 Found 350 hits of ic50 for UniProtKB: P29089
Found 350 hits of ic50 for UniProtKB: P29089 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Type-1 angiotensin II receptor B
(RAT) | BDBM50282363
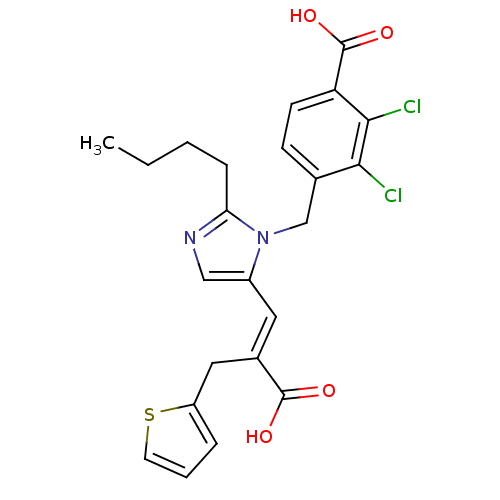
(4-[2-Butyl-5-((E)-2-carboxy-3-thiophen-2-yl-propen...)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(C(O)=O)c(Cl)c1Cl Show InChI InChI=1S/C23H22Cl2N2O4S/c1-2-3-6-19-26-12-16(10-15(22(28)29)11-17-5-4-9-32-17)27(19)13-14-7-8-18(23(30)31)21(25)20(14)24/h4-5,7-10,12H,2-3,6,11,13H2,1H3,(H,28,29)(H,30,31)/b15-10+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for inhibition of [125I]- AII specific binding to rat mesenteric arteries, expressed as IC50 |
Bioorg Med Chem Lett 4: 23-28 (1994)
Article DOI: 10.1016/S0960-894X(01)81116-1
BindingDB Entry DOI: 10.7270/Q29G5MRF |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50030727
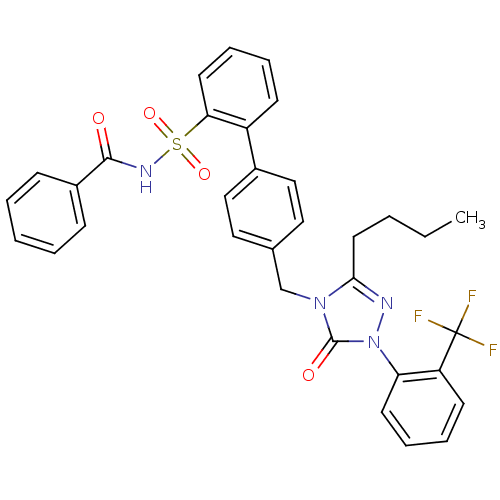
(4''-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,...)Show SMILES CCCCc1nn(-c2ccccc2C(F)(F)F)c(=O)n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)c1ccccc1 Show InChI InChI=1S/C33H29F3N4O4S/c1-2-3-17-30-37-40(28-15-9-8-14-27(28)33(34,35)36)32(42)39(30)22-23-18-20-24(21-19-23)26-13-7-10-16-29(26)45(43,44)38-31(41)25-11-5-4-6-12-25/h4-16,18-21H,2-3,17,22H2,1H3,(H,38,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of the compound towards Angiotensin II receptor, type 1 to displace 125I[Sar,Ile] from rat brain tissue preparation |
Bioorg Med Chem Lett 4: 115-120 (1994)
Article DOI: 10.1016/S0960-894X(01)81132-X
BindingDB Entry DOI: 10.7270/Q2057FT4 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50030727

(4''-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,...)Show SMILES CCCCc1nn(-c2ccccc2C(F)(F)F)c(=O)n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)c1ccccc1 Show InChI InChI=1S/C33H29F3N4O4S/c1-2-3-17-30-37-40(28-15-9-8-14-27(28)33(34,35)36)32(42)39(30)22-23-18-20-24(21-19-23)26-13-7-10-16-29(26)45(43,44)38-31(41)25-11-5-4-6-12-25/h4-16,18-21H,2-3,17,22H2,1H3,(H,38,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of the compound towards Angiotensin II receptor, type 1 to displace 125I[Sar,Ile] from rat adrenal tissue preparation |
Bioorg Med Chem Lett 4: 115-120 (1994)
Article DOI: 10.1016/S0960-894X(01)81132-X
BindingDB Entry DOI: 10.7270/Q2057FT4 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50009718
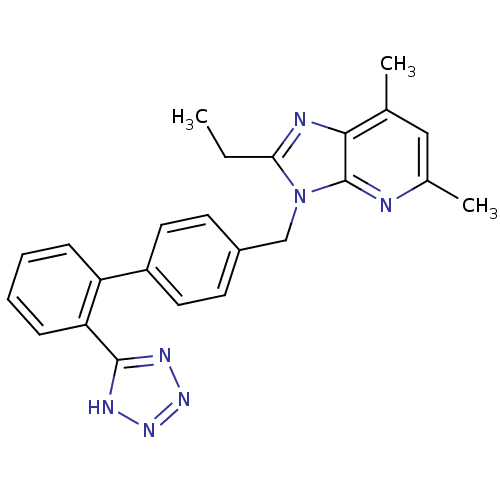
(2-Ethyl-5,7-dimethyl-3-[2'-(1H-tetrazol-5-yl)-biph...)Show SMILES CCc1nc2c(C)cc(C)nc2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H23N7/c1-4-21-26-22-15(2)13-16(3)25-24(22)31(21)14-17-9-11-18(12-10-17)19-7-5-6-8-20(19)23-27-29-30-28-23/h5-13H,4,14H2,1-3H3,(H,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Siena
Curated by ChEMBL
| Assay Description
Binding affinity for rat angiotensin II receptor, type 1 |
J Med Chem 47: 2574-86 (2004)
Article DOI: 10.1021/jm031100t
BindingDB Entry DOI: 10.7270/Q25X28DX |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50287294
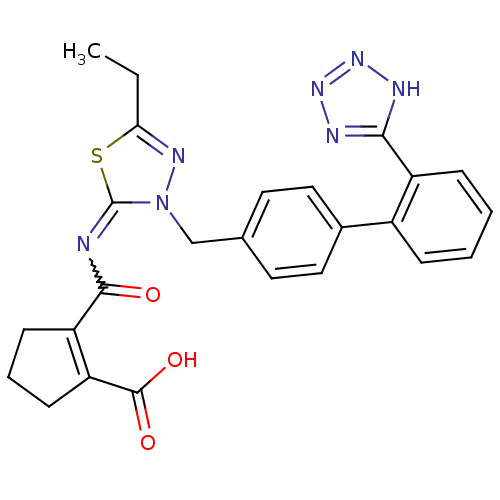
(2-[5-Ethyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylm...)Show SMILES CCc1nn(Cc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c(=NC(=O)C2=C(CCC2)C(O)=O)s1 |w:24.27,t:30| Show InChI InChI=1S/C25H23N7O3S/c1-2-21-29-32(25(36-21)26-23(33)19-8-5-9-20(19)24(34)35)14-15-10-12-16(13-11-15)17-6-3-4-7-18(17)22-27-30-31-28-22/h3-4,6-7,10-13H,2,5,8-9,14H2,1H3,(H,34,35)(H,27,28,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for the ability to displace the specific binding of [125I]-A II from receptors in rat liver membrane(type 1 receptor) |
Bioorg Med Chem Lett 6: 1469-1474 (1996)
Article DOI: 10.1016/S0960-894X(96)00250-8
BindingDB Entry DOI: 10.7270/Q2SN08XQ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50287290
(CHEMBL34866 | KRH-594 | Potassium; 2-[5-ethyl-3-[2...)Show SMILES CCc1nn(Cc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c(=NC(=O)C2=C(CCC2)C([O-])=O)s1 |w:24.27,t:30| Show InChI InChI=1S/C25H23N7O3S/c1-2-21-29-32(25(36-21)26-23(33)19-8-5-9-20(19)24(34)35)14-15-10-12-16(13-11-15)17-6-3-4-7-18(17)22-27-30-31-28-22/h3-4,6-7,10-13H,2,5,8-9,14H2,1H3,(H,34,35)(H,27,28,30,31)/p-1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for the ability to displace the specific binding of [125I]-A II from receptors in rat liver membrane(type 1 receptor) |
Bioorg Med Chem Lett 6: 1469-1474 (1996)
Article DOI: 10.1016/S0960-894X(96)00250-8
BindingDB Entry DOI: 10.7270/Q2SN08XQ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50285171
(2-{2,6-Dioxo-4-propyl-3-[2'-(1H-tetrazol-5-yl)-bip...)Show SMILES CCCc1cc(=O)n(CC(=O)N(C)C)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C25H27N7O3/c1-4-7-19-14-22(33)32(16-23(34)30(2)3)25(35)31(19)15-17-10-12-18(13-11-17)20-8-5-6-9-21(20)24-26-28-29-27-24/h5-6,8-14H,4,7,15-16H2,1-3H3,(H,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for binding affinity against angiotensin II receptor in rat adrenal cortex |
Bioorg Med Chem Lett 5: 2071-2076 (1995)
Article DOI: 10.1016/0960-894X(95)00370-9
BindingDB Entry DOI: 10.7270/Q2SF2W4J |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50287291

