 Found 1526 hits of ic50 for UniProtKB: P07858
Found 1526 hits of ic50 for UniProtKB: P07858 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cathepsin B
(Homo sapiens (Human)) | BDBM50602545

(CHEMBL4596647)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.149 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM420296
(Advanced SARS-CoV-2 Inhibitor 11a | MPI10 | acs.jm...)Show SMILES O=C[C@H](C[C@@H]1CCNC1=O)NC(=O)[C@H](CC1CCCCC1)NC(=O)c1cc2ccccc2[nH]1 Show InChI InChI=1S/C25H32N4O4/c30-15-19(13-18-10-11-26-23(18)31)27-24(32)21(12-16-6-2-1-3-7-16)29-25(33)22-14-17-8-4-5-9-20(17)28-22/h4-5,8-9,14-16,18-19,21,28H,1-3,6-7,10-13H2,(H,26,31)(H,27,32)(H,29,33)/t18-,19-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
FRET-based enzymatic assay. |
Citation and Details
Article DOI: 10.1021/jacs.1c08060
BindingDB Entry DOI: 10.7270/Q2BK1GHG |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50602542

(CHEMBL4594870)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC1CCCCC1)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.278 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50602544

(CHEMBL4594757)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.416 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM509972

(Advanced SARS-CoV-2 Inhibitor 6e | acs.jmedchem.1c...)Show SMILES CCCCC1CCC(CC1)OC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C=O |wU:22.22,24.24,wD:14.14,(-2.6,-5.27,;-1.26,-6.04,;.07,-5.27,;1.41,-6.04,;2.74,-5.27,;2.74,-3.73,;4.07,-2.96,;5.41,-3.73,;5.41,-5.27,;4.07,-6.04,;6.73,-2.93,;8.07,-3.67,;8.1,-5.21,;9.39,-2.88,;10.74,-3.62,;10.77,-5.16,;12.12,-5.91,;12.15,-7.45,;13.44,-5.11,;12.06,-2.83,;12.03,-1.29,;13.41,-3.57,;14.73,-2.78,;14.7,-1.24,;16.01,-.44,;17.41,-1.09,;18.46,.03,;17.72,1.38,;16.2,1.09,;15.08,2.14,;16.07,-3.52,;16.1,-5.06,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
FRET-based enzymatic assay. |
Citation and Details
Article DOI: 10.1021/jacs.1c08060
BindingDB Entry DOI: 10.7270/Q2BK1GHG |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM509973
(Advanced SARS-CoV-2 Inhibitor 6j | acs.jmedchem.1c...)Show SMILES CC(C)C[C@H](NC(=O)OCC1CCC(F)(F)CC1)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
FRET-based enzymatic assay. |
Citation and Details
Article DOI: 10.1021/jacs.1c08060
BindingDB Entry DOI: 10.7270/Q2BK1GHG |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50201701
(CHEMBL390474 | cis-4-(2,3-dimethylphenoxy)-6-oxa-1...)Show InChI InChI=1S/C14H17NO3/c1-9-4-3-5-11(10(9)2)17-12-6-7-15-8-13(12)18-14(15)16/h3-5,12-13H,6-8H2,1-2H3/t12-,13+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin B |
Bioorg Med Chem Lett 17: 1254-9 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.014
BindingDB Entry DOI: 10.7270/Q2QN66DT |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM16510
((2S,3S)-3-[[(1S)-1-(isoamylcarbamoyl)-3-methyl-but...)Show SMILES CC(C)CCNC(=O)[C@H](CC(C)C)NC(=O)[C@H]1O[C@@H]1C(O)=O |r| Show InChI InChI=1S/C15H26N2O5/c1-8(2)5-6-16-13(18)10(7-9(3)4)17-14(19)11-12(22-11)15(20)21/h8-12H,5-7H2,1-4H3,(H,16,18)(H,17,19)(H,20,21)/t10-,11-,12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PCBioAssay
| n/a | n/a | 1.53 | n/a | n/a | n/a | n/a | 6.8 | 23 |
PCMD
Curated by PubChem BioAssay
| Assay Description
Screening Center: Penn Center for Molecular Discovery Center Affiliation: University of Pennsylvania Network: Molecular Library Screening Center Netw... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q25T3HTJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin B
(Homo sapiens (Human)) | BDBM50107633
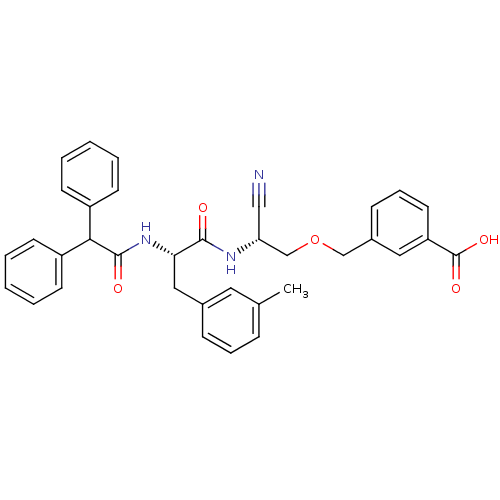
(3-[2-Cyano-2-(2-diphenylacetylamino-3-m-tolyl-prop...)Show SMILES Cc1cccc(C[C@H](NC(=O)C(c2ccccc2)c2ccccc2)C(=O)N[C@@H](COCc2cccc(c2)C(O)=O)C#N)c1 Show InChI InChI=1S/C35H33N3O5/c1-24-10-8-11-25(18-24)20-31(38-34(40)32(27-13-4-2-5-14-27)28-15-6-3-7-16-28)33(39)37-30(21-36)23-43-22-26-12-9-17-29(19-26)35(41)42/h2-19,30-32H,20,22-23H2,1H3,(H,37,39)(H,38,40)(H,41,42)/t30-,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human cathepsin B expressed in baculovirus expression system using Z-Arg-Arg-AMC as substrate incubated for 20 mins by fluo... |
Bioorg Med Chem 27: 1-15 (2019)
Article DOI: 10.1016/j.bmc.2018.10.017
BindingDB Entry DOI: 10.7270/Q21G0QK2 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50107639

