

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50615386 (CHEMBL5283195) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | UniChem | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50087158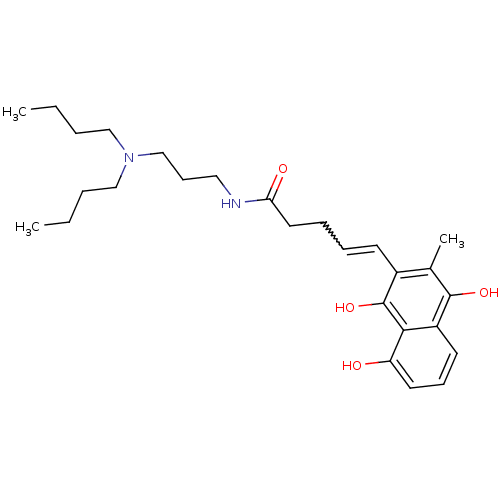 (5-(8-Hydroxy-3-methyl-1,4-dioxo-1,4-dihydro-naphth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Lille II Curated by ChEMBL | Assay Description Concentration required for inhibition of Trypanothione reductase (TcTR) from Trypanosoma cruzi in the presence of 50 uM TS2 as substrate | Bioorg Med Chem Lett 10: 631-5 (2000) BindingDB Entry DOI: 10.7270/Q2V9879F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50087157 (5-(8-Hydroxy-3-methyl-1,4-dioxo-1,4-dihydro-naphth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 8525 CNRS - Université Lille II Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione disulfide reductase, assay in presence of 57 microM T(S)2. | J Med Chem 44: 548-65 (2001) BindingDB Entry DOI: 10.7270/Q2MC8Z7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50087157 (5-(8-Hydroxy-3-methyl-1,4-dioxo-1,4-dihydro-naphth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Lille II Curated by ChEMBL | Assay Description Concentration required for inhibition of Trypanothione reductase (TcTR) from Trypanosoma cruzi in the presence of 50 uM TS2 as substrate | Bioorg Med Chem Lett 10: 631-5 (2000) BindingDB Entry DOI: 10.7270/Q2V9879F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50070270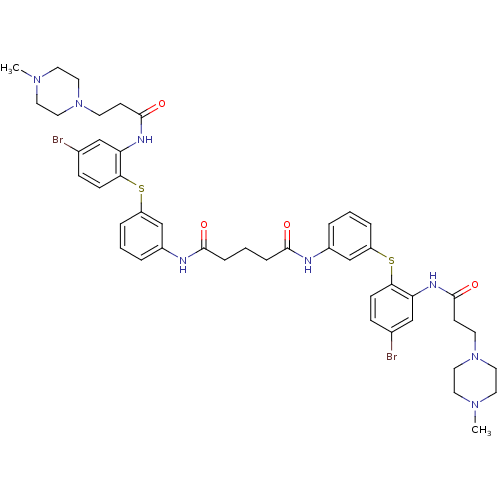 (CHEMBL279374 | Pentanedioic acid bis-[(3-{4-bromo-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50070270 (CHEMBL279374 | Pentanedioic acid bis-[(3-{4-bromo-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
URA CNRS 1309 Curated by ChEMBL | Assay Description Inhibition of trypanothione reductase from T. cruzi, in the presence 57 microM of trypanothione T(SH)2 | Bioorg Med Chem Lett 8: 1175-80 (1999) BindingDB Entry DOI: 10.7270/Q2ZC821D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50087156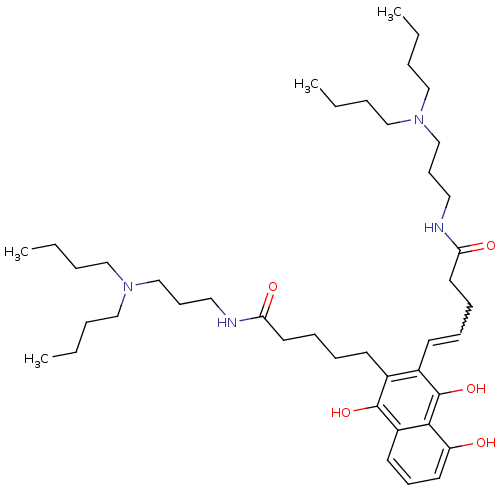 (5-{3-[4-(3-Dibutylamino-propylcarbamoyl)-butyl]-8-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Lille II Curated by ChEMBL | Assay Description Concentration