(CHEMBL35381 | N-[5-Ethyl-3-[2'-(1H-tetrazol-5-yl)-...)Show SMILES CCc1nn(Cc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c(=NC(=O)c2ccccc2C(O)=O)s1 |w:24.27| Show InChI InChI=1S/C26H21N7O3S/c1-2-22-30-33(26(37-22)27-24(34)20-9-5-6-10-21(20)25(35)36)15-16-11-13-17(14-12-16)18-7-3-4-8-19(18)23-28-31-32-29-23/h3-14H,2,15H2,1H3,(H,35,36)(H,28,29,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for the ability to displace the specific binding of [125I]-A II from receptors in rat liver membrane(type 1 receptor) |
Bioorg Med Chem Lett 6: 1469-1474 (1996)
Article DOI: 10.1016/S0960-894X(96)00250-8
BindingDB Entry DOI: 10.7270/Q2SN08XQ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50009712

(5,7-Dimethyl-2-propyl-3-[2'-(2H-tetrazol-5-yl)-bip...)Show SMILES CCCc1nc2c(C)cc(C)nc2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C25H25N7/c1-4-7-22-27-23-16(2)14-17(3)26-25(23)32(22)15-18-10-12-19(13-11-18)20-8-5-6-9-21(20)24-28-30-31-29-24/h5-6,8-14H,4,7,15H2,1-3H3,(H,28,29,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Siena
Curated by ChEMBL
| Assay Description
Binding affinity for rat angiotensin II receptor, type 1 |
J Med Chem 47: 2574-86 (2004)
Article DOI: 10.1021/jm031100t
BindingDB Entry DOI: 10.7270/Q25X28DX |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282260

(7-Butyl-8-[2'-(2H-tetrazol-5-yl)-biphenyl-4-ylmeth...)Show SMILES CCCCC1=NC2(CCCC2)CC(=O)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 |t:4| Show InChI InChI=1S/C26H30N6O/c1-2-3-10-23-27-26(15-6-7-16-26)17-24(33)32(23)18-19-11-13-20(14-12-19)21-8-4-5-9-22(21)25-28-30-31-29-25/h4-5,8-9,11-14H,2-3,6-7,10,15-18H2,1H3,(H,28,29,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of specific binding of [125I]-AII to Angiotensin II receptor, type 1 from rat liver membrane |
Bioorg Med Chem Lett 4: 157-162 (1994)
Article DOI: 10.1016/S0960-894X(01)81139-2
BindingDB Entry DOI: 10.7270/Q2QN66QV |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50011977
((E)-2-butyl-1-(p-carboxybenzyl)-alpha-2-thenylimid...)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H24N2O4S/c1-2-3-6-21-24-14-19(12-18(23(28)29)13-20-5-4-11-30-20)25(21)15-16-7-9-17(10-8-16)22(26)27/h4-5,7-12,14H,2-3,6,13,15H2,1H3,(H,26,27)(H,28,29)/b18-12+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for inhibition of [125I]- AII specific binding to rat mesenteric arteries, expressed as IC50 |
Bioorg Med Chem Lett 4: 23-28 (1994)
Article DOI: 10.1016/S0960-894X(01)81116-1
BindingDB Entry DOI: 10.7270/Q29G5MRF |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50048078

(4-[2-Butyl-5-((E)-2-carboxy-3-thiophen-2-yl-propen...)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(C(O)=O)c(Cl)c1 Show InChI InChI=1S/C23H23ClN2O4S/c1-2-3-6-21-25-13-17(11-16(22(27)28)12-18-5-4-9-31-18)26(21)14-15-7-8-19(23(29)30)20(24)10-15/h4-5,7-11,13H,2-3,6,12,14H2,1H3,(H,27,28)(H,29,30)/b16-11+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for inhibition of [125I]- AII specific binding to rat mesenteric arteries, expressed as IC50 |
Bioorg Med Chem Lett 4: 23-28 (1994)
Article DOI: 10.1016/S0960-894X(01)81116-1
BindingDB Entry DOI: 10.7270/Q29G5MRF |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50009719

(7-Methyl-2-propyl-3-[2'-(2H-tetrazol-5-yl)-bipheny...)Show SMILES CCCc1nc2c(C)ccnc2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H23N7/c1-3-6-21-26-22-16(2)13-14-25-24(22)31(21)15-17-9-11-18(12-10-17)19-7-4-5-8-20(19)23-27-29-30-28-23/h4-5,7-14H,3,6,15H2,1-2H3,(H,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Siena
Curated by ChEMBL
| Assay Description
Binding affinity for rat angiotensin II receptor, type 1 |
J Med Chem 47: 2574-86 (2004)
Article DOI: 10.1021/jm031100t
BindingDB Entry DOI: 10.7270/Q25X28DX |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50041701