(3-{2-[2-(4-Chloro-2-fluoro-benzoylamino)-3-m-tolyl...)Show SMILES Cc1cccc(C[C@H](NC(=O)c2ccc(Cl)cc2F)C(=O)N[C@@H](COCc2cccc(c2)C(O)=O)C#N)c1 Show InChI InChI=1S/C28H25ClFN3O5/c1-17-4-2-5-18(10-17)12-25(33-26(34)23-9-8-21(29)13-24(23)30)27(35)32-22(14-31)16-38-15-19-6-3-7-20(11-19)28(36)37/h2-11,13,22,25H,12,15-16H2,1H3,(H,32,35)(H,33,34)(H,36,37)/t22-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation
Curated by ChEMBL
| Assay Description
Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. |
J Med Chem 44: 4524-34 (2001)
BindingDB Entry DOI: 10.7270/Q2GQ6X2J |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50201705
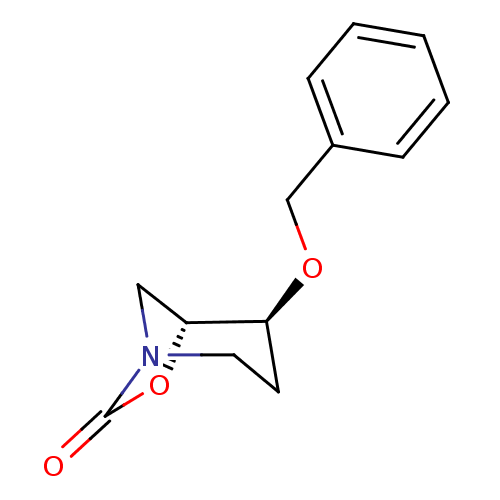
((1R,2S)-2-(benzyloxy)-7-oxa-5-aza-bicyclo[3.2.1]oc...)Show InChI InChI=1S/C13H15NO3/c15-13-14-7-6-11(12(8-14)17-13)16-9-10-4-2-1-3-5-10/h1-5,11-12H,6-9H2/t11-,12+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin B |
Bioorg Med Chem Lett 17: 1254-9 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.014
BindingDB Entry DOI: 10.7270/Q2QN66DT |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM16509
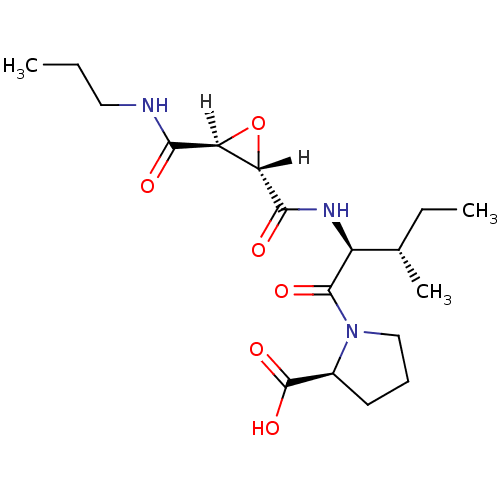
((2S)-1-[(2S,3S)-3-methyl-2-{[(2S,3S)-3-(propylcarb...)Show SMILES [H][C@@]1(O[C@]1([H])C(=O)N[C@@H]([C@@H](C)CC)C(=O)N1CCC[C@H]1C(O)=O)C(=O)NCCC |r| Show InChI InChI=1S/C18H29N3O6/c1-4-8-19-15(22)13-14(27-13)16(23)20-12(10(3)5-2)17(24)21-9-6-7-11(21)18(25)26/h10-14H,4-9H2,1-3H3,(H,19,22)(H,20,23)(H,25,26)/t10-,11-,12-,13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin B (unknown origin) assessed as reduction in 7-amino-4-methylcoumarin formation using Z-Arg-Arg-MCA as substrate preincubated ... |
Bioorg Med Chem 27: 1-15 (2019)
Article DOI: 10.1016/j.bmc.2018.10.017
BindingDB Entry DOI: 10.7270/Q21G0QK2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin B
(Homo sapiens (Human)) | BDBM16508
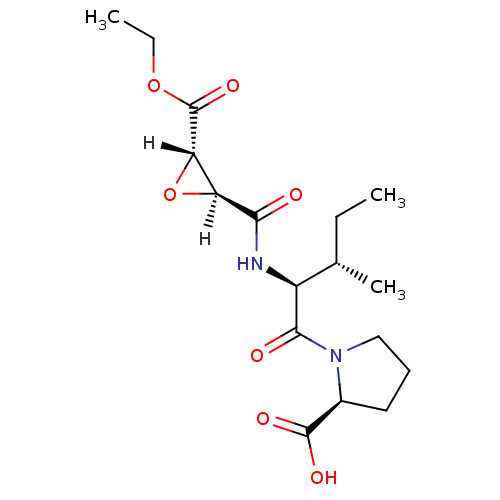
((2S)-1-[(2S,3S)-2-{[(2S,3S)-3-(ethoxycarbonyl)oxir...)Show SMILES [H][C@@]1(O[C@]1([H])C(=O)OCC)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C17H26N2O7/c1-4-9(3)11(15(21)19-8-6-7-10(19)16(22)23)18-14(20)12-13(26-12)17(24)25-5-2/h9-13H,4-8H2,1-3H3,(H,18,20)(H,22,23)/t9-,10-,11-,12-,13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin B (unknown origin) assessed as reduction in 7-amino-4-methylcoumarin formation using Z-Arg-Arg-MCA as substrate preincubated ... |
Bioorg Med Chem 27: 1-15 (2019)
Article DOI: 10.1016/j.bmc.2018.10.017
BindingDB Entry DOI: 10.7270/Q21G0QK2 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50163831
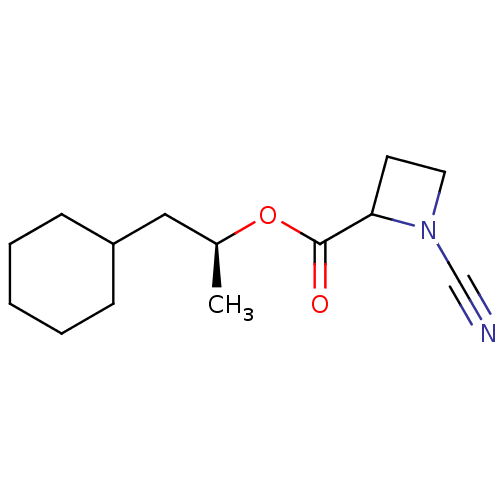
((2S)-1-cyclohexylpropan-2-yl 1-cyanoazetidine-2-ca...)Show InChI InChI=1S/C14H22N2O2/c1-11(9-12-5-3-2-4-6-12)18-14(17)13-7-8-16(13)10-15/h11-13H,2-9H2,1H3/t11-,13?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human cathepsin B using 10 uM Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 1815-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.033
BindingDB Entry DOI: 10.7270/Q2DR2V1G |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM16509

