 Found 6385 hits of ic50 for UniProtKB: P10635
Found 6385 hits of ic50 for UniProtKB: P10635 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50161093
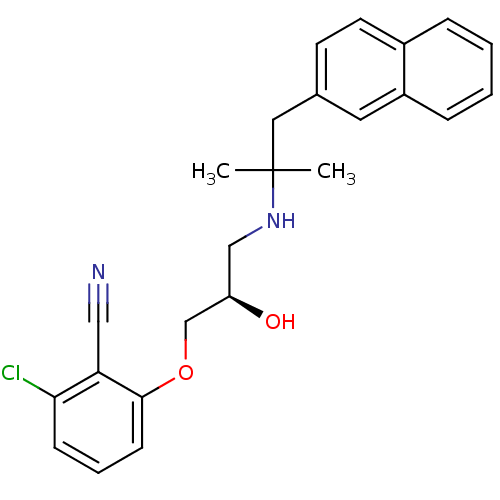
((R)-2-chloro-6-(2-hydroxy-3-(2-methyl-1-(naphthale...)Show SMILES CC(C)(Cc1ccc2ccccc2c1)NC[C@@H](O)COc1cccc(Cl)c1C#N |r| Show InChI InChI=1S/C24H25ClN2O2/c1-24(2,13-17-10-11-18-6-3-4-7-19(18)12-17)27-15-20(28)16-29-23-9-5-8-22(25)21(23)14-26/h3-12,20,27-28H,13,15-16H2,1-2H3/t20-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
J Med Chem 52: 6599-605 (2009)
Article DOI: 10.1021/jm900563e
BindingDB Entry DOI: 10.7270/Q2WM1FB4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50175387
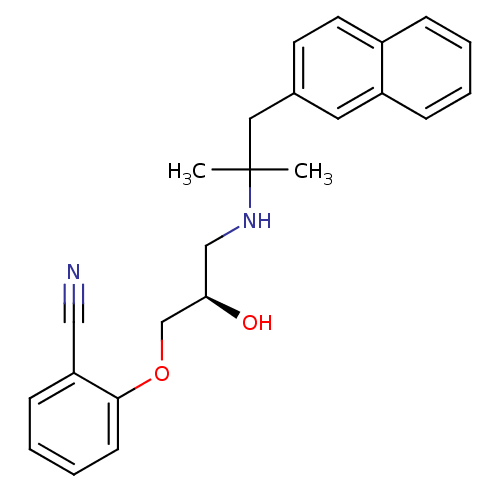
((R)-2-(2-hydroxy-3-(2-methyl-1-(naphthalen-2-yl)pr...)Show SMILES CC(C)(Cc1ccc2ccccc2c1)NC[C@@H](O)COc1ccccc1C#N Show InChI InChI=1S/C24H26N2O2/c1-24(2,14-18-11-12-19-7-3-4-8-20(19)13-18)26-16-22(27)17-28-23-10-6-5-9-21(23)15-25/h3-13,22,26-27H,14,16-17H2,1-2H3/t22-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
J Med Chem 52: 6599-605 (2009)
Article DOI: 10.1021/jm900563e
BindingDB Entry DOI: 10.7270/Q2WM1FB4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50200841

(CHEMBL220360 | MK-0364 | MK-0634 | N-((2S,3S)-4-(4...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1ccc(cn1)C(F)(F)F)[C@@H](Cc1ccc(Cl)cc1)c1cccc(c1)C#N |r| Show InChI InChI=1S/C27H25ClF3N3O2/c1-17(34-25(35)26(2,3)36-24-12-9-21(16-33-24)27(29,30)31)23(14-18-7-10-22(28)11-8-18)20-6-4-5-19(13-20)15-32/h4-13,16-17,23H,14H2,1-3H3,(H,34,35)/t17-,23+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes after 30 mins |
Bioorg Med Chem 18: 6377-88 (2010)
Article DOI: 10.1016/j.bmc.2010.07.013
BindingDB Entry DOI: 10.7270/Q2XG9RBT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50268077
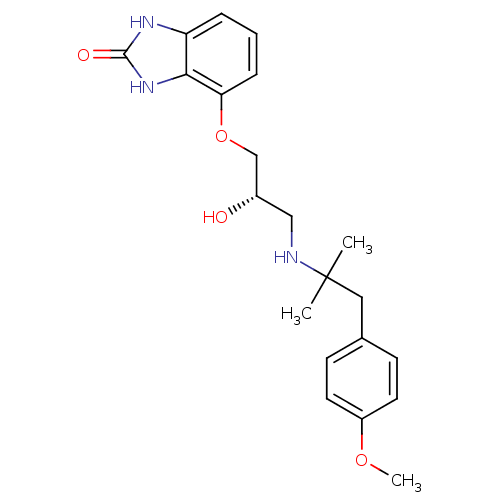
((S)-4-(2-hydroxy-3-(1-(4-methoxyphenyl)-2-methylpr...)Show SMILES COc1ccc(CC(C)(C)NC[C@H](O)COc2cccc3[nH]c(=O)[nH]c23)cc1 |r| Show InChI InChI=1S/C21H27N3O4/c1-21(2,11-14-7-9-16(27-3)10-8-14)22-12-15(25)13-28-18-6-4-5-17-19(18)24-20(26)23-17/h4-10,15,22,25H,11-13H2,1-3H3,(H2,23,24,26)/t15-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
J Med Chem 52: 6599-605 (2009)
Article DOI: 10.1021/jm900563e
BindingDB Entry DOI: 10.7270/Q2WM1FB4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM8610

(1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...)Show SMILES [H][C@]1(COc2ccc(cc2)N2CCN(CC2)C(C)=O)CO[C@@](Cn2ccnc2)(O1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116894
BindingDB Entry DOI: 10.7270/Q2474FVC |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50121975