(2-{2-Butyl-4-oxo-3-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCCc1nc2ccn(CC(N)=O)c(=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C26H26N8O2/c1-2-3-8-23-28-21-13-14-33(16-22(27)35)26(36)24(21)34(23)15-17-9-11-18(12-10-17)19-6-4-5-7-20(19)25-29-31-32-30-25/h4-7,9-14H,2-3,8,15-16H2,1H3,(H2,27,35)(H,29,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck
Curated by ChEMBL
| Assay Description
Binding affinity against AT1 receptor in the presence of 0.01% BSA |
J Med Chem 37: 1632-45 (1994)
BindingDB Entry DOI: 10.7270/Q2RR1X9M |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50042235

(2-butyl-3-{[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4...)Show SMILES CCCCC1=NC2(CCCC2)C(=O)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 |t:4| Show InChI InChI=1S/C25H28N6O/c1-2-3-10-22-26-25(15-6-7-16-25)24(32)31(22)17-18-11-13-19(14-12-18)20-8-4-5-9-21(20)23-27-29-30-28-23/h4-5,8-9,11-14H,2-3,6-7,10,15-17H2,1H3,(H,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of specific binding of [125I]-AII to Angiotensin II receptor, type 1 from rat liver membrane |
Bioorg Med Chem Lett 4: 157-162 (1994)
Article DOI: 10.1016/S0960-894X(01)81139-2
BindingDB Entry DOI: 10.7270/Q2QN66QV |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50042235

(2-butyl-3-{[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4...)Show SMILES CCCCC1=NC2(CCCC2)C(=O)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 |t:4| Show InChI InChI=1S/C25H28N6O/c1-2-3-10-22-26-25(15-6-7-16-25)24(32)31(22)17-18-11-13-19(14-12-18)20-8-4-5-9-21(20)23-27-29-30-28-23/h4-5,8-9,11-14H,2-3,6-7,10,15-17H2,1H3,(H,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 measured by ability to displace [125I]- A II from its specific binding site in rat liver membran... |
J Med Chem 36: 3371-80 (1993)
BindingDB Entry DOI: 10.7270/Q22Z14M0 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50041688

(CHEMBL43500 | {2-Butyl-4-oxo-3-[2'-(1H-tetrazol-5-...)Show SMILES CCCCc1nc2ccn(CC(=O)OC)c(=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C27H27N7O3/c1-3-4-9-23-28-22-14-15-33(17-24(35)37-2)27(36)25(22)34(23)16-18-10-12-19(13-11-18)20-7-5-6-8-21(20)26-29-31-32-30-26/h5-8,10-15H,3-4,9,16-17H2,1-2H3,(H,29,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck
Curated by ChEMBL
| Assay Description
Binding affinity against AT1 receptor of rat adrenal cortical membranes |
J Med Chem 37: 1632-45 (1994)
BindingDB Entry DOI: 10.7270/Q2RR1X9M |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50285164
(1-Cyclohexyl-3-(2-{2,6-dioxo-4-propyl-3-[2'-(1H-te...)Show SMILES CCCc1cc(=O)n(CCNC(=O)NC2CCCCC2)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C30H36N8O3/c1-2-8-24-19-27(39)37(18-17-31-29(40)32-23-9-4-3-5-10-23)30(41)38(24)20-21-13-15-22(16-14-21)25-11-6-7-12-26(25)28-33-35-36-34-28/h6-7,11-16,19,23H,2-5,8-10,17-18,20H2,1H3,(H2,31,32,40)(H,33,34,35,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for binding affinity against angiotensin II receptor in rat adrenal cortex |
Bioorg Med Chem Lett 5: 2071-2076 (1995)
Article DOI: 10.1016/0960-894X(95)00370-9
BindingDB Entry DOI: 10.7270/Q2SF2W4J |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50406795
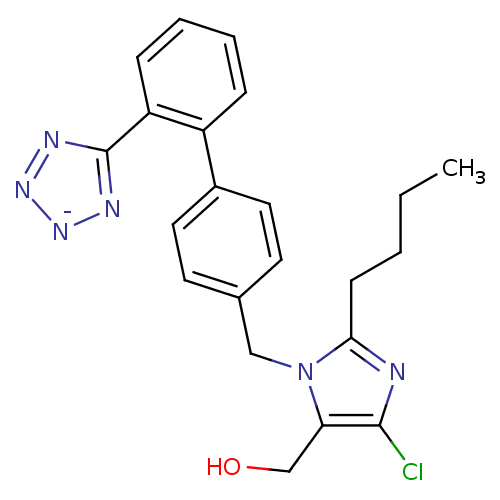
(Cozaar | LOSARTAN POTASSIUM)Show SMILES CCCCc1nc(Cl)c(CO)n1Cc1ccc(cc1)-c1ccccc1-c1nn[n-]n1 Show InChI InChI=1S/C22H22ClN6O/c1-2-3-8-20-24-21(23)19(14-30)29(20)13-15-9-11-16(12-10-15)17-6-4-5-7-18(17)22-25-27-28-26-22/h4-7,9-12,30H,2-3,8,13-14H2,1H3/q-1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck
Curated by ChEMBL
| Assay Description
Binding affinity against AT1 receptor in the presence of 0.01% BSA |
J Med Chem 37: 1632-45 (1994)
BindingDB Entry DOI: 10.7270/Q2RR1X9M |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50011975

(4-[2-Butyl-5-((E)-2-carboxy-3-thiophen-2-yl-propen...)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1Cl)C(O)=O Show InChI InChI=1S/C23H23ClN2O4S/c1-2-3-6-21-25-13-18(10-17(23(29)30)11-19-5-4-9-31-19)26(21)14-16-8-7-15(22(27)28)12-20(16)24/h4-5,7-10,12-13H,2-3,6,11,14H2,1H3,(H,27,28)(H,29,30)/b17-10+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for inhibition of [125I]- AII specific binding to rat mesenteric arteries, expressed as IC50 |
Bioorg Med Chem Lett 4: 23-28 (1994)
Article DOI: 10.1016/S0960-894X(01)81116-1
BindingDB Entry DOI: 10.7270/Q29G5MRF |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50041689