((2S)-1-[(2S,3S)-3-methyl-2-{[(2S,3S)-3-(propylcarb...)Show SMILES [H][C@@]1(O[C@]1([H])C(=O)N[C@@H]([C@@H](C)CC)C(=O)N1CCC[C@H]1C(O)=O)C(=O)NCCC |r| Show InChI InChI=1S/C18H29N3O6/c1-4-8-19-15(22)13-14(27-13)16(23)20-12(10(3)5-2)17(24)21-9-6-7-11(21)18(25)26/h10-14H,4-9H2,1-3H3,(H,19,22)(H,20,23)(H,25,26)/t10-,11-,12-,13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of cathepsin B in human SH-SY5Y cells using RR-AMC as substrate by fluorescence microplate reader assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.06.008
BindingDB Entry DOI: 10.7270/Q2736VMH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin B
(Homo sapiens (Human)) | BDBM50250153
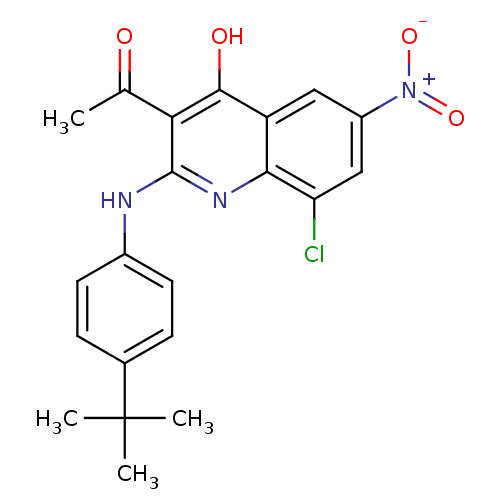
(3-acetyl-2-(4-tert-butylphenylamino)-8-chloro-6-ni...)Show SMILES CC(=O)c1c(Nc2ccc(cc2)C(C)(C)C)nc2c(Cl)cc(cc2c1O)[N+]([O-])=O Show InChI InChI=1S/C21H20ClN3O4/c1-11(26)17-19(27)15-9-14(25(28)29)10-16(22)18(15)24-20(17)23-13-7-5-12(6-8-13)21(2,3)4/h5-10H,1-4H3,(H2,23,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.11 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin B after 30 mins by fluorometric end-point assay |
J Med Chem 52: 3093-7 (2009)
Article DOI: 10.1021/jm8014734
BindingDB Entry DOI: 10.7270/Q2KK9CQ4 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM16509

((2S)-1-[(2S,3S)-3-methyl-2-{[(2S,3S)-3-(propylcarb...)Show SMILES [H][C@@]1(O[C@]1([H])C(=O)N[C@@H]([C@@H](C)CC)C(=O)N1CCC[C@H]1C(O)=O)C(=O)NCCC |r| Show InChI InChI=1S/C18H29N3O6/c1-4-8-19-15(22)13-14(27-13)16(23)20-12(10(3)5-2)17(24)21-9-6-7-11(21)18(25)26/h10-14H,4-9H2,1-3H3,(H,19,22)(H,20,23)(H,25,26)/t10-,11-,12-,13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of cathepsin B (unknown origin) using RR-AMC as substrate preincubated for 30 mins followed by substrate addition and further incubated fo... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.06.008
BindingDB Entry DOI: 10.7270/Q2736VMH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin B
(Homo sapiens (Human)) | BDBM50135541
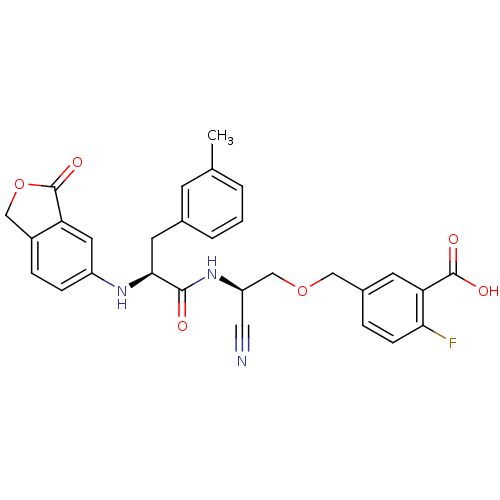
(5-{(R)-2-Cyano-2-[(S)-2-(3-oxo-1,3-dihydro-isobenz...)Show SMILES Cc1cccc(C[C@H](Nc2ccc3COC(=O)c3c2)C(=O)N[C@@H](COCc2ccc(F)c(c2)C(O)=O)C#N)c1 Show InChI InChI=1S/C29H26FN3O6/c1-17-3-2-4-18(9-17)11-26(32-21-7-6-20-15-39-29(37)23(20)12-21)27(34)33-22(13-31)16-38-14-19-5-8-25(30)24(10-19)28(35)36/h2-10,12,22,26,32H,11,14-16H2,1H3,(H,33,34)(H,35,36)/t22-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibtitory activity against cathepsin B (catB) |
Bioorg Med Chem Lett 13: 4121-4 (2003)
BindingDB Entry DOI: 10.7270/Q2BC3XZC |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM16509

((2S)-1-[(2S,3S)-3-methyl-2-{[(2S,3S)-3-(propylcarb...)Show SMILES [H][C@@]1(O[C@]1([H])C(=O)N[C@@H]([C@@H](C)CC)C(=O)N1CCC[C@H]1C(O)=O)C(=O)NCCC |r| Show InChI InChI=1S/C18H29N3O6/c1-4-8-19-15(22)13-14(27-13)16(23)20-12(10(3)5-2)17(24)21-9-6-7-11(21)18(25)26/h10-14H,4-9H2,1-3H3,(H,19,22)(H,20,23)(H,25,26)/t10-,11-,12-,13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Ewha Womans University, Seoul 120-750, Republic of Korea.
| Assay Description
Inhibition of cathepsin B was assayed in reaction buffer (50 mM NaOAc-HCl, 2 mM dithiothreitol, 2 mM EDTA, pH 5.5) containing 20 µM substrate an... |
Bioorg Chem 51: 24-30 (2013)
Article DOI: 10.1016/j.bioorg.2013.09.002
BindingDB Entry DOI: 10.7270/Q2NP233R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin B
(Homo sapiens (Human)) | BDBM50157741