((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...)Show SMILES COc1ccc2nccc([C@H](O)[C@H]3C[C@@H]4CCN3C[C@@H]4C=C)c2c1 |r,THB:20:19:12.13:16.15,10:12:18.19:16.15| Show InChI InChI=1S/C20H24N2O2/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3/t13-,14-,19+,20-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant microsomal CYP2D6 using {3-[2-(N,N-diethyl-N-methylamino)ethyl]-7-methoxy-4-methylcoumarin} as substrate assessed as ... |
J Med Chem 56: 7851-61 (2013)
Article DOI: 10.1021/jm400766k
BindingDB Entry DOI: 10.7270/Q290257C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50314267
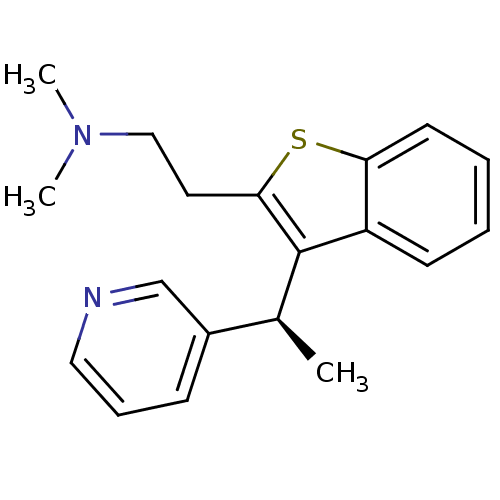
((+)-(S)-N,N-dimethyl-2-(3-(1-(pyridin-3-yl)ethyl)b...)Show InChI InChI=1S/C19H22N2S/c1-14(15-7-6-11-20-13-15)19-16-8-4-5-9-17(16)22-18(19)10-12-21(2)3/h4-9,11,13-14H,10,12H2,1-3H3/t14-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP2D6 after 30 mins by fluorescence assay |
Bioorg Med Chem Lett 20: 2316-20 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.134
BindingDB Entry DOI: 10.7270/Q2P55PG0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50297374

(3-(3-(tert-butylthio)-5-(pyridin-2-ylmethoxy)-1-(4...)Show SMILES CC(C)(C)Sc1c(CC(C)(C)C(O)=O)n(Cc2ccc(cc2)-c2cccnc2)c2ccc(OCc3ccccn3)cc12 Show InChI InChI=1S/C35H37N3O3S/c1-34(2,3)42-32-29-19-28(41-23-27-10-6-7-18-37-27)15-16-30(29)38(31(32)20-35(4,5)33(39)40)22-24-11-13-25(14-12-24)26-9-8-17-36-21-26/h6-19,21H,20,22-23H2,1-5H3,(H,39,40) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
J Med Chem 52: 5803-15 (2009)
Article DOI: 10.1021/jm900945d
BindingDB Entry DOI: 10.7270/Q2G44QB1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM493606
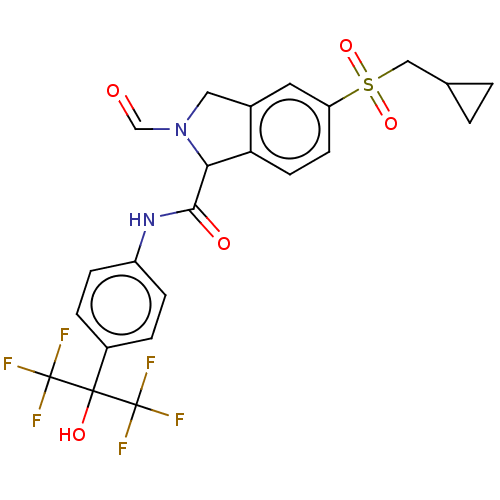
(US10988445, Example 202)Show SMILES OC(c1ccc(NC(=O)C2N(Cc3cc(ccc23)S(=O)(=O)CC2CC2)C=O)cc1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C23H20F6N2O5S/c24-22(25,26)21(34,23(27,28)29)15-3-5-16(6-4-15)30-20(33)19-18-8-7-17(9-14(18)10-31(19)12-32)37(35,36)11-13-1-2-13/h3-9,12-13,19,34H,1-2,10-11H2,(H,30,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2D6 in human liver microsomes |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01197
BindingDB Entry DOI: 10.7270/Q22Z19BG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50378146
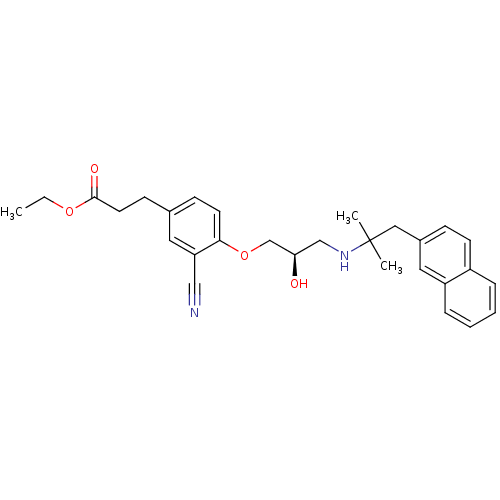
(CHEMBL1204009)Show SMILES CCOC(=O)CCc1ccc(OC[C@H](O)CNC(C)(C)Cc2ccc3ccccc3c2)c(c1)C#N |r| Show InChI InChI=1S/C29H34N2O4/c1-4-34-28(33)14-11-21-10-13-27(25(15-21)18-30)35-20-26(32)19-31-29(2,3)17-22-9-12-23-7-5-6-8-24(23)16-22/h5-10,12-13,15-16,26,31-32H,4,11,14,17,19-20H2,1-3H3/t26-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
J Med Chem 52: 6599-605 (2009)
Article DOI: 10.1021/jm900563e
BindingDB Entry DOI: 10.7270/Q2WM1FB4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50121975

((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...)Show SMILES COc1ccc2nccc([C@H](O)[C@H]3C[C@@H]4CCN3C[C@@H]4C=C)c2c1 |r,THB:20:19:12.13:16.15,10:12:18.19:16.15| Show InChI InChI=1S/C20H24N2O2/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3/t13-,14-,19+,20-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit
Curated by ChEMBL
| Assay Description
Inhibition of MAMC O-dealkylation mediated by human Cytochrome P450 2D6 expressed in human lymphoblastoid cell line |
J Med Chem 46: 74-86 (2002)
Article DOI: 10.1021/jm0209578
BindingDB Entry DOI: 10.7270/Q2K64JSD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50297370