required for inhibition of Trypanothione reductase (TcTR) from Trypanosoma cruzi in the presence of 50 uM TS2 as substrate | Bioorg Med Chem Lett 10: 631-5 (2000) BindingDB Entry DOI: 10.7270/Q2V9879F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50615388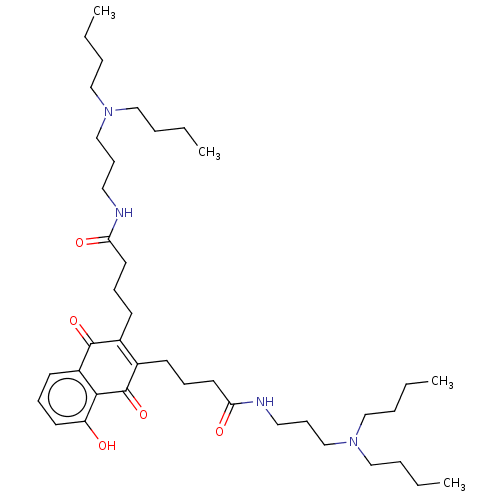 (CHEMBL5280255) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | UniChem | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50178811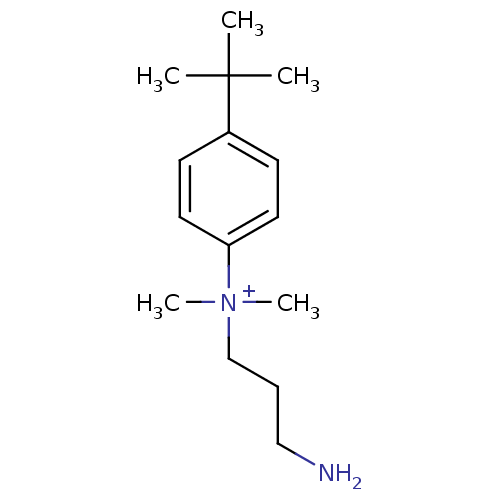 (CHEMBL199020 | N-(3-aminopropyl)-4-tert-butyl-N,N-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity aganist trypanothione reductase from Trypanosoma cruzi using 0.12 mM TSST substrate | J Med Chem 48: 8087-97 (2005) Article DOI: 10.1021/jm050819t BindingDB Entry DOI: 10.7270/Q2V69J65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091160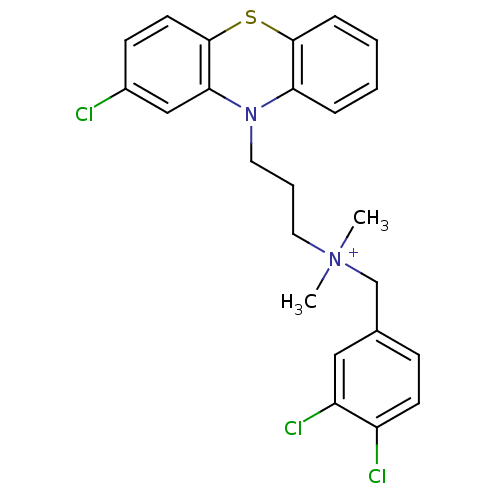 (CHEMBL106127 | [3-(2-Chloro-phenothiazin-10-yl)-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50615389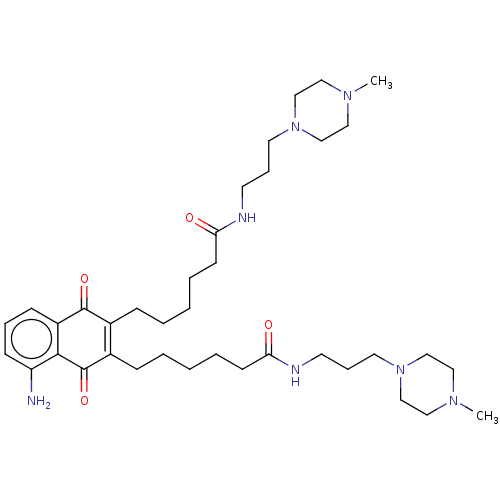 (CHEMBL5283514) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | UniChem | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50096063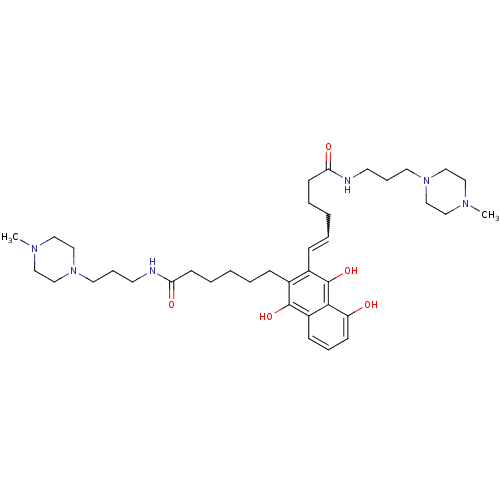 (6-(5-Hydroxy-3-{5-[3-(4-methyl-piperazin-1-yl)-pro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 8525 CNRS - Université Lille II Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione disulfide reductase, assay in presence of 57 microM T(S)2. | J Med Chem 44: 548-65 (2001) BindingDB Entry DOI: 10.7270/Q2MC8Z7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50615385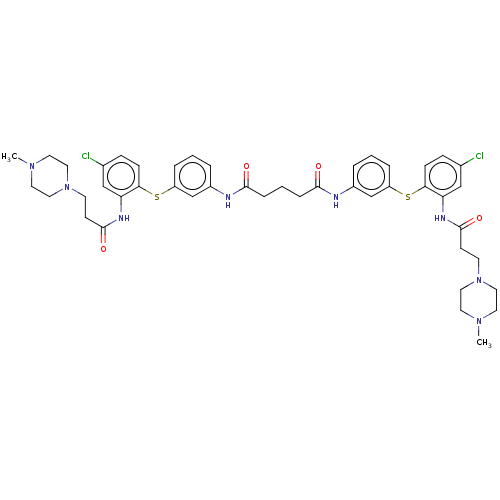 (CHEMBL5270809) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | UniChem | n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50185388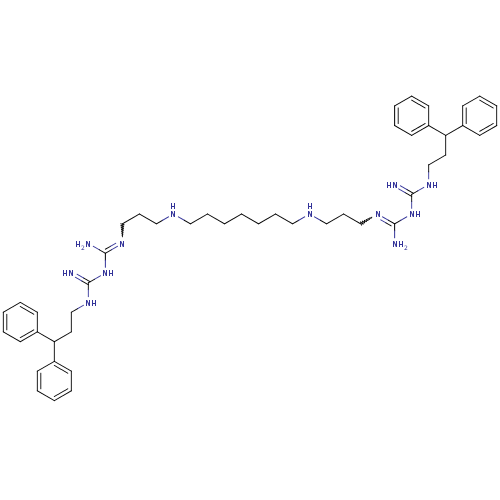 (CHEMBL378900 | N-(3,3-diphenylpropyl)-1-3-[3-({7-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi TR | Bioorg Med Chem Lett 16: 3229-32 (2006) Article DOI: 10.1016/j.bmcl.2006.03.048 BindingDB Entry DOI: 10.7270/Q2JH3KS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50335534 (1-(1-benzylpiperidin-4-yl)-3-(pyridin-3-yl)thioure...