(CHEMBL374508 | E-64 | E64)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10-,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00316j
BindingDB Entry DOI: 10.7270/Q23200VF |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50135543

(3-{(R)-2-Cyano-2-[(S)-2-(2-methyl-1,3-dioxo-2,3-di...)Show SMILES CN1C(=O)c2ccc(N[C@@H](Cc3cccc(C)c3)C(=O)N[C@@H](COCc3cccc(c3)C(O)=O)C#N)cc2C1=O Show InChI InChI=1S/C30H28N4O6/c1-18-5-3-6-19(11-18)13-26(32-22-9-10-24-25(14-22)29(37)34(2)28(24)36)27(35)33-23(15-31)17-40-16-20-7-4-8-21(12-20)30(38)39/h3-12,14,23,26,32H,13,16-17H2,1-2H3,(H,33,35)(H,38,39)/t23-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibtitory activity against cathepsin B (catB) |
Bioorg Med Chem Lett 13: 4121-4 (2003)
BindingDB Entry DOI: 10.7270/Q2BC3XZC |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50135533

((S)-N-[(R)-Cyano-(3-tetrazol-1-yl-benzyloxymethyl)...)Show SMILES Cc1cccc(C[C@H](Nc2ccc3COC(=O)c3c2)C(=O)N[C@@H](COCc2cccc(c2)-n2cnnn2)C#N)c1 Show InChI InChI=1S/C29H27N7O4/c1-19-4-2-5-20(10-19)12-27(32-23-9-8-22-16-40-29(38)26(22)13-23)28(37)33-24(14-30)17-39-15-21-6-3-7-25(11-21)36-18-31-34-35-36/h2-11,13,18,24,27,32H,12,15-17H2,1H3,(H,33,37)/t24-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibtitory activity against cathepsin B (catB) |
Bioorg Med Chem Lett 13: 4121-4 (2003)
BindingDB Entry DOI: 10.7270/Q2BC3XZC |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50107633

(3-[2-Cyano-2-(2-diphenylacetylamino-3-m-tolyl-prop...)Show SMILES Cc1cccc(C[C@H](NC(=O)C(c2ccccc2)c2ccccc2)C(=O)N[C@@H](COCc2cccc(c2)C(O)=O)C#N)c1 Show InChI InChI=1S/C35H33N3O5/c1-24-10-8-11-25(18-24)20-31(38-34(40)32(27-13-4-2-5-14-27)28-15-6-3-7-16-28)33(39)37-30(21-36)23-43-22-26-12-9-17-29(19-26)35(41)42/h2-19,30-32H,20,22-23H2,1H3,(H,37,39)(H,38,40)(H,41,42)/t30-,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation
Curated by ChEMBL
| Assay Description
Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. |
J Med Chem 44: 4524-34 (2001)
BindingDB Entry DOI: 10.7270/Q2GQ6X2J |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50107633

(3-[2-Cyano-2-(2-diphenylacetylamino-3-m-tolyl-prop...)Show SMILES Cc1cccc(C[C@H](NC(=O)C(c2ccccc2)c2ccccc2)C(=O)N[C@@H](COCc2cccc(c2)C(O)=O)C#N)c1 Show InChI InChI=1S/C35H33N3O5/c1-24-10-8-11-25(18-24)20-31(38-34(40)32(27-13-4-2-5-14-27)28-15-6-3-7-16-28)33(39)37-30(21-36)23-43-22-26-12-9-17-29(19-26)35(41)42/h2-19,30-32H,20,22-23H2,1H3,(H,37,39)(H,38,40)(H,41,42)/t30-,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation
Curated by ChEMBL
| Assay Description
Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. |
J Med Chem 44: 4524-34 (2001)
BindingDB Entry DOI: 10.7270/Q2GQ6X2J |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50201700
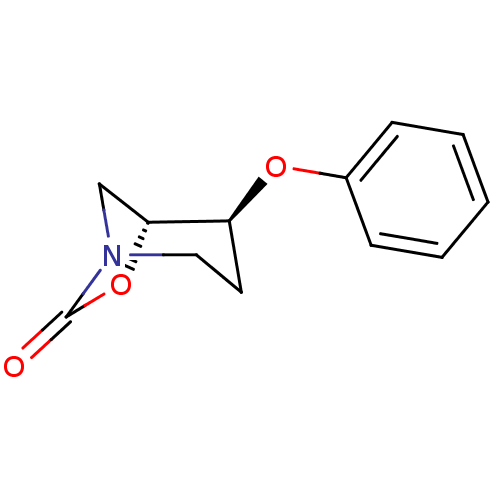
((1R,2S)-2-phenoxy-7-oxa-5-aza-bicyclo[3.2.1]octan-...)Show InChI InChI=1S/C12H13NO3/c14-12-13-7-6-10(11(8-13)16-12)15-9-4-2-1-3-5-9/h1-5,10-11H,6-8H2/t10-,11+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin B |
Bioorg Med Chem Lett 17: 1254-9 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.014
BindingDB Entry DOI: 10.7270/Q2QN66DT |
More data for this
Ligand-Target Pair | |
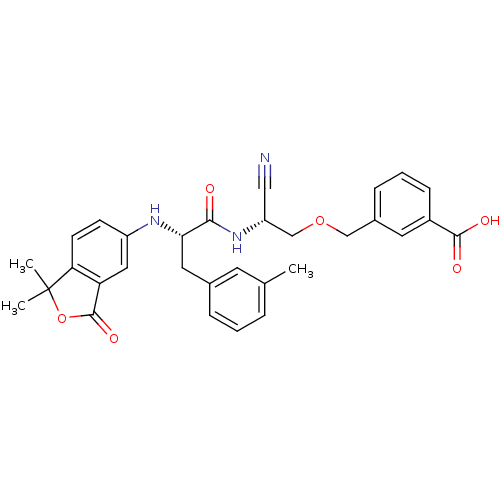
Cathepsin B
(Homo sapiens (Human)) | BDBM50135535
(3-{(R)-2-Cyano-2-[(S)-2-(1,1-dimethyl-3-oxo-1,3-di...)Show SMILES Cc1cccc(C[C@H](Nc2ccc3c(c2)C(=O)OC3(C)C)C(=O)N[C@@H](COCc2cccc(c2)C(O)=O)C#N)c1 Show InChI InChI=1S/C31H31N3O6/c1-19-6-4-7-20(12-19)14-27(33-23-10-11-26-25(15-23)30(38)40-31(26,2)3)28(35)34-24(16-32)18-39-17-21-8-5-9-22(13-21)29(36)37/h4-13,15,24,27,33H,14,17-18H2,1-3H3,(H,34,35)(H,36,37)/t24-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibtitory activity against cathepsin B (catB) |
Bioorg Med Chem Lett 13: 4121-4 (2003)
BindingDB Entry DOI: 10.7270/Q2BC3XZC |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50135537