(3-(3-(tert-butylthio)-5-(pyridin-2-ylmethoxy)-1-(4...)Show SMILES CC(C)(C)Sc1c(CC(C)(C)C(O)=O)n(Cc2ccc(cc2)-c2nccs2)c2ccc(OCc3ccccn3)cc12 Show InChI InChI=1S/C33H35N3O3S2/c1-32(2,3)41-29-26-18-25(39-21-24-8-6-7-15-34-24)13-14-27(26)36(28(29)19-33(4,5)31(37)38)20-22-9-11-23(12-10-22)30-35-16-17-40-30/h6-18H,19-21H2,1-5H3,(H,37,38) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
J Med Chem 52: 5803-15 (2009)
Article DOI: 10.1021/jm900945d
BindingDB Entry DOI: 10.7270/Q2G44QB1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM247367
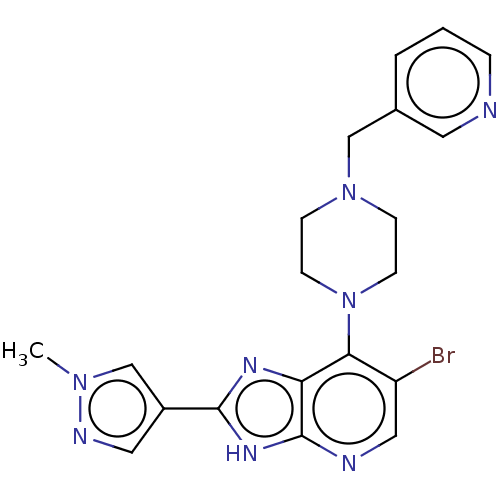
(US9447092, Comparator 1, Example 56)Show SMILES Cn1cc(cn1)-c1nc2c(N3CCN(Cc4cccnc4)CC3)c(Br)cnc2[nH]1 Show InChI InChI=1S/C20H21BrN8/c1-27-13-15(10-24-27)19-25-17-18(16(21)11-23-20(17)26-19)29-7-5-28(6-8-29)12-14-3-2-4-22-9-14/h2-4,9-11,13H,5-8,12H2,1H3,(H,23,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED
US Patent
| Assay Description
Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit... |
US Patent US9447092 (2016)
BindingDB Entry DOI: 10.7270/Q2PV6J9C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50121975

((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...)Show SMILES COc1ccc2nccc([C@H](O)[C@H]3C[C@@H]4CCN3C[C@@H]4C=C)c2c1 |r,THB:20:19:12.13:16.15,10:12:18.19:16.15| Show InChI InChI=1S/C20H24N2O2/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3/t13-,14-,19+,20-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | <6 | n/a | n/a | n/a | n/a | n/a | n/a |
Chung-Ang University
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsome |
Bioorg Med Chem Lett 24: 4271-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.026
BindingDB Entry DOI: 10.7270/Q261122X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50121975

((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...)Show SMILES COc1ccc2nccc([C@H](O)[C@H]3C[C@@H]4CCN3C[C@@H]4C=C)c2c1 |r,THB:20:19:12.13:16.15,10:12:18.19:16.15| Show InChI InChI=1S/C20H24N2O2/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3/t13-,14-,19+,20-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 using luciferin as substrate preincubated for 10 mins followed by substrate addition and measured after 50 min... |
Bioorg Med Chem 25: 5341-5354 (2017)
Article DOI: 10.1016/j.bmc.2017.07.058
BindingDB Entry DOI: 10.7270/Q29W0K0D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50121975

((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...)Show SMILES COc1ccc2nccc([C@H](O)[C@H]3C[C@@H]4CCN3C[C@@H]4C=C)c2c1 |r,THB:20:19:12.13:16.15,10:12:18.19:16.15| Show InChI InChI=1S/C20H24N2O2/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3/t13-,14-,19+,20-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) expressed in insect cell microsomes using dibenzylfluorescein substrate by fluorescence based assay |
J Med Chem 63: 7721-7739 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00564
BindingDB Entry DOI: 10.7270/Q2WD444S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50413014

(CHEMBL497171)Show InChI InChI=1S/C15H17N3/c1-2-3-9-16-15-14-11-6-4-5-7-12(11)18-13(14)8-10-17-15/h4-8,10,18H,2-3,9H2,1H3,(H,16,17) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.94 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 18: 4936-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.054
BindingDB Entry DOI: 10.7270/Q2GH9K5T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50334783
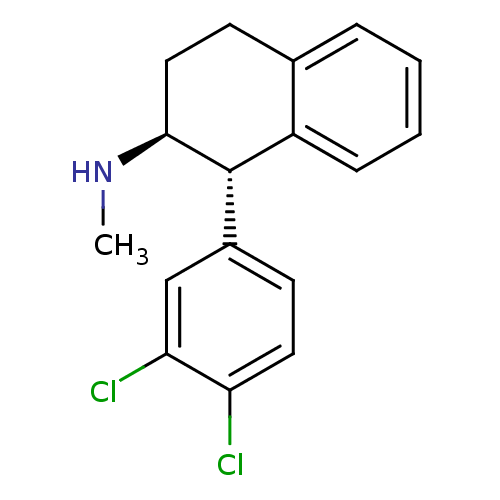
(CHEMBL1642914 | trans-(1S,2S)-1-(3,4-dichloropheny...)Show SMILES CN[C@H]1CCc2ccccc2[C@@H]1c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C17H17Cl2N/c1-20-16-9-7-11-4-2-3-5-13(11)17(16)12-6-8-14(18)15(19)10-12/h2-6,8,10,16-17,20H,7,9H2,1H3/t16-,17-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Sepracor Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem 19: 663-76 (2011)
Article DOI: 10.1016/j.bmc.2010.10.034
BindingDB Entry DOI: 10.7270/Q2639Q0M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50121975

((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...)Show SMILES COc1ccc2nccc([C@H](O)[C@H]3C[C@@H]4CCN3C[C@@H]4C=C)c2c1 |r,THB:20:19:12.13:16.15,10:12:18.19:16.15| Show InChI InChI=1S/C20H24N2O2/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3/t13-,14-,19+,20-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant CYP2D6 |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.9b00062
BindingDB Entry DOI: 10.7270/Q23F4T9H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50013515

((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...)Show SMILES COC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15-,17-,18-,19+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leicester
Curated by ChEMBL
| Assay Description
Inhibitory concentration against cytochrome P450 2D6 |
J Med Chem 47: 5340-6 (2004)
Article DOI: 10.1021/jm049934e
BindingDB Entry DOI: 10.7270/Q2R49RJH |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50121975

((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...)Show SMILES COc1ccc2nccc([C@H](O)[C@H]3C[C@@H]4CCN3C[C@@H]4C=C)c2c1 |r,THB:20:19:12.13:16.15,10:12:18.19:16.15| Show InChI InChI=1S/C20H24N2O2/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3/t13-,14-,19+,20-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Perugia
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate after 10 mins by LC-MS analysis |
J Med Chem 59: 3340-52 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00030
BindingDB Entry DOI: 10.7270/Q29K4D50 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM190405
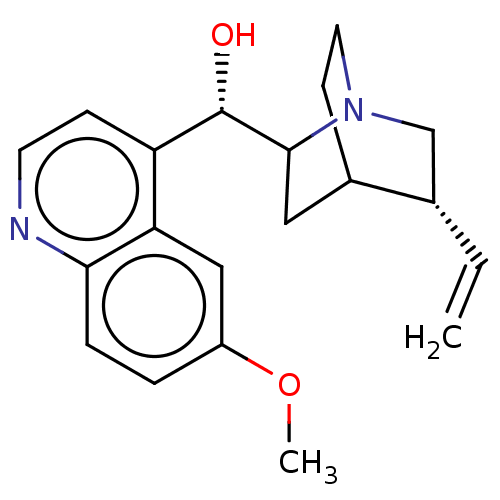
(US9180183, Quinidine)Show SMILES COc1ccc2nccc([C@H](O)C3CC4CCN3C[C@@H]4C=C)c2c1 |r,TLB:10:12:16.15:19.18,THB:20:19:12.13:16.15| Show InChI InChI=1S/C20H24N2O2/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3/t13-,14?,19?,20-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | 37 |
Telormedix SA
US Patent
| Assay Description
The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w... |
US Patent US9180183 (2015)
BindingDB Entry DOI: 10.7270/Q2B27T2W |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM190405

