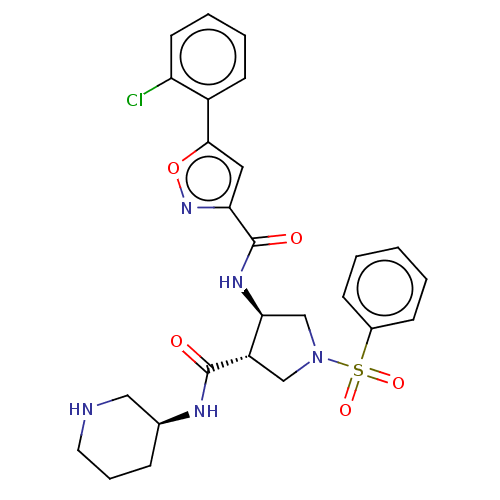
(CHEMBL4639111)Show SMILES Clc1ccccc1-c1cc(no1)C(=O)N[C@H]1CN(C[C@@H]1C(=O)N[C@H]1CCCNC1)S(=O)(=O)c1ccccc1 |r| Show InChI InChI=1S/C26H28ClN5O5S/c27-21-11-5-4-10-19(21)24-13-22(31-37-24)26(34)30-23-16-32(38(35,36)18-8-2-1-3-9-18)15-20(23)25(33)29-17-7-6-12-28-14-17/h1-5,8-11,13,17,20,23,28H,6-7,12,14-16H2,(H,29,33)(H,30,34)/t17-,20-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | <1.00E+5 | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Agonist activity at rat KOR expressed in CHOK1 cells assessed as increase in cAMP accumulation measured after 10 mins by HTRF assay |
J Med Chem 63: 9705-9730 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00828
BindingDB Entry DOI: 10.7270/Q2445R3K |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50544372

(CHEMBL4640438)Show SMILES O=C(N[C@H]1CCCNC1)[C@H]1CN(C[C@@H]1NC(=O)c1cc(on1)-c1ccccc1)S(=O)(=O)c1ccccc1 |r| Show InChI InChI=1S/C26H29N5O5S/c32-25(28-19-10-7-13-27-15-19)21-16-31(37(34,35)20-11-5-2-6-12-20)17-23(21)29-26(33)22-14-24(36-30-22)18-8-3-1-4-9-18/h1-6,8-9,11-12,14,19,21,23,27H,7,10,13,15-17H2,(H,28,32)(H,29,33)/t19-,21-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | <1.00E+5 | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Agonist activity at rat KOR expressed in CHOK1 cells assessed as increase in cAMP accumulation measured after 10 mins by HTRF assay |
J Med Chem 63: 9705-9730 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00828
BindingDB Entry DOI: 10.7270/Q2445R3K |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
