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville campus) Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi trypanothione reductase assessed as inhibition of thionitrobenzoate formation by microplate reader analysis | Antimicrob Agents Chemother 53: 2824-33 (2009) Article DOI: 10.1128/AAC.01568-08 BindingDB Entry DOI: 10.7270/Q2QV3MSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50615383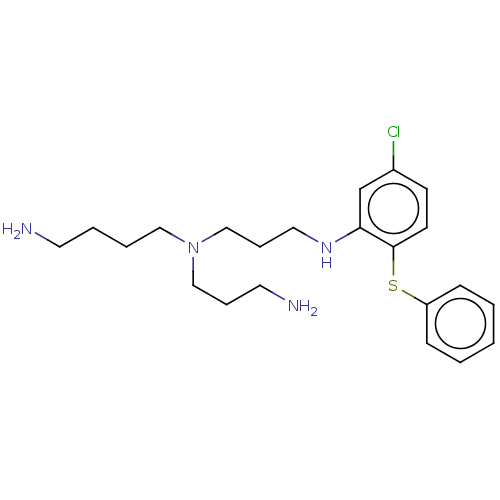 (CHEMBL5273760) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | UniChem | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50335534 (1-(1-benzylpiperidin-4-yl)-3-(pyridin-3-yl)thioure...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville campus) Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi trypanothione reductase assessed as inhibition of thionitrobenzoate formation by microplate reader analysis | Antimicrob Agents Chemother 53: 2824-33 (2009) Article DOI: 10.1128/AAC.01568-08 BindingDB Entry DOI: 10.7270/Q2QV3MSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50087155 (5-(3-Methyl-1,4-dioxo-1,4-dihydro-naphthalen-2-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Lille II Curated by ChEMBL | Assay Description Concentration required for inhibition of Trypanothione reductase (TcTR) from Trypanosoma cruzi in the presence of 50 uM TS2 as substrate | Bioorg Med Chem Lett 10: 631-5 (2000) BindingDB Entry DOI: 10.7270/Q2V9879F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50096043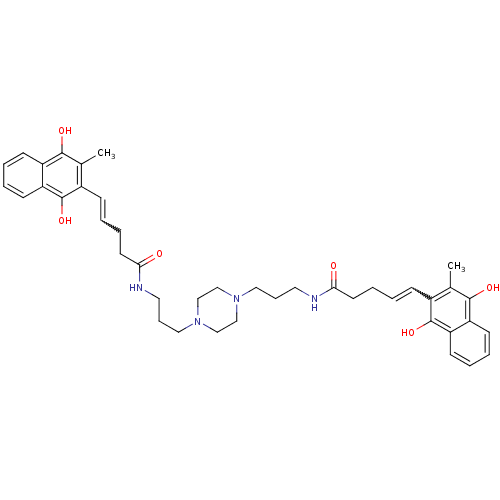 (5-(3-Methyl-1,4-dioxo-1,4-dihydro-naphthalen-2-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 8525 CNRS - Université Lille II Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione disulfide reductase, assay in presence of 57 microM T(S)2. | J Med Chem 44: 548-65 (2001) BindingDB Entry DOI: 10.7270/Q2MC8Z7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091163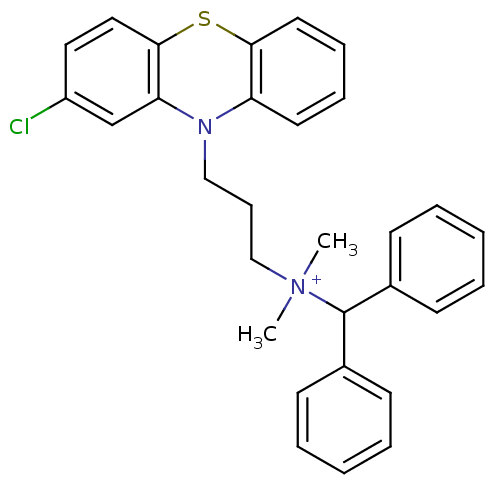 (Benzhydryl-[3-(2-chloro-phenothiazin-10-yl)-propyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50096064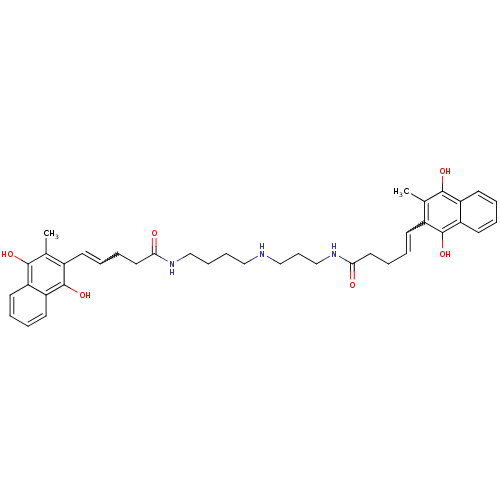 (5-(3-Methyl-1,4-dioxo-1,4-dihydro-naphthalen-2-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 8525 CNRS - Université Lille II Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione disulfide reductase, assay