(US9180183, Quinidine)Show SMILES COc1ccc2nccc([C@H](O)C3CC4CCN3C[C@@H]4C=C)c2c1 |r,TLB:10:12:16.15:19.18,THB:20:19:12.13:16.15| Show InChI InChI=1S/C20H24N2O2/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3/t13-,14?,19?,20-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Telormedix SA
US Patent
| Assay Description
The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w... |
US Patent US9173935 (2015)
BindingDB Entry DOI: 10.7270/Q2JS9P8S |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM155255
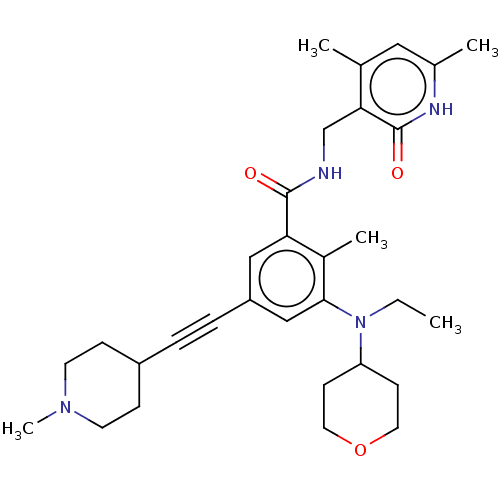
(US10098888, Compound 105 | US11642348, Compound 10...)Show SMILES CCN(C1CCOCC1)c1cc(cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C)C#CC1CCN(C)CC1 Show InChI InChI=1S/C31H42N4O3/c1-6-35(26-11-15-38-16-12-26)29-19-25(8-7-24-9-13-34(5)14-10-24)18-27(23(29)4)30(36)32-20-28-21(2)17-22(3)33-31(28)37/h17-19,24,26H,6,9-16,20H2,1-5H3,(H,32,36)(H,33,37) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 9.67 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc.
US Patent
| Assay Description
The potential inhibition of enzyme activities of human cytochromes P450 (CYP) of Compound 1, 2, or 105 was evaluated using pooled human liver microso... |
US Patent US10098888 (2018)
BindingDB Entry DOI: 10.7270/Q2BK1FDQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50314262
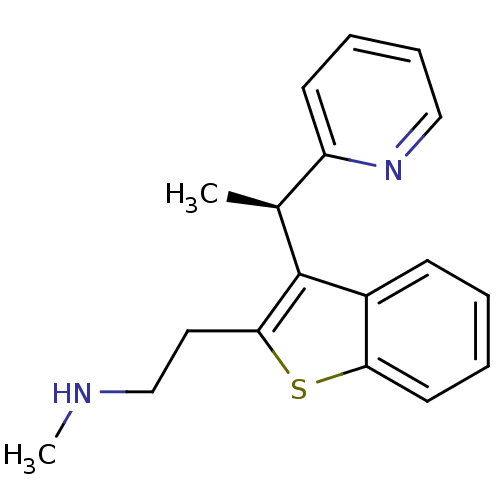
((-)-(R)-N-methyl-2-(3-(1-(pyridin-2-yl)ethyl)benzo...)Show InChI InChI=1S/C18H20N2S/c1-13(15-8-5-6-11-20-15)18-14-7-3-4-9-16(14)21-17(18)10-12-19-2/h3-9,11,13,19H,10,12H2,1-2H3/t13-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP2D6 after 30 mins by fluorescence assay |
Bioorg Med Chem Lett 20: 2316-20 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.134
BindingDB Entry DOI: 10.7270/Q2P55PG0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM493605
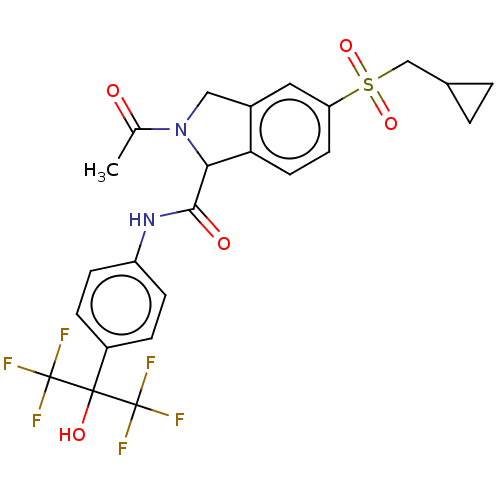
(US10988445, Example 201)Show SMILES CC(=O)N1Cc2cc(ccc2C1C(=O)Nc1ccc(cc1)C(O)(C(F)(F)F)C(F)(F)F)S(=O)(=O)CC1CC1 Show InChI InChI=1S/C24H22F6N2O5S/c1-13(33)32-11-15-10-18(38(36,37)12-14-2-3-14)8-9-19(15)20(32)21(34)31-17-6-4-16(5-7-17)22(35,23(25,26)27)24(28,29)30/h4-10,14,20,35H,2-3,11-12H2,1H3,(H,31,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2D6 in human liver microsomes |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01197
BindingDB Entry DOI: 10.7270/Q22Z19BG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50210012