(3-{(R)-2-Cyano-2-[(S)-2-(3-oxo-indan-5-ylamino)-3-...)Show SMILES Cc1cccc(C[C@H](Nc2ccc3CCC(=O)c3c2)C(=O)N[C@@H](COCc2cccc(c2)C(O)=O)C#N)c1 Show InChI InChI=1S/C30H29N3O5/c1-19-4-2-5-20(12-19)14-27(32-24-10-8-22-9-11-28(34)26(22)15-24)29(35)33-25(16-31)18-38-17-21-6-3-7-23(13-21)30(36)37/h2-8,10,12-13,15,25,27,32H,9,11,14,17-18H2,1H3,(H,33,35)(H,36,37)/t25-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibtitory activity against cathepsin B (catB) |
Bioorg Med Chem Lett 13: 4121-4 (2003)
BindingDB Entry DOI: 10.7270/Q2BC3XZC |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50107646

(3-[4-Cyano-4-(2-diphenylacetylamino-3-m-tolyl-prop...)Show SMILES Cc1cccc(C[C@H](NC(=O)C(c2ccccc2)c2ccccc2)C(=O)N[C@@H](CC#Cc2cccc(c2)C(O)=O)C#N)c1 Show InChI InChI=1S/C36H31N3O4/c1-25-11-8-14-27(21-25)23-32(39-35(41)33(28-15-4-2-5-16-28)29-17-6-3-7-18-29)34(40)38-31(24-37)20-10-13-26-12-9-19-30(22-26)36(42)43/h2-9,11-12,14-19,21-22,31-33H,20,23H2,1H3,(H,38,40)(H,39,41)(H,42,43)/t31-,32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation
Curated by ChEMBL
| Assay Description
Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. |
J Med Chem 44: 4524-34 (2001)
BindingDB Entry DOI: 10.7270/Q2GQ6X2J |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50229129
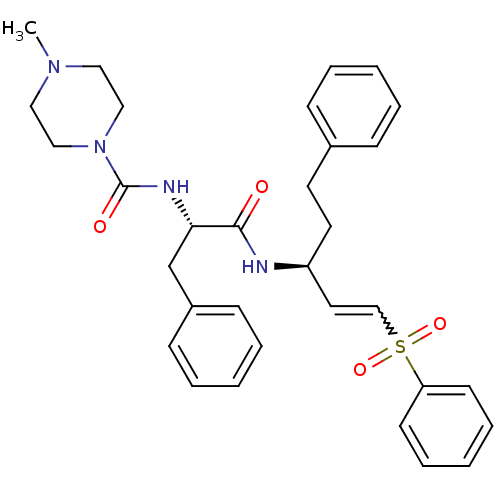
(4-Methyl-piperazine-1-carboxylic acid [(S)-1-((E)-...)Show SMILES CN1CCN(CC1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCc1ccccc1)C=CS(=O)(=O)c1ccccc1 |r,w:31.34| Show InChI InChI=1S/C32H38N4O4S/c1-35-20-22-36(23-21-35)32(38)34-30(25-27-13-7-3-8-14-27)31(37)33-28(18-17-26-11-5-2-6-12-26)19-24-41(39,40)29-15-9-4-10-16-29/h2-16,19,24,28,30H,17-18,20-23,25H2,1H3,(H,33,37)(H,34,38)/t28-,30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University
Curated by ChEMBL
| Assay Description
Inhibition of human Cathepsin B using Cbz-Phe-Arg-AMC as substrate by fluorescence assay |
J Med Chem 63: 3298-3316 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02078
BindingDB Entry DOI: 10.7270/Q2PV6PQD |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50135546
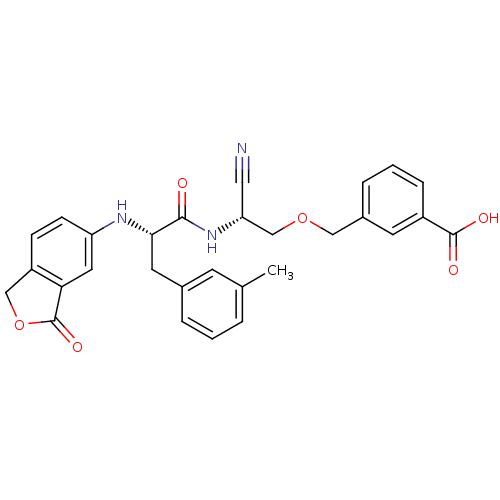
(3-{(R)-2-Cyano-2-[(S)-2-(3-oxo-1,3-dihydro-isobenz...)Show SMILES Cc1cccc(C[C@H](Nc2ccc3COC(=O)c3c2)C(=O)N[C@@H](COCc2cccc(c2)C(O)=O)C#N)c1 Show InChI InChI=1S/C29H27N3O6/c1-18-4-2-5-19(10-18)12-26(31-23-9-8-22-16-38-29(36)25(22)13-23)27(33)32-24(14-30)17-37-15-20-6-3-7-21(11-20)28(34)35/h2-11,13,24,26,31H,12,15-17H2,1H3,(H,32,33)(H,34,35)/t24-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibtitory activity against cathepsin B (catB) |
Bioorg Med Chem Lett 13: 4121-4 (2003)
BindingDB Entry DOI: 10.7270/Q2BC3XZC |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50107626