in presence of 57 microM T(S)2. | J Med Chem 44: 548-65 (2001) BindingDB Entry DOI: 10.7270/Q2MC8Z7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50096024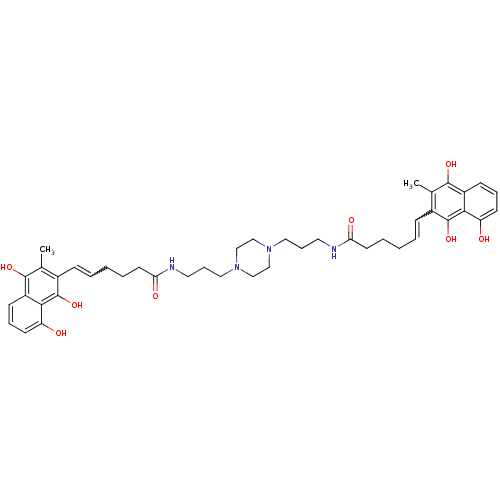 (6-(8-Hydroxy-3-methyl-1,4-dioxo-1,4-dihydro-naphth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 8525 CNRS - Université Lille II Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione disulfide reductase, assay in presence of 57 microM T(S)2. | J Med Chem 44: 548-65 (2001) BindingDB Entry DOI: 10.7270/Q2MC8Z7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50096061 (6-(8-Hydroxy-3-methyl-1,4-dioxo-1,4-dihydro-naphth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 8525 CNRS - Université Lille II Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione disulfide reductase, assay in presence of 57 microM T(S)2. | J Med Chem 44: 548-65 (2001) BindingDB Entry DOI: 10.7270/Q2MC8Z7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50185389 (CHEMBL209039 | N-(2,2-diphenylethyl)-1-3-[3-({7-[(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi TR | Bioorg Med Chem Lett 16: 3229-32 (2006) Article DOI: 10.1016/j.bmcl.2006.03.048 BindingDB Entry DOI: 10.7270/Q2JH3KS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50096076 (5-(8-Hydroxy-3-methyl-1,4-dioxo-1,4-dihydro-naphth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 8525 CNRS - Université Lille II Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione disulfide reductase, assay in presence of 57 microM T(S)2. | J Med Chem 44: 548-65 (2001) BindingDB Entry DOI: 10.7270/Q2MC8Z7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50096012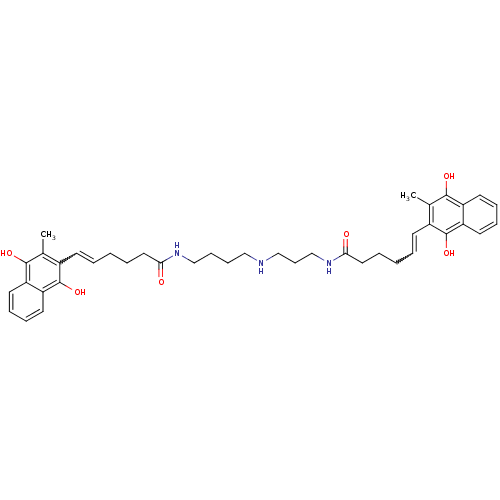 (6-(3-Methyl-1,4-dioxo-1,4-dihydro-naphthalen-2-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 8525 CNRS - Université Lille II Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione disulfide reductase, assay in presence of 57 microM T(S)2. | J Med Chem 44: 548-65 (2001) BindingDB Entry DOI: 10.7270/Q2MC8Z7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50070275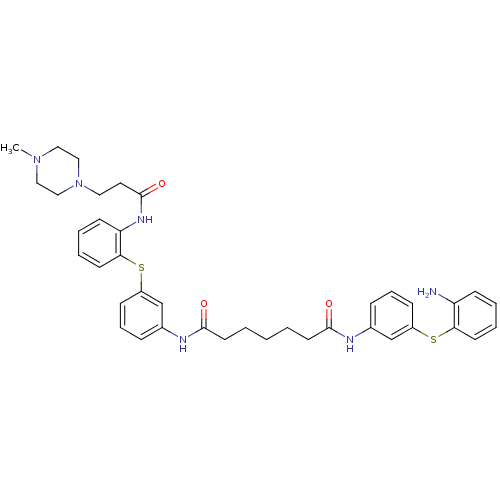 (CHEMBL17665 | Heptanedioic acid [3-(2-amino-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