((3R,4R)-methyl 3-(((S)-3-(4-fluorobenzyl)piperidin...)Show SMILES COC(=O)N1CC[C@@H](NC(=O)Nc2nc(C)c(s2)C(C)=O)[C@H](CN2CCC[C@@H](Cc3ccc(F)cc3)C2)C1 Show InChI InChI=1S/C27H36FN5O4S/c1-17-24(18(2)34)38-26(29-17)31-25(35)30-23-10-12-33(27(36)37-3)16-21(23)15-32-11-4-5-20(14-32)13-19-6-8-22(28)9-7-19/h6-9,20-21,23H,4-5,10-16H2,1-3H3,(H2,29,30,31,35)/t20-,21+,23+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
Bioorg Med Chem Lett 17: 2992-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.065
BindingDB Entry DOI: 10.7270/Q2V987SQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50367247
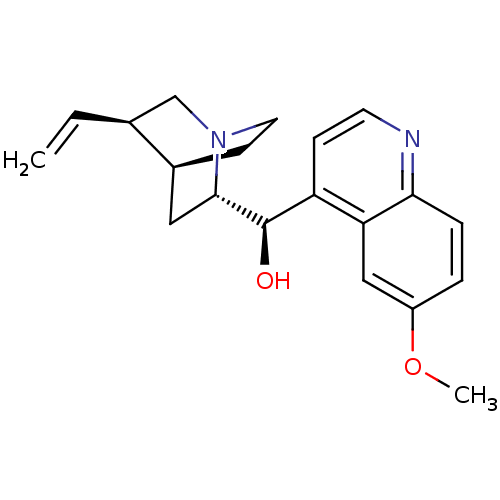
(QUININE | Quinamm | Quinsan | cid_3034034)Show SMILES COc1ccc2nccc([C@@H](O)[C@@H]3C[C@@H]4CCN3C[C@@H]4C=C)c2c1 |THB:20:19:12.13:16.15,10:12:19.18:16.15| Show InChI InChI=1S/C20H24N2O2/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3/t13-,14-,19-,20+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 preincubated for 5 mins followed by NADPH addition and measured after 45 mins by luminescence based microplate reader anal... |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111857
BindingDB Entry DOI: 10.7270/Q2833W92 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM143355
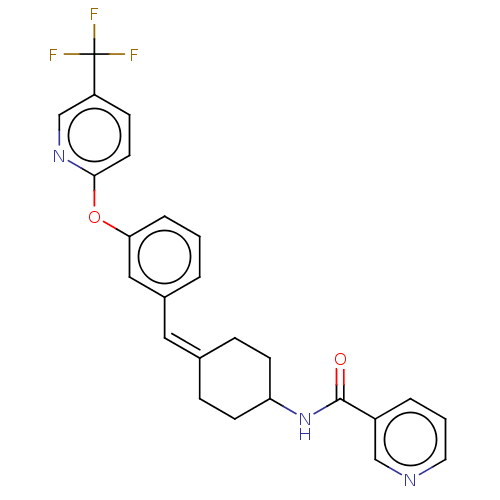
(US9682953, 20.A-1)Show SMILES CCOC(=O)Cn1nc2C(=O)N(C(c2c1C(C)C)c1ccc(Cl)cc1C)c1cc(Cl)ccc1C Show InChI InChI=1S/C25H22F3N3O2/c26-25(27,28)20-8-11-23(30-16-20)33-22-5-1-3-18(14-22)13-17-6-9-21(10-7-17)31-24(32)19-4-2-12-29-15-19/h1-5,8,11-16,21H,6-7,9-10H2,(H,31,32)/b17-13- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Limited
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate preincubated for 5 mins followed by NADPH cofactor addition and me... |
Bioorg Med Chem Lett 29: 238-243 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.048
BindingDB Entry DOI: 10.7270/Q2125X14 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50121975

((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...)Show SMILES COc1ccc2nccc([C@H](O)[C@H]3C[C@@H]4CCN3C[C@@H]4C=C)c2c1 |r,THB:20:19:12.13:16.15,10:12:18.19:16.15| Show InChI InChI=1S/C20H24N2O2/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3/t13-,14-,19+,20-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Southwestern
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 by fluorescence method |
J Med Chem 62: 10124-10143 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00952
BindingDB Entry DOI: 10.7270/Q2QF8XB9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50121975

((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...)Show SMILES COc1ccc2nccc([C@H](O)[C@H]3C[C@@H]4CCN3C[C@@H]4C=C)c2c1 |r,THB:20:19:12.13:16.15,10:12:18.19:16.15| Show InChI InChI=1S/C20H24N2O2/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3/t13-,14-,19+,20-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Southwestern
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 by fluorescence method |
J Med Chem 62: 10124-10143 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00952
BindingDB Entry DOI: 10.7270/Q2QF8XB9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50121975

((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...)Show SMILES COc1ccc2nccc([C@H](O)[C@H]3C[C@@H]4CCN3C[C@@H]4C=C)c2c1 |r,THB:20:19:12.13:16.15,10:12:18.19:16.15| Show InChI InChI=1S/C20H24N2O2/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3/t13-,14-,19+,20-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 in presence of NADPH by luciferase reporter gene assay |
J Med Chem 62: 1330-1347 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01464
BindingDB Entry DOI: 10.7270/Q2CF9TKG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50121975

((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...)Show SMILES COc1ccc2nccc([C@H](O)[C@H]3C[C@@H]4CCN3C[C@@H]4C=C)c2c1 |r,THB:20:19:12.13:16.15,10:12:18.19:16.15| Show InChI InChI=1S/C20H24N2O2/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3/t13-,14-,19+,20-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 in presence of NADPH by luciferase reporter gene assay |
J Med Chem 62: 1330-1347 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01464
BindingDB Entry DOI: 10.7270/Q2CF9TKG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50297371
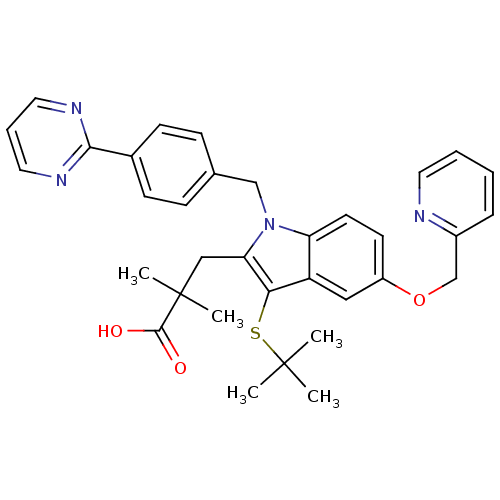
(3-(3-(tert-butylthio)-5-(pyridin-2-ylmethoxy)-1-(4...)Show SMILES CC(C)(C)Sc1c(CC(C)(C)C(O)=O)n(Cc2ccc(cc2)-c2ncccn2)c2ccc(OCc3ccccn3)cc12 Show InChI InChI=1S/C34H36N4O3S/c1-33(2,3)42-30-27-19-26(41-22-25-9-6-7-16-35-25)14-15-28(27)38(29(30)20-34(4,5)32(39)40)21-23-10-12-24(13-11-23)31-36-17-8-18-37-31/h6-19H,20-22H2,1-5H3,(H,39,40) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
J Med Chem 52: 5803-15 (2009)
Article DOI: 10.1021/jm900945d
BindingDB Entry DOI: 10.7270/Q2G44QB1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50297377