(5-Benzyl-2-butyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCCc1nc2ccn(Cc3ccccc3)c(=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C31H29N7O/c1-2-3-13-28-32-27-18-19-37(20-22-9-5-4-6-10-22)31(39)29(27)38(28)21-23-14-16-24(17-15-23)25-11-7-8-12-26(25)30-33-35-36-34-30/h4-12,14-19H,2-3,13,20-21H2,1H3,(H,33,34,35,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck
Curated by ChEMBL
| Assay Description
Binding affinity against AT1 receptor of rat adrenal cortical membranes |
J Med Chem 37: 1632-45 (1994)
BindingDB Entry DOI: 10.7270/Q2RR1X9M |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50049186
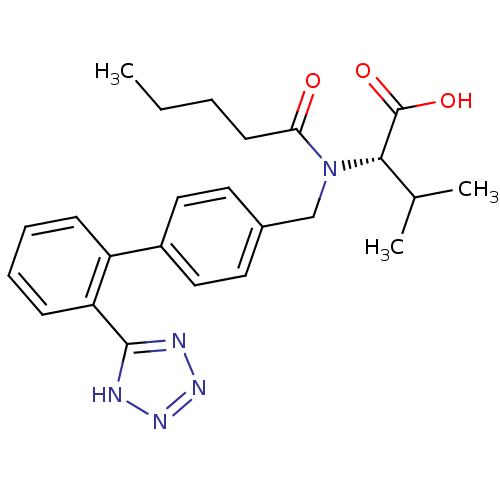
((S)-3-Methyl-2-{pentanoyl-[2'-(1H-tetrazol-5-yl)-b...)Show SMILES CCCCC(=O)N(Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)[C@@H](C(C)C)C(O)=O Show InChI InChI=1S/C24H29N5O3/c1-4-5-10-21(30)29(22(16(2)3)24(31)32)15-17-11-13-18(14-12-17)19-8-6-7-9-20(19)23-25-27-28-26-23/h6-9,11-14,16,22H,4-5,10,15H2,1-3H3,(H,31,32)(H,25,26,27,28)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Siena
Curated by ChEMBL
| Assay Description
Binding affinity for rat angiotensin II receptor, type 1 |
J Med Chem 47: 2574-86 (2004)
Article DOI: 10.1021/jm031100t
BindingDB Entry DOI: 10.7270/Q25X28DX |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282261
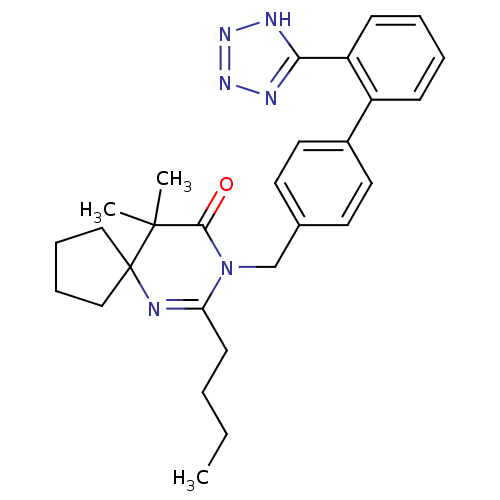
(7-Butyl-10,10-dimethyl-8-[2'-(2H-tetrazol-5-yl)-bi...)Show SMILES CCCCC1=NC2(CCCC2)C(C)(C)C(=O)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 |t:4| Show InChI InChI=1S/C28H34N6O/c1-4-5-12-24-29-28(17-8-9-18-28)27(2,3)26(35)34(24)19-20-13-15-21(16-14-20)22-10-6-7-11-23(22)25-30-32-33-31-25/h6-7,10-11,13-16H,4-5,8-9,12,17-19H2,1-3H3,(H,30,31,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of specific binding of [125I]-AII to Angiotensin II receptor, type 1 from rat liver membrane |
Bioorg Med Chem Lett 4: 157-162 (1994)
Article DOI: 10.1016/S0960-894X(01)81139-2
BindingDB Entry DOI: 10.7270/Q2QN66QV |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50285165
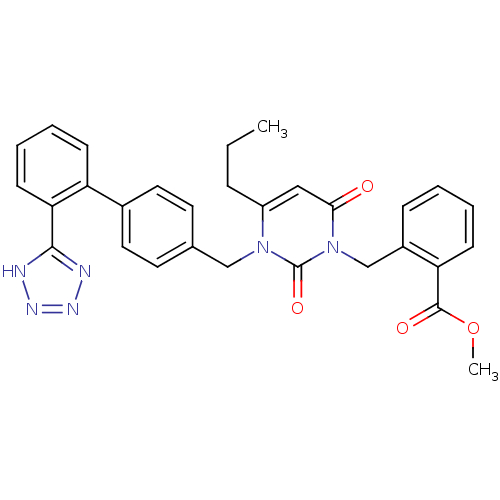
(2-{2,6-Dioxo-4-propyl-3-[2'-(1H-tetrazol-5-yl)-bip...)Show SMILES CCCc1cc(=O)n(Cc2ccccc2C(=O)OC)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C30H28N6O4/c1-3-8-23-17-27(37)36(19-22-9-4-5-11-25(22)29(38)40-2)30(39)35(23)18-20-13-15-21(16-14-20)24-10-6-7-12-26(24)28-31-33-34-32-28/h4-7,9-17H,3,8,18-19H2,1-2H3,(H,31,32,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for binding affinity against angiotensin II receptor in rat adrenal cortex |
Bioorg Med Chem Lett 5: 2071-2076 (1995)
Article DOI: 10.1016/0960-894X(95)00370-9
BindingDB Entry DOI: 10.7270/Q2SF2W4J |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50009338
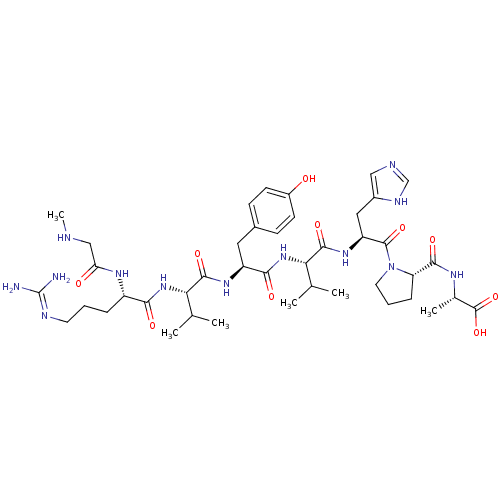
((S)-2-((S)-1-((S)-2-((S)-2-((S)-2-((S)-2-((S)-5-(d...)Show SMILES CNCC(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(O)=O |r,wU:60.63,wD:24.23,36.36,43.43,56.59,17.16,6.5,(6.84,-31.4,;5.3,-31.4,;4.53,-30.07,;2.99,-30.07,;2.22,-31.4,;2.22,-28.73,;2.99,-27.4,;4.53,-27.4,;5.3,-26.07,;4.53,-24.73,;5.3,-23.4,;6.84,-23.4,;7.61,-24.73,;7.61,-22.07,;2.22,-26.07,;2.99,-24.73,;.68,-26.07,;-.09,-24.73,;.68,-23.4,;2.22,-23.4,;-.09,-22.07,;-1.63,-24.73,;-2.4,-23.4,;-2.4,-26.07,;-3.94,-26.07,;-4.71,-24.73,;-6.25,-24.73,;-7.02,-23.4,;-8.56,-23.4,;-9.33,-24.73,;-10.87,-24.73,;-8.56,-26.07,;-7.02,-26.07,;-4.71,-27.4,;-6.25,-27.4,;-3.94,-28.73,;-4.71,-30.07,;-6.25,-30.07,;-7.02,-28.73,;-7.02,-31.4,;-3.94,-31.4,;-2.4,-31.4,;-4.71,-32.73,;-3.94,-34.07,;-4.71,-35.4,;-6.25,-35.4,;-7.15,-34.16,;-8.62,-34.63,;-8.62,-36.17,;-7.15,-36.65,;-2.4,-34.07,;-1.63,-32.73,;-1.63,-35.4,;-2.26,-36.81,;-1.12,-37.84,;.22,-37.07,;-.1,-35.56,;.93,-34.42,;.45,-32.95,;2.42,-34.82,;3.5,-33.73,;3.11,-32.24,;5,-34.13,;6.09,-33.04,;5.39,-35.61,)| Show InChI InChI=1S/C42H65N13O10/c1-22(2)33(53-35(58)28(50-32(57)20-45-6)9-7-15-47-42(43)44)38(61)51-29(17-25-11-13-27(56)14-12-25)36(59)54-34(23(3)4)39(62)52-30(18-26-19-46-21-48-26)40(63)55-16-8-10-31(55)37(60)49-24(5)41(64)65/h11-14,19,21-24,28-31,33-34,45,56H,7-10,15-18,20H2,1-6H3,(H,46,48)(H,49,60)(H,50,57)(H,51,61)(H,52,62)(H,53,58)(H,54,59)(H,64,65)(H4,43,44,47)/t24-,28-,29-,30-,31-,33-,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 measured by ability to displace [125I]- A II from its specific binding site in rat liver membran... |
J Med Chem 36: 3371-80 (1993)
BindingDB Entry DOI: 10.7270/Q22Z14M0 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50285163