(3-{(R)-2-Cyano-2-[(S)-2-(2,4-difluoro-benzoylamino...)Show SMILES Cc1cccc(C[C@H](NC(=O)c2ccc(F)cc2F)C(=O)N[C@@H](COCc2cccc(c2)C(O)=O)C#N)c1 Show InChI InChI=1S/C28H25F2N3O5/c1-17-4-2-5-18(10-17)12-25(33-26(34)23-9-8-21(29)13-24(23)30)27(35)32-22(14-31)16-38-15-19-6-3-7-20(11-19)28(36)37/h2-11,13,22,25H,12,15-16H2,1H3,(H,32,35)(H,33,34)(H,36,37)/t22-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation
Curated by ChEMBL
| Assay Description
Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. |
J Med Chem 44: 4524-34 (2001)
BindingDB Entry DOI: 10.7270/Q2GQ6X2J |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50033762

(Gallinamide A)Show SMILES CC[C@H](C)[C@H](N(C)C)C(=O)O[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)\C=C\C(=O)N1[C@@H](C)C(OC)=CC1=O |r,c:38| Show InChI InChI=1S/C31H52N4O7/c1-12-20(6)28(34(9)10)31(40)42-25(16-19(4)5)30(39)33-23(15-18(2)3)29(38)32-21(7)13-14-26(36)35-22(8)24(41-11)17-27(35)37/h13-14,17-23,25,28H,12,15-16H2,1-11H3,(H,32,38)(H,33,39)/b14-13+/t20-,21-,22-,23-,25-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50201699
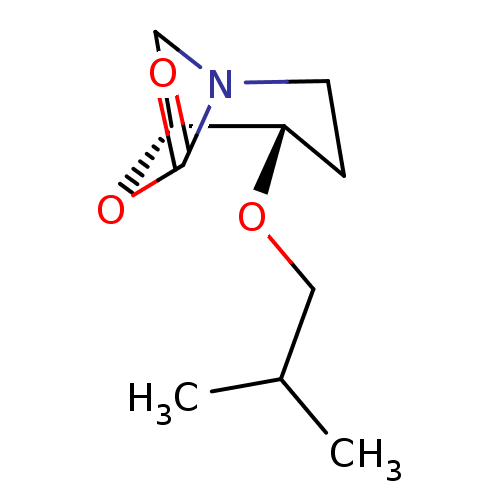
((1R,2S)-2-isobutoxy-7-oxa-5-aza-bicyclo[3.2.1]octa...)Show InChI InChI=1S/C10H17NO3/c1-7(2)6-13-8-3-4-11-5-9(8)14-10(11)12/h7-9H,3-6H2,1-2H3/t8-,9+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin B |
Bioorg Med Chem Lett 17: 1254-9 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.014
BindingDB Entry DOI: 10.7270/Q2QN66DT |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50135532
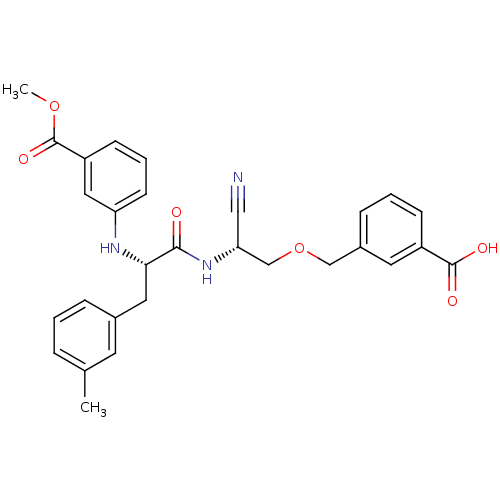
(3-{2-cyano-2-[1-(3-methyloxycarbonylanilino)-2-(3-...)Show SMILES COC(=O)c1cccc(N[C@@H](Cc2cccc(C)c2)C(=O)N[C@@H](COCc2cccc(c2)C(O)=O)C#N)c1 Show InChI InChI=1S/C29H29N3O6/c1-19-6-3-7-20(12-19)14-26(31-24-11-5-10-23(15-24)29(36)37-2)27(33)32-25(16-30)18-38-17-21-8-4-9-22(13-21)28(34)35/h3-13,15,25-26,31H,14,17-18H2,1-2H3,(H,32,33)(H,34,35)/t25-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibtitory activity against cathepsin B (catB) |
Bioorg Med Chem Lett 13: 4121-4 (2003)
BindingDB Entry DOI: 10.7270/Q2BC3XZC |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50135544

(2-Chloro-5-[(R)-2-cyano-2-((S)-2-phenylamino-3-m-t...)Show SMILES Cc1cccc(C[C@H](Nc2ccccc2)C(=O)N[C@@H](COCc2ccc(Cl)c(c2)C(O)=O)C#N)c1 Show InChI InChI=1S/C27H26ClN3O4/c1-18-6-5-7-19(12-18)14-25(30-21-8-3-2-4-9-21)26(32)31-22(15-29)17-35-16-20-10-11-24(28)23(13-20)27(33)34/h2-13,22,25,30H,14,16-17H2,1H3,(H,31,32)(H,33,34)/t22-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibtitory activity against cathepsin B (catB) |
Bioorg Med Chem Lett 13: 4121-4 (2003)
BindingDB Entry DOI: 10.7270/Q2BC3XZC |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50549374

(CHEMBL4746374)Show SMILES C[C@@H](OCc1ccccc1)[C@H](NC(=O)[C@H](Cc1cccc(Cl)c1)NC(=O)c1cccc(c1)-c1cccnc1)C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human cathepsin B at pH 6 using Z-Phe-Arg-AMC fluorogenic peptide as substrate preincubated for 2 mins followed by substrate addition a... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115827
BindingDB Entry DOI: 10.7270/Q2CR5XZ7 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50331787
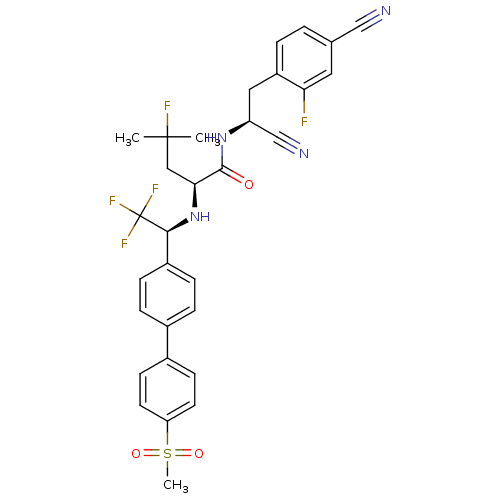
((S)-N-((S)-1-cyano-2-(4-cyano-2-fluorophenyl)ethyl...)Show SMILES CC(C)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)N[C@@H](Cc1ccc(cc1F)C#N)C#N |r| Show InChI InChI=1S/C31H29F5N4O3S/c1-30(2,33)16-27(29(41)39-24(18-38)15-23-5-4-19(17-37)14-26(23)32)40-28(31(34,35)36)22-8-6-20(7-9-22)21-10-12-25(13-11-21)44(3,42)43/h4-14,24,27-28,40H,15-16H2,1-3H3,(H,39,41)/t24-,27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Cat B in HepG2 cells |
Bioorg Med Chem Lett 20: 7444-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.015
BindingDB Entry DOI: 10.7270/Q29S1R8D |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50331787