(3-[3-tert-Butylsulfanyl-1-[4-(6-methylpyridin-3-yl...)Show SMILES Cc1ccc(cn1)-c1ccc(Cn2c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c3cc(OCc4ccccn4)ccc23)cc1 Show InChI InChI=1S/C36H39N3O3S/c1-24-10-13-27(21-38-24)26-14-11-25(12-15-26)22-39-31-17-16-29(42-23-28-9-7-8-18-37-28)19-30(31)33(43-35(2,3)4)32(39)20-36(5,6)34(40)41/h7-19,21H,20,22-23H2,1-6H3,(H,40,41) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
J Med Chem 52: 5803-15 (2009)
Article DOI: 10.1021/jm900945d
BindingDB Entry DOI: 10.7270/Q2G44QB1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50297384
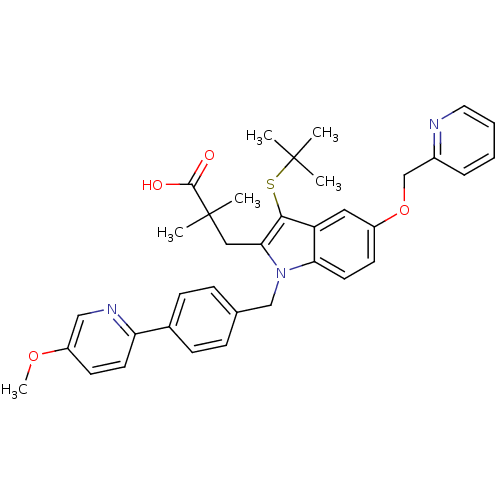
(3-(3-(tert-butylthio)-1-(4-(5-methoxypyridin-2-yl)...)Show SMILES COc1ccc(nc1)-c1ccc(Cn2c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c3cc(OCc4ccccn4)ccc23)cc1 Show InChI InChI=1S/C36H39N3O4S/c1-35(2,3)44-33-29-19-27(43-23-26-9-7-8-18-37-26)15-17-31(29)39(32(33)20-36(4,5)34(40)41)22-24-10-12-25(13-11-24)30-16-14-28(42-6)21-38-30/h7-19,21H,20,22-23H2,1-6H3,(H,40,41) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
J Med Chem 52: 5803-15 (2009)
Article DOI: 10.1021/jm900945d
BindingDB Entry DOI: 10.7270/Q2G44QB1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50121975

((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...)Show SMILES COc1ccc2nccc([C@H](O)[C@H]3C[C@@H]4CCN3C[C@@H]4C=C)c2c1 |r,THB:20:19:12.13:16.15,10:12:18.19:16.15| Show InChI InChI=1S/C20H24N2O2/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3/t13-,14-,19+,20-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50121975

((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...)Show SMILES COc1ccc2nccc([C@H](O)[C@H]3C[C@@H]4CCN3C[C@@H]4C=C)c2c1 |r,THB:20:19:12.13:16.15,10:12:18.19:16.15| Show InChI InChI=1S/C20H24N2O2/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3/t13-,14-,19+,20-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 after 30 mins by fluorometric assay |
J Nat Prod 74: 102-5 (2011)
Article DOI: 10.1021/np100665j
BindingDB Entry DOI: 10.7270/Q2H70GT7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50121975

((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...)Show SMILES COc1ccc2nccc([C@H](O)[C@H]3C[C@@H]4CCN3C[C@@H]4C=C)c2c1 |r,THB:20:19:12.13:16.15,10:12:18.19:16.15| Show InChI InChI=1S/C20H24N2O2/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3/t13-,14-,19+,20-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leicester
Curated by ChEMBL
| Assay Description
Inhibitory concentration against cytochrome P450 2D6 |
J Med Chem 47: 5340-6 (2004)
Article DOI: 10.1021/jm049934e
BindingDB Entry DOI: 10.7270/Q2R49RJH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50026727
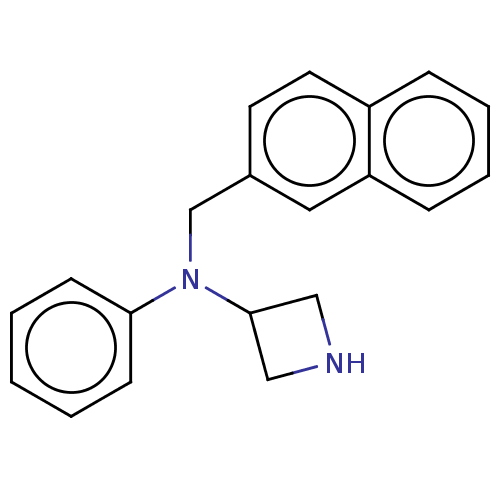
(CHEMBL3331469)Show InChI InChI=1S/C20H20N2.ClH/c1-2-8-19(9-3-1)22(20-13-21-14-20)15-16-10-11-17-6-4-5-7-18(17)12-16;/h1-12,20-21H,13-15H2;1H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 incubated for 5 mins by fluorescence assay |
ACS Med Chem Lett 5: 999-1004 (2014)
Article DOI: 10.1021/ml500187a
BindingDB Entry DOI: 10.7270/Q2MW2JRD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50089334

(CHEMBL3577939)Show SMILES CC(C)CC[C@@H]1C[C@@H](CC[C@@H]1NC(=O)CNC(=O)c1cccc(c1)C(F)(F)F)N(C)C(C)C |r| Show InChI InChI=1S/C25H38F3N3O2/c1-16(2)9-10-18-14-21(31(5)17(3)4)11-12-22(18)30-23(32)15-29-24(33)19-7-6-8-20(13-19)25(26,27)28/h6-8,13,16-18,21-22H,9-12,14-15H2,1-5H3,(H,29,33)(H,30,32)/t18-,21-,22+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
ACS Med Chem Lett 6: 439-44 (2015)
Article DOI: 10.1021/ml500505q
BindingDB Entry DOI: 10.7270/Q2668FWM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM8610