(2-{4-Butyl-2,6-dioxo-3-[2'-(1H-tetrazol-5-yl)-biph...)Show SMILES CCCCc1cc(=O)n(Cc2ccccc2C(=O)OC)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C31H30N6O4/c1-3-4-10-24-18-28(38)37(20-23-9-5-6-12-26(23)30(39)41-2)31(40)36(24)19-21-14-16-22(17-15-21)25-11-7-8-13-27(25)29-32-34-35-33-29/h5-9,11-18H,3-4,10,19-20H2,1-2H3,(H,32,33,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for binding affinity against angiotensin II receptor in rat adrenal cortex |
Bioorg Med Chem Lett 5: 2071-2076 (1995)
Article DOI: 10.1016/0960-894X(95)00370-9
BindingDB Entry DOI: 10.7270/Q2SF2W4J |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50043244
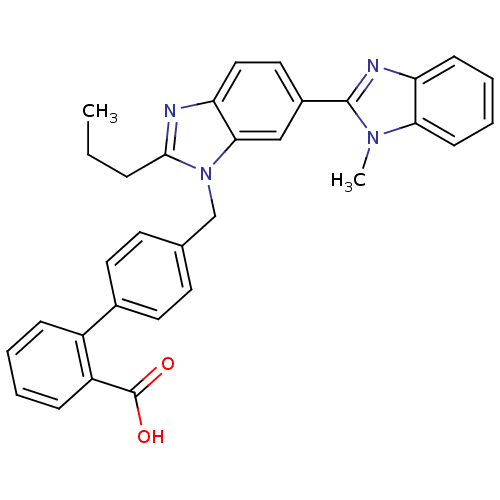
(4'-(1-Methyl-2'-propyl-1H-[2,5']bibenzoimidazolyl-...)Show SMILES CCCc1nc2ccc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)-c1nc2ccccc2n1C Show InChI InChI=1S/C32H28N4O2/c1-3-8-30-33-27-18-17-23(31-34-26-11-6-7-12-28(26)35(31)2)19-29(27)36(30)20-21-13-15-22(16-14-21)24-9-4-5-10-25(24)32(37)38/h4-7,9-19H,3,8,20H2,1-2H3,(H,37,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [125I]-angiotensin-II to angiotensin 1 receptor in rat lung membrane preparation |
J Med Chem 36: 4040-51 (1994)
BindingDB Entry DOI: 10.7270/Q2V98746 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50043280

(4'-((1,4'-dimethyl-2'-propyl(2,6'-bi-1H-benzimidaz...)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)-c1nc2ccccc2n1C Show InChI InChI=1S/C33H30N4O2/c1-4-9-30-35-31-21(2)18-24(32-34-27-12-7-8-13-28(27)36(32)3)19-29(31)37(30)20-22-14-16-23(17-15-22)25-10-5-6-11-26(25)33(38)39/h5-8,10-19H,4,9,20H2,1-3H3,(H,38,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [125I]-angiotensin-II to angiotensin 1 receptor in rat lung membrane preparation |
J Med Chem 36: 4040-51 (1994)
BindingDB Entry DOI: 10.7270/Q2V98746 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50043260