((S)-N-((S)-1-cyano-2-(4-cyano-2-fluorophenyl)ethyl...)Show SMILES CC(C)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)N[C@@H](Cc1ccc(cc1F)C#N)C#N |r| Show InChI InChI=1S/C31H29F5N4O3S/c1-30(2,33)16-27(29(41)39-24(18-38)15-23-5-4-19(17-37)14-26(23)32)40-28(31(34,35)36)22-8-6-20(7-9-22)21-10-12-25(13-11-21)44(3,42)43/h4-14,24,27-28,40H,15-16H2,1-3H3,(H,39,41)/t24-,27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo
Curated by ChEMBL
| Assay Description
Reversible inhibition of human cathepsin B |
J Med Chem 62: 10497-10525 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00683
BindingDB Entry DOI: 10.7270/Q2K93BT1 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50135547

(3-{(R)-2-Cyano-2-[(S)-2-(2-methyl-3-oxo-2,3-dihydr...)Show SMILES CN1Cc2ccc(N[C@@H](Cc3cccc(C)c3)C(=O)N[C@@H](COCc3cccc(c3)C(O)=O)C#N)cc2C1=O Show InChI InChI=1S/C30H30N4O5/c1-19-5-3-6-20(11-19)13-27(32-24-10-9-23-16-34(2)29(36)26(23)14-24)28(35)33-25(15-31)18-39-17-21-7-4-8-22(12-21)30(37)38/h3-12,14,25,27,32H,13,16-18H2,1-2H3,(H,33,35)(H,37,38)/t25-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibtitory activity against cathepsin B (catB) |
Bioorg Med Chem Lett 13: 4121-4 (2003)
BindingDB Entry DOI: 10.7270/Q2BC3XZC |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50286441

((S)-2-((S)-2-Acetylamino-4-(S)-methyl-pentanoylami...)Show SMILES CC(C)C[C@H](NC(C)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O Show InChI InChI=1S/C20H38N6O5/c1-11(2)9-15(24-13(5)27)17(28)26-16(10-12(3)4)18(29)25-14(19(30)31)7-6-8-23-20(21)22/h11-12,14-16H,6-10H2,1-5H3,(H,24,27)(H,25,29)(H,26,28)(H,30,31)(H4,21,22,23)/t14-,15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin B |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50107620

(3-[2-(2-Benzoylamino-3-m-tolyl-propionylamino)-2-c...)Show SMILES Cc1cccc(C[C@H](NC(=O)c2ccccc2)C(=O)N[C@@H](COCc2cccc(c2)C(O)=O)C#N)c1 Show InChI InChI=1S/C28H27N3O5/c1-19-7-5-8-20(13-19)15-25(31-26(32)22-10-3-2-4-11-22)27(33)30-24(16-29)18-36-17-21-9-6-12-23(14-21)28(34)35/h2-14,24-25H,15,17-18H2,1H3,(H,30,33)(H,31,32)(H,34,35)/t24-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation
Curated by ChEMBL
| Assay Description
Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. |
J Med Chem 44: 4524-34 (2001)
BindingDB Entry DOI: 10.7270/Q2GQ6X2J |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50157741

(CHEMBL374508 | E-64 | E64)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10-,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin B after 10 mins |
J Biol Chem 282: 20836-46 (2007)
Article DOI: 10.1074/jbc.M702615200
BindingDB Entry DOI: 10.7270/Q2125TJM |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50107620

(3-[2-(2-Benzoylamino-3-m-tolyl-propionylamino)-2-c...)Show SMILES Cc1cccc(C[C@H](NC(=O)c2ccccc2)C(=O)N[C@@H](COCc2cccc(c2)C(O)=O)C#N)c1 Show InChI InChI=1S/C28H27N3O5/c1-19-7-5-8-20(13-19)15-25(31-26(32)22-10-3-2-4-11-22)27(33)30-24(16-29)18-36-17-21-9-6-12-23(14-21)28(34)35/h2-14,24-25H,15,17-18H2,1H3,(H,30,33)(H,31,32)(H,34,35)/t24-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human cathepsin B expressed in baculovirus expression system using Z-Arg-Arg-AMC as substrate incubated for 20 mins by fluo... |
Bioorg Med Chem 27: 1-15 (2019)
Article DOI: 10.1016/j.bmc.2018.10.017
BindingDB Entry DOI: 10.7270/Q21G0QK2 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50107629

(3-[2-Cyano-2-(2-pentanoylamino-3-m-tolyl-propionyl...)Show SMILES CCCCC(=O)N[C@@H](Cc1cccc(C)c1)C(=O)N[C@@H](COCc1cccc(c1)C(O)=O)C#N Show InChI InChI=1S/C26H31N3O5/c1-3-4-11-24(30)29-23(14-19-8-5-7-18(2)12-19)25(31)28-22(15-27)17-34-16-20-9-6-10-21(13-20)26(32)33/h5-10,12-13,22-23H,3-4,11,14,16-17H2,1-2H3,(H,28,31)(H,29,30)(H,32,33)/t22-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human cathepsin B expressed in baculovirus expression system using Z-Arg-Arg-AMC as substrate incubated for 20 mins by fluo... |
Bioorg Med Chem 27: 1-15 (2019)
Article DOI: 10.1016/j.bmc.2018.10.017
BindingDB Entry DOI: 10.7270/Q21G0QK2 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50107626