URA CNRS 1309 Curated by ChEMBL | Assay Description Inhibition of trypanothione reductase from T. cruzi, in the presence 57 microM of trypanothione T(SH)2 | Bioorg Med Chem Lett 8: 1175-80 (1999) BindingDB Entry DOI: 10.7270/Q2ZC821D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50096023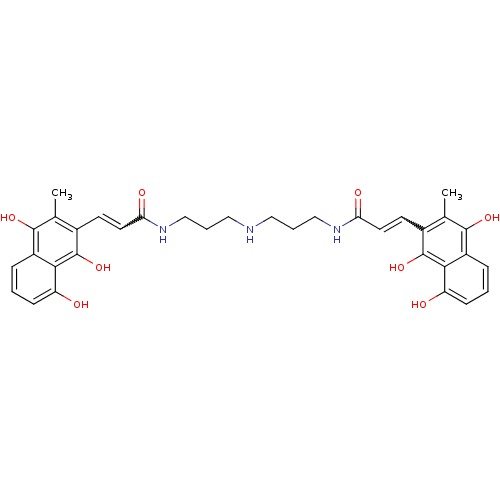 (3-(8-Hydroxy-3-methyl-1,4-dioxo-1,4-dihydro-naphth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 8525 CNRS - Université Lille II Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione disulfide reductase, assay in presence of 57 microM T(S)2. | J Med Chem 44: 548-65 (2001) BindingDB Entry DOI: 10.7270/Q2MC8Z7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50096021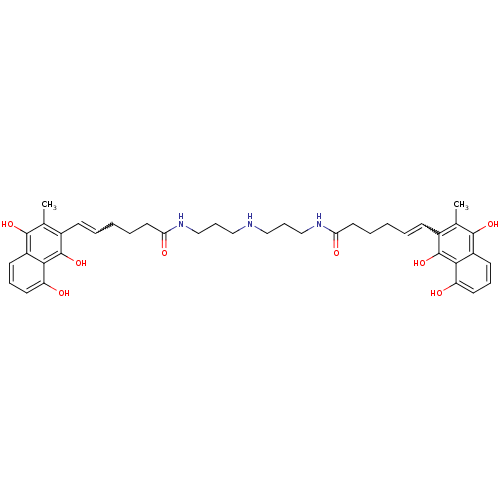 (6-(8-Hydroxy-3-methyl-1,4-dioxo-1,4-dihydro-naphth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 8525 CNRS - Université Lille II Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione disulfide reductase, assay in presence of 57 microM T(S)2. | J Med Chem 44: 548-65 (2001) BindingDB Entry DOI: 10.7270/Q2MC8Z7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50335534 (1-(1-benzylpiperidin-4-yl)-3-(pyridin-3-yl)thioure...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville campus) Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi X10/1 trypanothione reductase assessed as inhibition of thionitrobenzoate formation by microplate reader analysis | Antimicrob Agents Chemother 53: 2824-33 (2009) Article DOI: 10.1128/AAC.01568-08 BindingDB Entry DOI: 10.7270/Q2QV3MSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50177103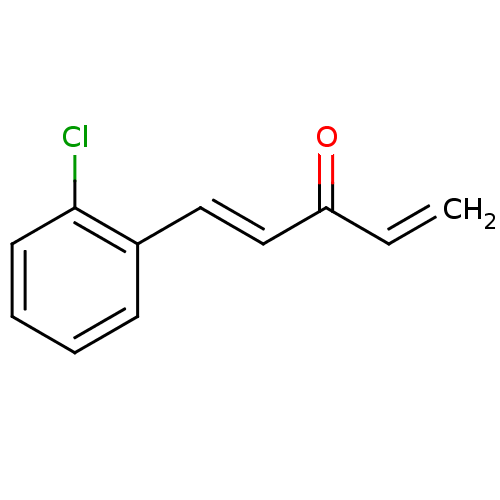 (1-(2'-chlorophenyl)penta-1,4-dien-3-one | CHEMBL22...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Biochemie-Zentrum der Universität Heidelberg Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi trypanothione reductase after 5 mins preincubation with NADPH in presence of 100 uM TS2 substrate | J Med Chem 48: 7400-10 (2005) Article DOI: 10.1021/jm0504860 BindingDB Entry DOI: 10.7270/Q23X8662 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50096059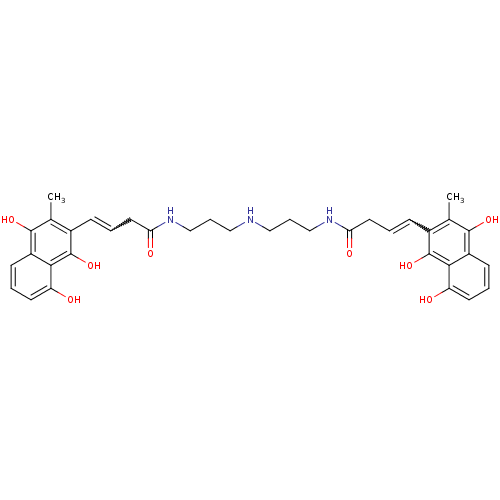 (4-(8-Hydroxy-3-methyl-1,4-dioxo-1,4-dihydro-naphth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 8525 