(2-Butyl-6-(1,1-dioxo-1lambda*6*-[1,2]thiazinan-2-y...)Show SMILES CCCCc1nc2ccc(cc2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)N1CCCCS1(=O)=O Show InChI InChI=1S/C29H31N7O2S/c1-2-3-10-28-30-26-16-15-23(36-17-6-7-18-39(36,37)38)19-27(26)35(28)20-21-11-13-22(14-12-21)24-8-4-5-9-25(24)29-31-33-34-32-29/h4-5,8-9,11-16,19H,2-3,6-7,10,17-18,20H2,1H3,(H,31,32,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [125I]-angiotensin-II to angiotensin 1 receptor in rat lung membrane preparation |
J Med Chem 36: 4040-51 (1994)
BindingDB Entry DOI: 10.7270/Q2V98746 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
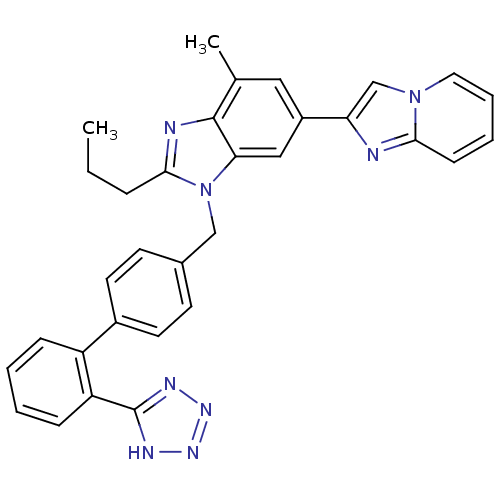
(RAT) | BDBM50043257
(6-Imidazo[1,2-a]pyridin-2-yl-4-methyl-2-propyl-1-[...)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)-c1cn2ccccc2n1 Show InChI InChI=1S/C32H28N8/c1-3-8-30-34-31-21(2)17-24(27-20-39-16-7-6-11-29(39)33-27)18-28(31)40(30)19-22-12-14-23(15-13-22)25-9-4-5-10-26(25)32-35-37-38-36-32/h4-7,9-18,20H,3,8,19H2,1-2H3,(H,35,36,37,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [125I]-angiotensin-II to angiotensin 1 receptor in rat lung membrane preparation |
J Med Chem 36: 4040-51 (1994)
BindingDB Entry DOI: 10.7270/Q2V98746 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
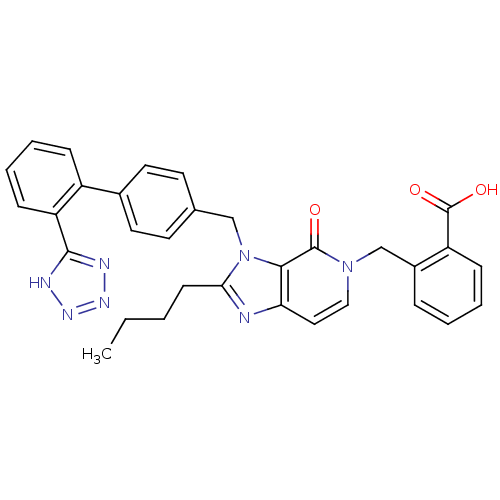
(RAT) | BDBM50041683
(2-{2-Butyl-4-oxo-3-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCCc1nc2ccn(Cc3ccccc3C(O)=O)c(=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C32H29N7O3/c1-2-3-12-28-33-27-17-18-38(20-23-8-4-5-10-25(23)32(41)42)31(40)29(27)39(28)19-21-13-15-22(16-14-21)24-9-6-7-11-26(24)30-34-36-37-35-30/h4-11,13-18H,2-3,12,19-20H2,1H3,(H,41,42)(H,34,35,36,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck
Curated by ChEMBL
| Assay Description
Binding affinity against AT1 receptor in the presence of 0.01% BSA |
J Med Chem 37: 1632-45 (1994)
BindingDB Entry DOI: 10.7270/Q2RR1X9M |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044356
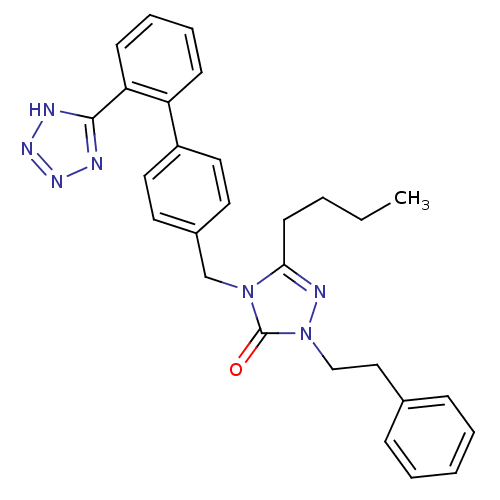
(5-Butyl-2-phenethyl-4-[2'-(1H-tetrazol-5-yl)-biphe...)Show SMILES CCCCc1nn(CCc2ccccc2)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C28H29N7O/c1-2-3-13-26-31-35(19-18-21-9-5-4-6-10-21)28(36)34(26)20-22-14-16-23(17-15-22)24-11-7-8-12-25(24)27-29-32-33-30-27/h4-12,14-17H,2-3,13,18-20H2,1H3,(H,29,30,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50285166
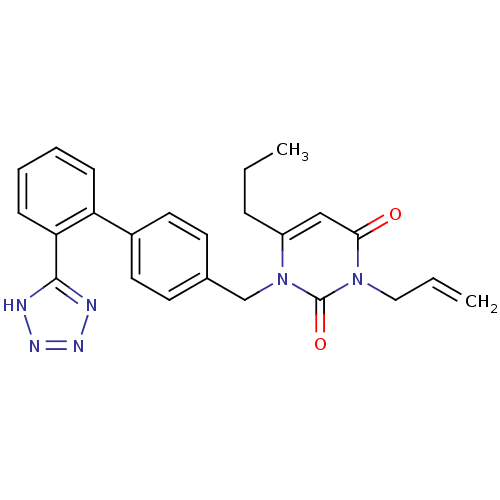
(3-Allyl-6-propyl-1-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCc1cc(=O)n(CC=C)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H24N6O2/c1-3-7-19-15-22(31)29(14-4-2)24(32)30(19)16-17-10-12-18(13-11-17)20-8-5-6-9-21(20)23-25-27-28-26-23/h4-6,8-13,15H,2-3,7,14,16H2,1H3,(H,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for binding affinity against angiotensin II receptor in rat adrenal cortex |
Bioorg Med Chem Lett 5: 2071-2076 (1995)
Article DOI: 10.1016/0960-894X(95)00370-9
BindingDB Entry DOI: 10.7270/Q2SF2W4J |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282270

(4'-(2-Butyl-4-cyclopentyl-4-methyl-5-oxo-4,5-dihyd...)Show SMILES CCCCC1=NC(C)(C2CCCC2)C(=O)N1Cc1ccc(cc1)-c1ccccc1C(O)=O |t:4| Show InChI InChI=1S/C27H32N2O3/c1-3-4-13-24-28-27(2,21-9-5-6-10-21)26(32)29(24)18-19-14-16-20(17-15-19)22-11-7-8-12-23(22)25(30)31/h7-8,11-12,14-17,21H,3-6,9-10,13,18H2,1-2H3,(H,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity at Angiotensin II receptor, type 1 from rat liver membrane by [125I]- A II displacement. |
Bioorg Med Chem Lett 4: 163-168 (1994)
Article DOI: 10.1016/S0960-894X(01)81140-9
BindingDB Entry DOI: 10.7270/Q2M045CQ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50285160
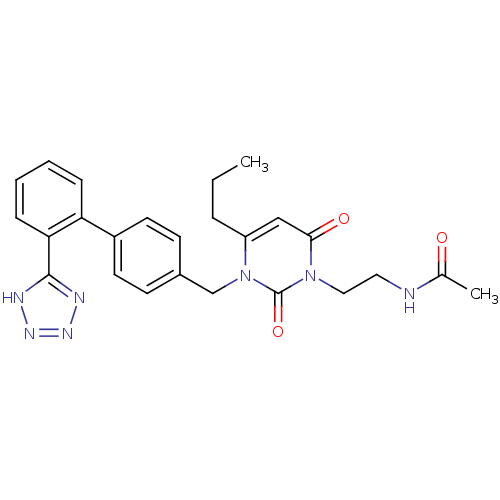
(CHEMBL292781 | N-(2-{2,6-Dioxo-4-propyl-3-[2'-(1H-...)Show SMILES CCCc1cc(=O)n(CCNC(C)=O)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C25H27N7O3/c1-3-6-20-15-23(34)31(14-13-26-17(2)33)25(35)32(20)16-18-9-11-19(12-10-18)21-7-4-5-8-22(21)24-27-29-30-28-24/h4-5,7-12,15H,3,6,13-14,16H2,1-2H3,(H,26,33)(H,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for binding affinity against angiotensin II receptor in rat adrenal cortex |
Bioorg Med Chem Lett 5: 2071-2076 (1995)
Article DOI: 10.1016/0960-894X(95)00370-9
BindingDB Entry DOI: 10.7270/Q2SF2W4J |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044345