(3-{(R)-2-Cyano-2-[(S)-2-(2,4-difluoro-benzoylamino...)Show SMILES Cc1cccc(C[C@H](NC(=O)c2ccc(F)cc2F)C(=O)N[C@@H](COCc2cccc(c2)C(O)=O)C#N)c1 Show InChI InChI=1S/C28H25F2N3O5/c1-17-4-2-5-18(10-17)12-25(33-26(34)23-9-8-21(29)13-24(23)30)27(35)32-22(14-31)16-38-15-19-6-3-7-20(11-19)28(36)37/h2-11,13,22,25H,12,15-16H2,1H3,(H,32,35)(H,33,34)(H,36,37)/t22-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human cathepsin B expressed in baculovirus expression system using Z-Arg-Arg-AMC as substrate incubated for 20 mins by fluo... |
Bioorg Med Chem 27: 1-15 (2019)
Article DOI: 10.1016/j.bmc.2018.10.017
BindingDB Entry DOI: 10.7270/Q21G0QK2 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50107639

(3-{2-[2-(4-Chloro-2-fluoro-benzoylamino)-3-m-tolyl...)Show SMILES Cc1cccc(C[C@H](NC(=O)c2ccc(Cl)cc2F)C(=O)N[C@@H](COCc2cccc(c2)C(O)=O)C#N)c1 Show InChI InChI=1S/C28H25ClFN3O5/c1-17-4-2-5-18(10-17)12-25(33-26(34)23-9-8-21(29)13-24(23)30)27(35)32-22(14-31)16-38-15-19-6-3-7-20(11-19)28(36)37/h2-11,13,22,25H,12,15-16H2,1H3,(H,32,35)(H,33,34)(H,36,37)/t22-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human cathepsin B expressed in baculovirus expression system using Z-Arg-Arg-AMC as substrate incubated for 20 mins by fluo... |
Bioorg Med Chem 27: 1-15 (2019)
Article DOI: 10.1016/j.bmc.2018.10.017
BindingDB Entry DOI: 10.7270/Q21G0QK2 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50107638

(CHEMBL336436 | N-(Benzyloxymethyl-cyano-methyl)-2-...)Show SMILES Cc1cccc(C[C@H](NC(=O)C(c2ccccc2)c2ccccc2)C(=O)N[C@@H](COCc2ccccc2)C#N)c1 Show InChI InChI=1S/C34H33N3O3/c1-25-12-11-15-27(20-25)21-31(33(38)36-30(22-35)24-40-23-26-13-5-2-6-14-26)37-34(39)32(28-16-7-3-8-17-28)29-18-9-4-10-19-29/h2-20,30-32H,21,23-24H2,1H3,(H,36,38)(H,37,39)/t30-,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation
Curated by ChEMBL
| Assay Description
Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. |
J Med Chem 44: 4524-34 (2001)
BindingDB Entry DOI: 10.7270/Q2GQ6X2J |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50252525
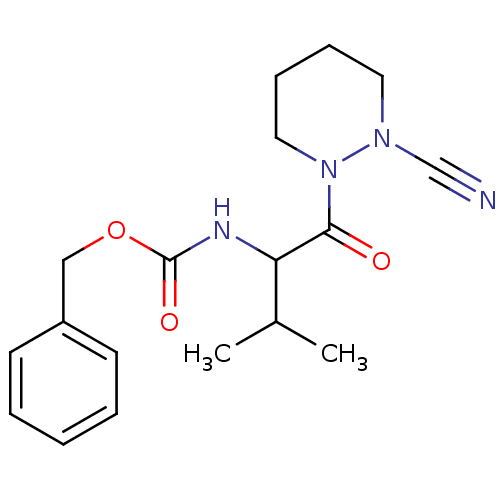
(CHEMBL493039 | benzyl 1-(2-cyanopiperazin-1-yl)-3-...)Show InChI InChI=1S/C18H24N4O3/c1-14(2)16(17(23)22-11-7-6-10-21(22)13-19)20-18(24)25-12-15-8-4-3-5-9-15/h3-5,8-9,14,16H,6-7,10-12H2,1-2H3,(H,20,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin B |
Bioorg Med Chem Lett 18: 3988-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.017
BindingDB Entry DOI: 10.7270/Q20V8CKF |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50107650

(CHEMBL138661 | N-Cyanomethyl-3-(3,5-dimethyl-pheny...)Show SMILES Cc1cc(C)cc(C[C@H](NC(=O)C(c2ccccc2)c2ccccc2)C(=O)NCC#N)c1 Show InChI InChI=1S/C27H27N3O2/c1-19-15-20(2)17-21(16-19)18-24(26(31)29-14-13-28)30-27(32)25(22-9-5-3-6-10-22)23-11-7-4-8-12-23/h3-12,15-17,24-25H,14,18H2,1-2H3,(H,29,31)(H,30,32)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 11.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation
Curated by ChEMBL
| Assay Description
Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. |
J Med Chem 44: 4524-34 (2001)
BindingDB Entry DOI: 10.7270/Q2GQ6X2J |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50510758
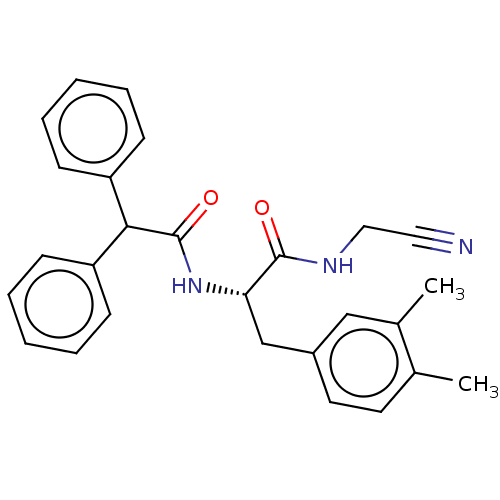
(CHEMBL4582255)Show SMILES Cc1ccc(C[C@H](NC(=O)C(c2ccccc2)c2ccccc2)C(=O)NCC#N)cc1C |r| Show InChI InChI=1S/C27H27N3O2/c1-19-13-14-21(17-20(19)2)18-24(26(31)29-16-15-28)30-27(32)25(22-9-5-3-6-10-22)23-11-7-4-8-12-23/h3-14,17,24-25H,16,18H2,1-2H3,(H,29,31)(H,30,32)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human cathepsin B expressed in baculovirus expression system using Z-Arg-Arg-AMC as substrate incubated for 20 mins by fluo... |
Bioorg Med Chem 27: 1-15 (2019)
Article DOI: 10.1016/j.bmc.2018.10.017
BindingDB Entry DOI: 10.7270/Q21G0QK2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data