CNRS - Université Lille II Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione disulfide reductase, assay in presence of 57 microM T(S)2. | J Med Chem 44: 548-65 (2001) BindingDB Entry DOI: 10.7270/Q2MC8Z7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50185394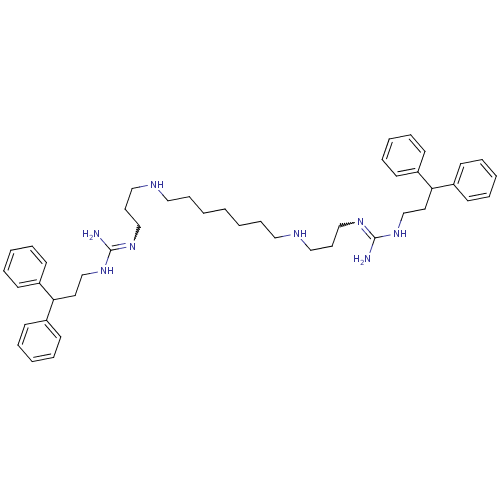 (CHEMBL379701 | N-(3,3-diphenyl-propyl)-N'-[3-(7-{3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi TR | Bioorg Med Chem Lett 16: 3229-32 (2006) Article DOI: 10.1016/j.bmcl.2006.03.048 BindingDB Entry DOI: 10.7270/Q2JH3KS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50096078 (4-(8-Hydroxy-3-methyl-1,4-dioxo-1,4-dihydro-naphth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 8525 CNRS - Université Lille II Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione disulfide reductase, assay in presence of 57 microM T(S)2. | J Med Chem 44: 548-65 (2001) BindingDB Entry DOI: 10.7270/Q2MC8Z7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50096017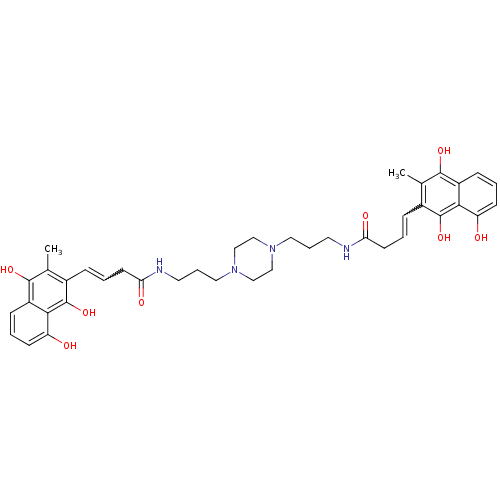 (4-(8-Hydroxy-3-methyl-1,4-dioxo-1,4-dihydro-naphth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 8525 CNRS - Université Lille II Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione disulfide reductase, assay in presence of 57 microM T(S)2. | J Med Chem 44: 548-65 (2001) BindingDB Entry DOI: 10.7270/Q2MC8Z7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50096075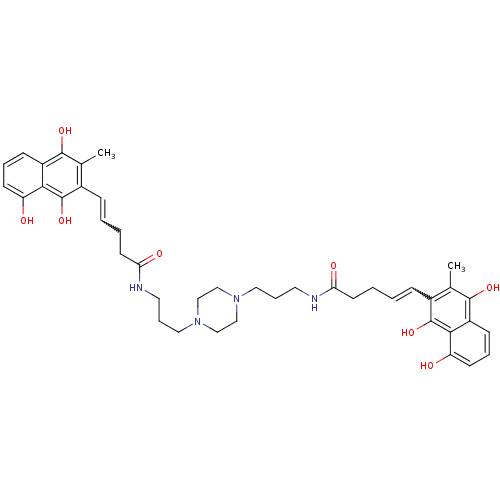 (5-(8-Hydroxy-3-methyl-1,4-dioxo-1,4-dihydro-naphth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 8525 CNRS - Université Lille II Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione disulfide reductase, assay in presence of 57 microM T(S)2. | J Med Chem 44: 548-65 (2001) BindingDB Entry DOI: 10.7270/Q2MC8Z7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50096048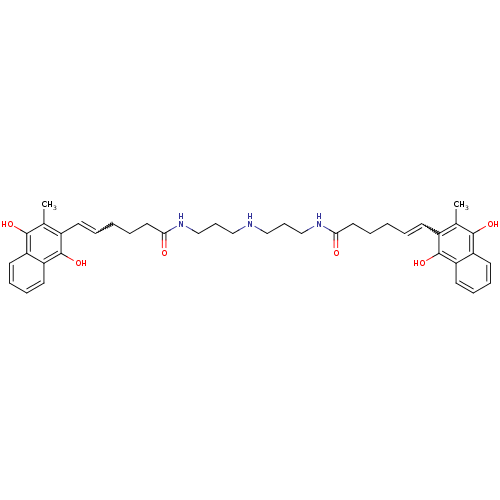 (6-(3-Methyl-1,4-dioxo-1,4-dihydro-naphthalen-2-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 8525 CNRS - Université Lille II Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione disulfide reductase, assay in presence of 57 microM T(S)2. | J Med Chem 44: 548-65 (2001) BindingDB Entry DOI: 10.7270/Q2MC8Z7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50096051 (3-(8-Hydroxy-3-methyl-1,4-dioxo-1,4-dihydro-naphth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 8525 CNRS - Université Lille II Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione disulfide reductase, assay in presence of 57 microM T(S)2. | J Med Chem 44: 548-65 (2001) BindingDB Entry DOI: 10.7270/Q2MC8Z7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50096052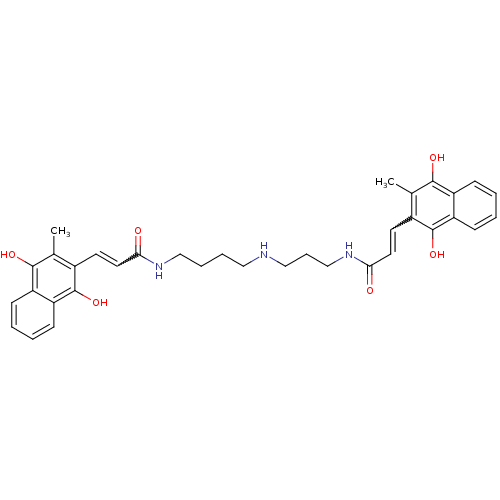 (3-(3-Methyl-1,4-dioxo-1,4-dihydro-naphthalen-2-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 8525 CNRS - Université Lille II Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione disulfide reductase, assay in presence of 57 microM T(S)2. | J Med Chem 44: 548-65 (2001) BindingDB Entry DOI: 10.7270/Q2MC8Z7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50185393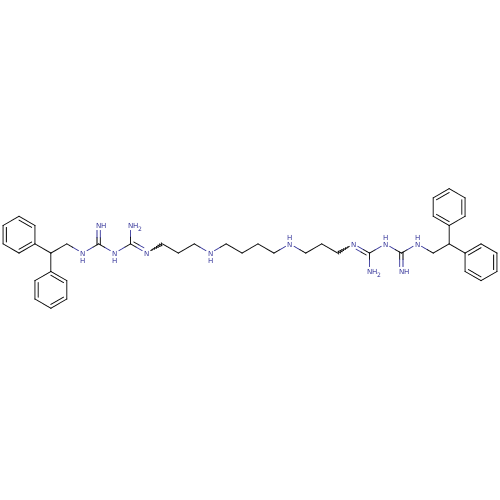 (CHEMBL210371 | N-(2,2-diphenylethyl)-1-3-[3-({4-[(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi TR | Bioorg Med Chem Lett 16: 3229-32 (2006) Article DOI: 10.1016/j.bmcl.2006.03.048 BindingDB Entry DOI: 10.7270/Q2JH3KS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50096036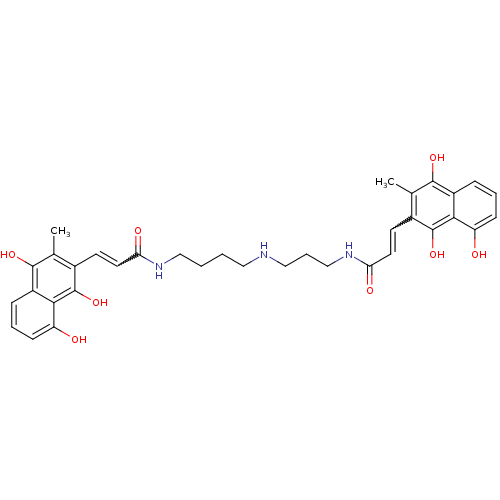 (3-(8-Hydroxy-3-methyl-1,4-dioxo-1,4-dihydro-naphth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 8525 CNRS - Université Lille II Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione disulfide reductase, assay in presence of 57 microM T(S)2. | J Med Chem 44: 548-65 (2001) BindingDB Entry DOI: 10.7270/Q2MC8Z7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50096056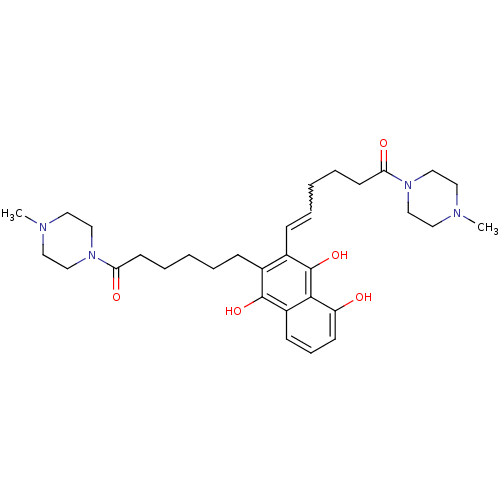 (5-Hydroxy-2,3-bis-[6-(4-methyl-piperazin-1-yl)-6-o...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 8525 CNRS - Université Lille II Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione disulfide reductase, assay in presence of 57 microM T(S)2. | J Med Chem 44: 548-65 (2001) BindingDB Entry DOI: 10.7270/Q2MC8Z7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091148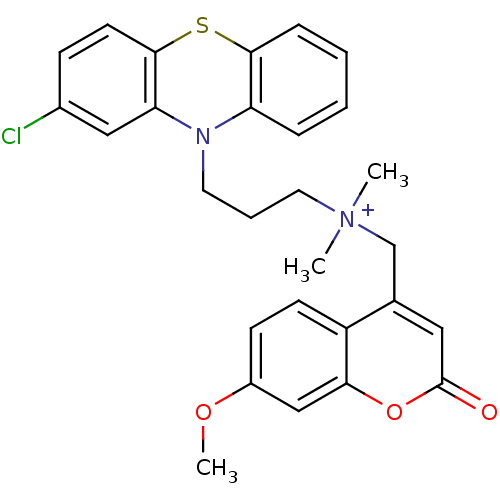 (CHEMBL106769 | [3-(2-Chloro-phenothiazin-10-yl)-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50185400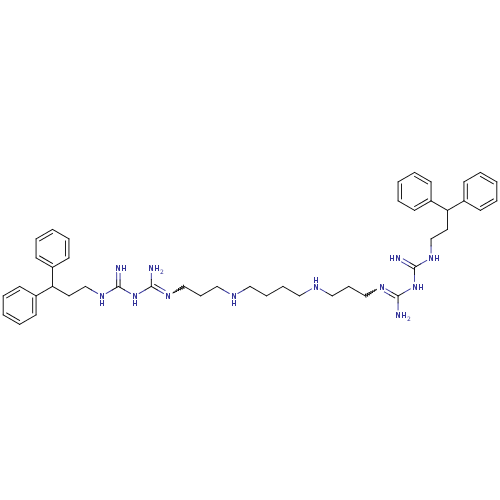 (CHEMBL378136 | N-(3,3-diphenylpropyl)-1-3-[3-({4-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi TR | Bioorg Med Chem Lett 16: 3229-32 (2006) Article DOI: 10.1016/j.bmcl.2006.03.048 BindingDB Entry DOI: 10.7270/Q2JH3KS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50185397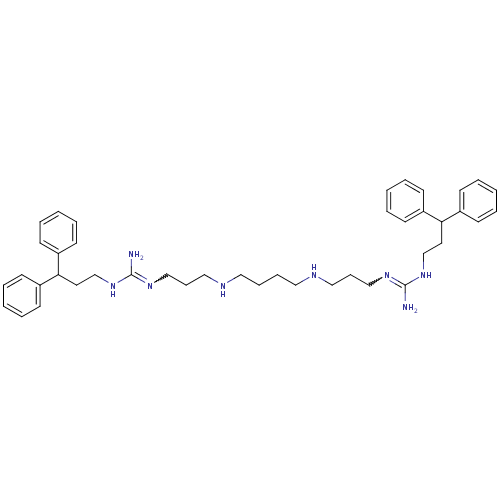 (CHEMBL380857 | N-(3,3-diphenyl-propyl)-N'-[3-(4-{3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi TR | Bioorg Med Chem Lett 16: 3229-32 (2006) Article DOI: 10.1016/j.bmcl.2006.03.048 BindingDB Entry DOI: 10.7270/Q2JH3KS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50096047 (6-(3-Methyl-1,4-dioxo-1,4-dihydro-naphthalen-2-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 8525 CNRS - Université Lille II Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione disulfide reductase, assay in presence of 57 microM T(S)2. | J Med Chem 44: 548-65 (2001) BindingDB Entry DOI: 10.7270/Q2MC8Z7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50104660 (CHEMBL90820 | Naphthalene-2-sulfonic acid [3-(6-ch...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against trypanothione reductase was determined | Bioorg Med Chem Lett 11: 2655-7 (2001) BindingDB Entry DOI: 10.7270/Q2154G90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50070268 (CHEMBL276829 | Heptanedioic acid (3-{4-bromo-2-[3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
URA CNRS 1309 Curated by ChEMBL | Assay Description Inhibition of trypanothione reductase from T. cruzi, in the presence 57 microM of trypanothione T(SH)2 | Bioorg Med Chem Lett 8: 1175-80 (1999) BindingDB Entry DOI: 10.7270/Q2ZC821D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50185401 (CHEMBL207163 | N-(2,2-diphenyl-ethyl)-N'-[3-(3-{3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi TR | Bioorg Med Chem Lett 16: 3229-32 (2006) Article DOI: 10.1016/j.bmcl.2006.03.048 BindingDB Entry DOI: 10.7270/Q2JH3KS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50185396 (CHEMBL207396 | N-(3,3-diphenylpropyl)-1-3-[3-({3-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi TR | Bioorg Med Chem Lett 16: 3229-32 (2006) Article DOI: 10.1016/j.bmcl.2006.03.048 BindingDB Entry DOI: 10.7270/Q2JH3KS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 242 total ) | Next | Last >> |