(5-Butyl-2-(2-hydroxy-2-phenyl-ethyl)-4-[2'-(1H-tet...)Show SMILES CCCCc1nn(CC(O)c2ccccc2)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C28H29N7O2/c1-2-3-13-26-31-35(19-25(36)22-9-5-4-6-10-22)28(37)34(26)18-20-14-16-21(17-15-20)23-11-7-8-12-24(23)27-29-32-33-30-27/h4-12,14-17,25,36H,2-3,13,18-19H2,1H3,(H,29,30,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50287289

(2-Chloro-N-[5-ethyl-3-[2'-(1H-tetrazol-5-yl)-biphe...)Show SMILES CCc1nn(Cc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c(=NC(=O)c2ccccc2Cl)s1 |w:24.27| Show InChI InChI=1S/C25H20ClN7OS/c1-2-22-30-33(25(35-22)27-24(34)20-9-5-6-10-21(20)26)15-16-11-13-17(14-12-16)18-7-3-4-8-19(18)23-28-31-32-29-23/h3-14H,2,15H2,1H3,(H,28,29,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for the ability to displace the specific binding of [125I]-A II from receptors in rat liver membrane(type 1 receptor) |
Bioorg Med Chem Lett 6: 1469-1474 (1996)
Article DOI: 10.1016/S0960-894X(96)00250-8
BindingDB Entry DOI: 10.7270/Q2SN08XQ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50043277

(1-{2-Butyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylm...)Show SMILES CCCCc1nc2ccc(cc2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)N1CCCCC1=O Show InChI InChI=1S/C30H31N7O/c1-2-3-10-28-31-26-17-16-23(36-18-7-6-11-29(36)38)19-27(26)37(28)20-21-12-14-22(15-13-21)24-8-4-5-9-25(24)30-32-34-35-33-30/h4-5,8-9,12-17,19H,2-3,6-7,10-11,18,20H2,1H3,(H,32,33,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [125I]-angiotensin-II to angiotensin 1 receptor in rat lung membrane preparation |
J Med Chem 36: 4040-51 (1994)
BindingDB Entry DOI: 10.7270/Q2V98746 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50145961

(7-Methyl-2-propyl-3-{4-[3-(2H-tetrazol-5-yl)-pyrid...)Show SMILES CCCc1nc2c(C)ccnc2n1Cc1ccc(cc1)-c1ccncc1-c1nnn[nH]1 Show InChI InChI=1S/C23H22N8/c1-3-4-20-26-21-15(2)9-12-25-23(21)31(20)14-16-5-7-17(8-6-16)18-10-11-24-13-19(18)22-27-29-30-28-22/h5-13H,3-4,14H2,1-2H3,(H,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Siena
Curated by ChEMBL
| Assay Description
Binding affinity for rat angiotensin II receptor, type 1 |
J Med Chem 47: 2574-86 (2004)
Article DOI: 10.1021/jm031100t
BindingDB Entry DOI: 10.7270/Q25X28DX |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50043246

(4'-(6-Imidazo[1,2-a]pyridin-2-yl-4-methyl-2-propyl...)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)-c1cn2ccccc2n1 Show InChI InChI=1S/C32H28N4O2/c1-3-8-30-34-31-21(2)17-24(27-20-35-16-7-6-11-29(35)33-27)18-28(31)36(30)19-22-12-14-23(15-13-22)25-9-4-5-10-26(25)32(37)38/h4-7,9-18,20H,3,8,19H2,1-2H3,(H,37,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [125I]-angiotensin-II to angiotensin 1 receptor in rat lung membrane preparation |
J Med Chem 36: 4040-51 (1994)
BindingDB Entry DOI: 10.7270/Q2V98746 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50145972

(2-Amino-4-[4-(5,7-dimethyl-2-propyl-imidazo[4,5-b]...)Show SMILES CCCc1nc2c(C)cc(C)nc2n1Cc1ccc(cc1)-c1c(C(O)=O)c(N)nc2ccccc12 Show InChI InChI=1S/C28H27N5O2/c1-4-7-22-32-25-16(2)14-17(3)30-27(25)33(22)15-18-10-12-19(13-11-18)23-20-8-5-6-9-21(20)31-26(29)24(23)28(34)35/h5-6,8-14H,4,7,15H2,1-3H3,(H2,29,31)(H,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Siena
Curated by ChEMBL
| Assay Description
Binding affinity for rat angiotensin II receptor, type 1 |
J Med Chem 47: 2574-86 (2004)
Article DOI: 10.1021/jm031100t
BindingDB Entry DOI: 10.7270/Q25X28DX |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50285177

(6-Propyl-1-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmet...)Show SMILES CCCc1cc(=O)[nH]c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C21H20N6O2/c1-2-5-16-12-19(28)22-21(29)27(16)13-14-8-10-15(11-9-14)17-6-3-4-7-18(17)20-23-25-26-24-20/h3-4,6-12H,2,5,13H2,1H3,(H,22,28,29)(H,23,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for binding affinity against angiotensin II receptor in rat adrenal cortex |
Bioorg Med Chem Lett 5: 2071-2076 (1995)
Article DOI: 10.1016/0960-894X(95)00370-9
BindingDB Entry DOI: 10.7270/Q2SF2W4J |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044334
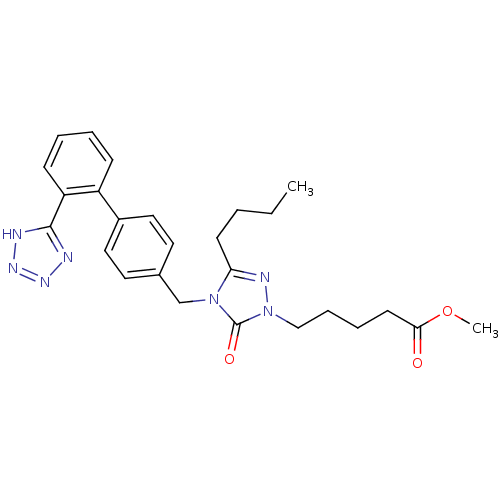
(5-{3-Butyl-5-oxo-4-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCCc1nn(CCCCC(=O)OC)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C26H31N7O3/c1-3-4-11-23-29-33(17-8-7-12-24(34)36-2)26(35)32(23)18-19-13-15-20(16-14-19)21-9-5-6-10-22(21)25-27-30-31-28-25/h5-6,9-10,13-16H,3-4,7-8,11-12,17-18H2,1-2H3,(H,27,28,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50285167
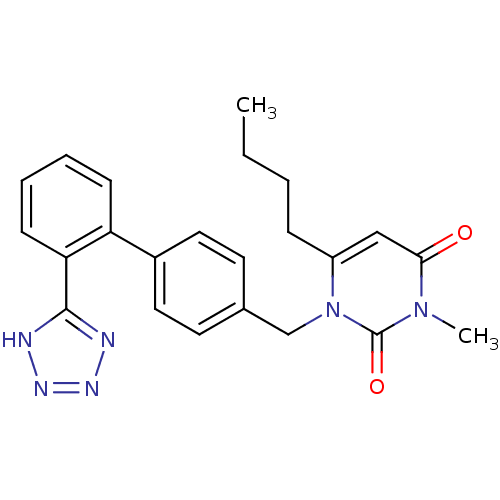
(6-Butyl-3-methyl-1-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCCc1cc(=O)n(C)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C23H24N6O2/c1-3-4-7-18-14-21(30)28(2)23(31)29(18)15-16-10-12-17(13-11-16)19-8-5-6-9-20(19)22-24-26-27-25-22/h5-6,8-14H,3-4,7,15H2,1-2H3,(H,24,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for binding affinity against angiotensin II receptor in rat adrenal cortex |
Bioorg Med Chem Lett 5: 2071-2076 (1995)
Article DOI: 10.1016/0960-894X(95)00370-9
BindingDB Entry DOI: 10.7270/Q2SF2W4J |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044355