(1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...)Show SMILES [H][C@]1(COc2ccc(cc2)N2CCN(CC2)C(C)=O)CO[C@@](Cn2ccnc2)(O1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) after 2 hrs by firefly luciferase-luminescence based P450-glo assay |
Eur J Med Chem 144: 716-729 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.053
BindingDB Entry DOI: 10.7270/Q2ZK5K6S |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
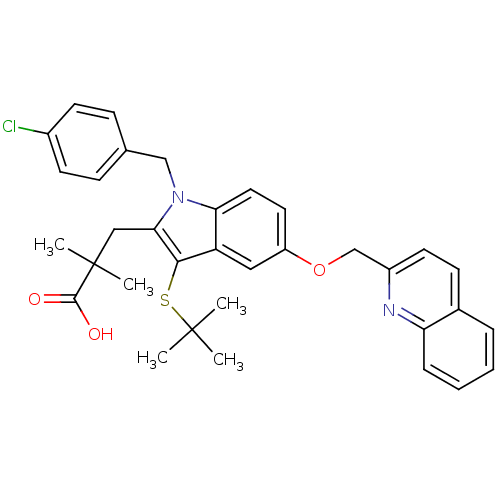
(Homo sapiens (Human)) | BDBM50029559
(2-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-5-(qui...)Show SMILES CC(C)(C)Sc1c(CC(C)(C)C(O)=O)n(Cc2ccc(Cl)cc2)c2ccc(OCc3ccc4ccccc4n3)cc12 Show InChI InChI=1S/C34H35ClN2O3S/c1-33(2,3)41-31-27-18-26(40-21-25-15-12-23-8-6-7-9-28(23)36-25)16-17-29(27)37(20-22-10-13-24(35)14-11-22)30(31)19-34(4,5)32(38)39/h6-18H,19-21H2,1-5H3,(H,38,39) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
J Med Chem 52: 5803-15 (2009)
Article DOI: 10.1021/jm900945d
BindingDB Entry DOI: 10.7270/Q2G44QB1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
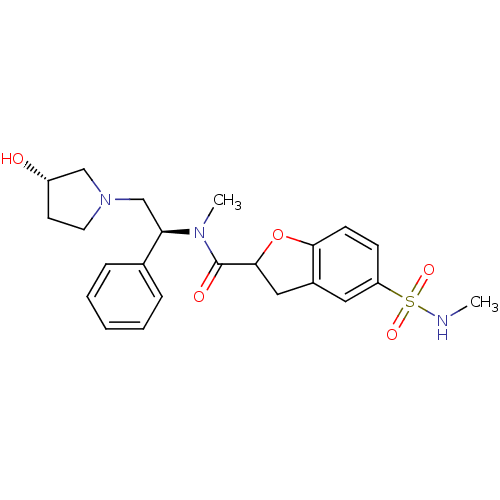
(Homo sapiens (Human)) | BDBM50174965
(5-Methylsulfamoyl-2,3-dihydro-benzofuran-2-carboxy...)Show SMILES CNS(=O)(=O)c1ccc2OC(Cc2c1)C(=O)N(C)[C@H](CN1CC[C@H](O)C1)c1ccccc1 Show InChI InChI=1S/C23H29N3O5S/c1-24-32(29,30)19-8-9-21-17(12-19)13-22(31-21)23(28)25(2)20(16-6-4-3-5-7-16)15-26-11-10-18(27)14-26/h3-9,12,18,20,22,24,27H,10-11,13-15H2,1-2H3/t18-,20+,22?/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against CYP2D6 |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50121975

((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...)Show SMILES COc1ccc2nccc([C@H](O)[C@H]3C[C@@H]4CCN3C[C@@H]4C=C)c2c1 |r,THB:20:19:12.13:16.15,10:12:18.19:16.15| Show InChI InChI=1S/C20H24N2O2/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3/t13-,14-,19+,20-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
J Med Chem 51: 2158-69 (2008)
Article DOI: 10.1021/jm701447v
BindingDB Entry DOI: 10.7270/Q29G5NPZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2D6
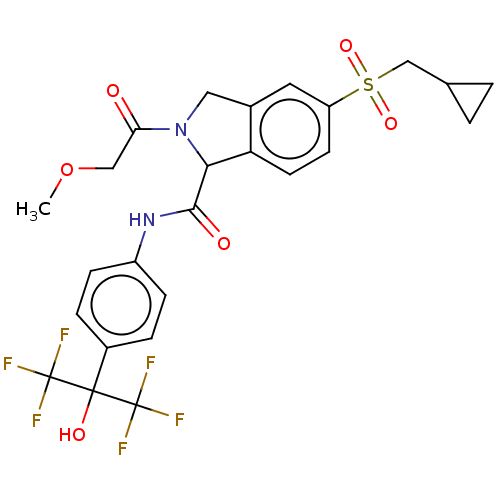
(Homo sapiens (Human)) | BDBM493607
(US10988445, Example 203)Show SMILES COCC(=O)N1Cc2cc(ccc2C1C(=O)Nc1ccc(cc1)C(O)(C(F)(F)F)C(F)(F)F)S(=O)(=O)CC1CC1 Show InChI InChI=1S/C25H24F6N2O6S/c1-39-12-20(34)33-11-15-10-18(40(37,38)13-14-2-3-14)8-9-19(15)21(33)22(35)32-17-6-4-16(5-7-17)23(36,24(26,27)28)25(29,30)31/h4-10,14,21,36H,2-3,11-13H2,1H3,(H,32,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2D6 in human liver microsomes |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01197
BindingDB Entry DOI: 10.7270/Q22Z19BG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM143359
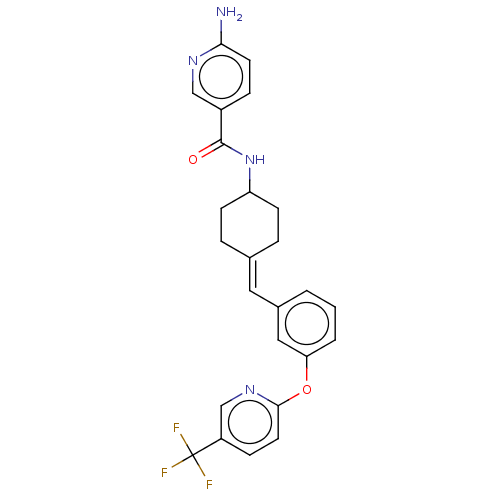
(US9682953, 20.A-3)Show SMILES Nc1ccc(cn1)C(=O)NC1CCC(CC1)=Cc1cccc(Oc2ccc(cn2)C(F)(F)F)c1 |(12.65,-1.17,;11.31,-1.94,;11.31,-3.48,;9.98,-4.25,;8.65,-3.48,;8.65,-1.94,;9.98,-1.17,;7.31,-4.25,;7.31,-5.79,;5.98,-3.48,;4.65,-4.25,;4.65,-5.79,;3.31,-6.56,;1.98,-5.79,;1.98,-4.25,;3.31,-3.48,;.65,-6.56,;-.69,-5.79,;-.69,-4.25,;-2.02,-3.48,;-3.36,-4.25,;-3.36,-5.79,;-4.69,-6.56,;-6.02,-5.79,;-6.02,-4.25,;-7.36,-3.48,;-8.69,-4.25,;-8.69,-5.79,;-7.36,-6.56,;-10.02,-3.48,;-11.36,-4.25,;-10.02,-1.94,;-10.02,-5.02,;-2.02,-6.56,)| Show InChI InChI=1S/C25H23F3N4O2/c26-25(27,28)19-7-11-23(31-15-19)34-21-3-1-2-17(13-21)12-16-4-8-20(9-5-16)32-24(33)18-6-10-22(29)30-14-18/h1-3,6-7,10-15,20H,4-5,8-9H2,(H2,29,30)(H,32,33)/b16-12- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >13 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Limited
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate preincubated for 5 mins followed by NADPH cofactor addition and me... |
Bioorg Med Chem Lett 29: 238-243 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.048
BindingDB Entry DOI: 10.7270/Q2125X14 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM143373