(2,5-Dibutyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-yl...)Show SMILES CCCCc1nn(CCCC)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H29N7O/c1-3-5-11-22-27-31(16-6-4-2)24(32)30(22)17-18-12-14-19(15-13-18)20-9-7-8-10-21(20)23-25-28-29-26-23/h7-10,12-15H,3-6,11,16-17H2,1-2H3,(H,25,26,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125-I]-labeled angiotensin II from Angiotensin II receptor, type 1 of rat uterine membranes |
Bioorg Med Chem Lett 4: 2591-2596 (1994)
Article DOI: 10.1016/S0960-894X(01)80290-0
BindingDB Entry DOI: 10.7270/Q29Z94VB |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50009714
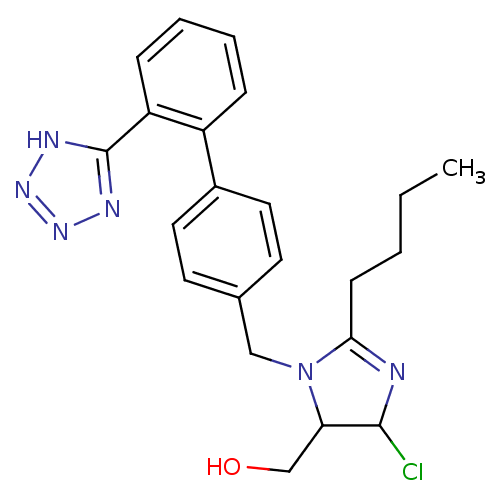
(CHEMBL191 | {2-Butyl-5-chloro-3-[2'-(2H-tetrazol-5...)Show SMILES CCCCC1=NC(Cl)C(CO)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 |t:4| Show InChI InChI=1S/C22H25ClN6O/c1-2-3-8-20-24-21(23)19(14-30)29(20)13-15-9-11-16(12-10-15)17-6-4-5-7-18(17)22-25-27-28-26-22/h4-7,9-12,19,21,30H,2-3,8,13-14H2,1H3,(H,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Binding affinity against angiotensin II receptor type 1 (AT1) from rat liver. |
J Med Chem 36: 1735-45 (1993)
BindingDB Entry DOI: 10.7270/Q28W3CCP |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50043279

(1-Methyl-2'-propyl-3'-[2'-(1H-tetrazol-5-yl)-biphe...)Show SMILES CCCc1nc2ccc(cc2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)-c1nc2ccccc2n1C Show InChI InChI=1S/C32H28N8/c1-3-8-30-33-27-18-17-23(32-34-26-11-6-7-12-28(26)39(32)2)19-29(27)40(30)20-21-13-15-22(16-14-21)24-9-4-5-10-25(24)31-35-37-38-36-31/h4-7,9-19H,3,8,20H2,1-2H3,(H,35,36,37,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [125I]-angiotensin-II to angiotensin 1 receptor in rat lung membrane preparation |
J Med Chem 36: 4040-51 (1994)
BindingDB Entry DOI: 10.7270/Q2V98746 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50043247

(4-Methyl-2-propyl-6-pyridin-2-yl-1-[2'-(1H-tetrazo...)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)-c1ccccn1 Show InChI InChI=1S/C30H27N7/c1-3-8-28-32-29-20(2)17-23(26-11-6-7-16-31-26)18-27(29)37(28)19-21-12-14-22(15-13-21)24-9-4-5-10-25(24)30-33-35-36-34-30/h4-7,9-18H,3,8,19H2,1-2H3,(H,33,34,35,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [125I]-angiotensin-II to angiotensin 1 receptor in rat lung membrane preparation |
J Med Chem 36: 4040-51 (1994)
BindingDB Entry DOI: 10.7270/Q2V98746 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044355

(2,5-Dibutyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-yl...)Show SMILES CCCCc1nn(CCCC)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H29N7O/c1-3-5-11-22-27-31(16-6-4-2)24(32)30(22)17-18-12-14-19(15-13-18)20-9-7-8-10-21(20)23-25-28-29-26-23/h7-10,12-15H,3-6,11,16-17H2,1-2H3,(H,25,26,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282264
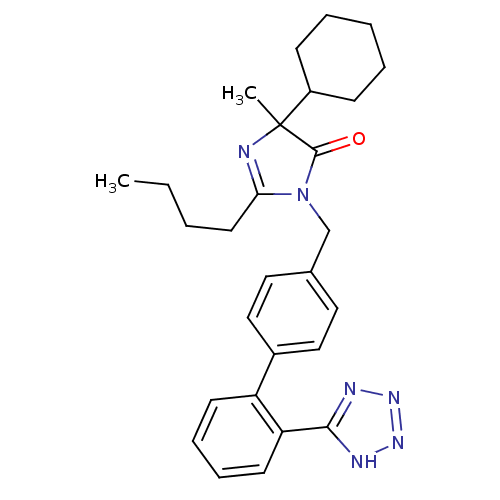
(2-Butyl-5-cyclohexyl-5-methyl-3-[2'-(1H-tetrazol-5...)Show SMILES CCCCC1=NC(C)(C2CCCCC2)C(=O)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 |t:4| Show InChI InChI=1S/C28H34N6O/c1-3-4-14-25-29-28(2,22-10-6-5-7-11-22)27(35)34(25)19-20-15-17-21(18-16-20)23-12-8-9-13-24(23)26-30-32-33-31-26/h8-9,12-13,15-18,22H,3-7,10-11,14,19H2,1-2H3,(H,30,31,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity at Angiotensin II receptor, type 1 from rat liver membrane by [125I]- A II displacement. |
Bioorg Med Chem Lett 4: 163-168 (1994)
Article DOI: 10.1016/S0960-894X(01)81140-9
BindingDB Entry DOI: 10.7270/Q2M045CQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data