(US9682953, 20.A-10 | US9682953, 20.A-9)Show SMILES Nc1ncc(cn1)C(=O)NC1CCC(CC1)=Cc1cccc(Oc2ccc(cn2)C(F)(F)F)c1 |(11.34,2.69,;10,1.93,;10,.38,;8.67,-.38,;7.34,.38,;7.34,1.93,;8.67,2.69,;6,-.38,;6,-1.93,;4.67,.38,;3.33,-.38,;3.33,-1.93,;2,-2.69,;.67,-1.93,;.67,-.38,;2,.38,;-.67,-2.69,;-2,-1.93,;-2,-.38,;-3.33,.38,;-4.67,-.38,;-4.67,-1.93,;-6,-2.69,;-7.34,-1.93,;-7.34,-.38,;-8.67,.38,;-10,-.38,;-10,-1.93,;-8.67,-2.69,;-11.34,.38,;-12.67,-.39,;-11.34,1.93,;-11.34,-1.16,;-3.33,-2.69,)| Show InChI InChI=1S/C24H22F3N5O2/c25-24(26,27)18-6-9-21(29-14-18)34-20-3-1-2-16(11-20)10-15-4-7-19(8-5-15)32-22(33)17-12-30-23(28)31-13-17/h1-3,6,9-14,19H,4-5,7-8H2,(H,32,33)(H2,28,30,31)/b15-10- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >13 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Limited
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate preincubated for 5 mins followed by NADPH cofactor addition and me... |
Bioorg Med Chem Lett 29: 238-243 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.048
BindingDB Entry DOI: 10.7270/Q2125X14 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM143362
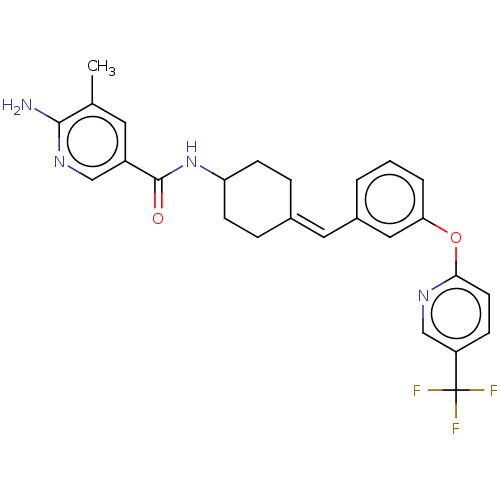
(US9682953, 20.A-5)Show SMILES Cc1cc(cnc1N)C(=O)NC1CCC(CC1)=Cc1cccc(Oc2ccc(cn2)C(F)(F)F)c1 |(11.34,-.39,;10,.38,;8.67,-.38,;7.34,.38,;7.34,1.93,;8.67,2.69,;10,1.93,;11.34,2.69,;6,-.38,;6,-1.93,;4.67,.38,;3.33,-.38,;3.33,-1.93,;2,-2.69,;.67,-1.93,;.67,-.38,;2,.38,;-.67,-2.69,;-2,-1.93,;-2,-.38,;-3.33,.38,;-4.67,-.38,;-4.67,-1.93,;-6,-2.69,;-7.34,-1.93,;-7.34,-.38,;-8.67,.38,;-10,-.38,;-10,-1.93,;-8.67,-2.69,;-11.34,.38,;-12.67,-.39,;-11.34,1.93,;-11.34,-1.16,;-3.33,-2.69,)| Show InChI InChI=1S/C26H25F3N4O2/c1-16-11-19(14-32-24(16)30)25(34)33-21-8-5-17(6-9-21)12-18-3-2-4-22(13-18)35-23-10-7-20(15-31-23)26(27,28)29/h2-4,7,10-15,21H,5-6,8-9H2,1H3,(H2,30,32)(H,33,34)/b17-12- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >13 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Limited
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate preincubated for 5 mins followed by NADPH cofactor addition and me... |
Bioorg Med Chem Lett 29: 238-243 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.048
BindingDB Entry DOI: 10.7270/Q2125X14 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50360603
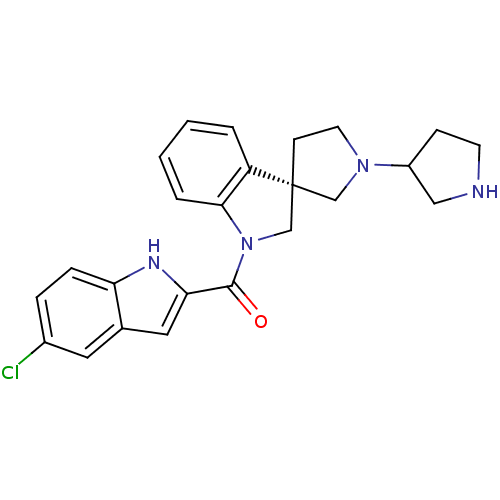
(CHEMBL1933536)Show SMILES Clc1ccc2[nH]c(cc2c1)C(=O)N1C[C@]2(CCN(C2)C2CCNC2)c2ccccc12 |r| Show InChI InChI=1S/C24H25ClN4O/c25-17-5-6-20-16(11-17)12-21(27-20)23(30)29-15-24(19-3-1-2-4-22(19)29)8-10-28(14-24)18-7-9-26-13-18/h1-6,11-12,18,26-27H,7-10,13-15H2/t18?,24-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 using dextromethorphan as substrate |
Bioorg Med Chem Lett 22: 190-3 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.036
BindingDB Entry DOI: 10.7270/Q2CF9QJS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data