 Found 170 hits of ki for UniProtKB: P56481
Found 170 hits of ki for UniProtKB: P56481 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50062005
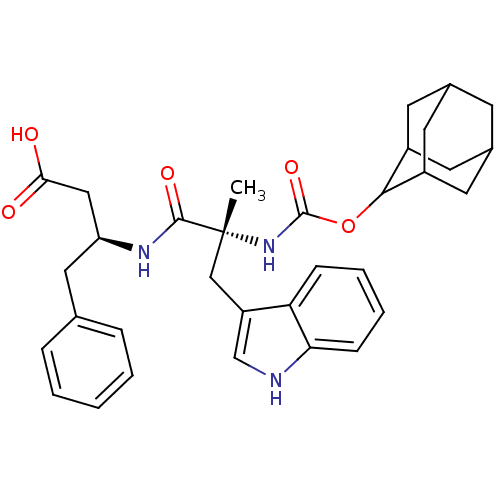
((S)-3-[(R)-2-(Adamantan-2-yloxycarbonylamino)-3-(1...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N[C@H](CC(O)=O)Cc1ccccc1 |wU:1.13,wD:29.33,1.0,TLB:15:16:18:21.22.20,THB:23:24:18:21.22.20,23:21:18:16.24.25,20:21:16:19.18.25,20:19:16:21.23.22,(10.8,-2.25,;10.81,-3.63,;10.9,-5.18,;12.26,-5.88,;11.02,-6.79,;11.49,-8.25,;13.03,-8.24,;14.06,-9.39,;15.56,-9.07,;16.04,-7.61,;15.01,-6.46,;13.52,-6.79,;9.43,-3.12,;8.15,-3.96,;8.17,-5.43,;6.76,-3.28,;5.47,-4.12,;5.46,-5.67,;4.44,-6.95,;3.04,-6.37,;1.53,-6.79,;2.74,-5.52,;4.05,-6.01,;2.72,-4.03,;4.08,-3.55,;3.02,-4.78,;12.1,-2.95,;12.07,-1.57,;13.48,-3.63,;14.76,-2.78,;14.67,-1.25,;15.43,.09,;14.65,1.43,;16.98,.11,;16.14,-3.47,;17.43,-2.62,;17.34,-1.25,;18.6,-.23,;20,-.92,;20.1,-2.46,;18.81,-3.31,)| Show InChI InChI=1S/C33H39N3O5/c1-33(18-25-19-34-28-10-6-5-9-27(25)28,31(39)35-26(17-29(37)38)16-20-7-3-2-4-8-20)36-32(40)41-30-23-12-21-11-22(14-23)15-24(30)13-21/h2-10,19,21-24,26,30,34H,11-18H2,1H3,(H,35,39)(H,36,40)(H,37,38)/t21?,22?,23?,24?,26-,30?,33+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Cholecystokinin type B receptor binding assay performed on homogenized cerebral cortex from male mouse |
J Med Chem 35: 1572-7 (1992)
BindingDB Entry DOI: 10.7270/Q26H4KM8 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50281737
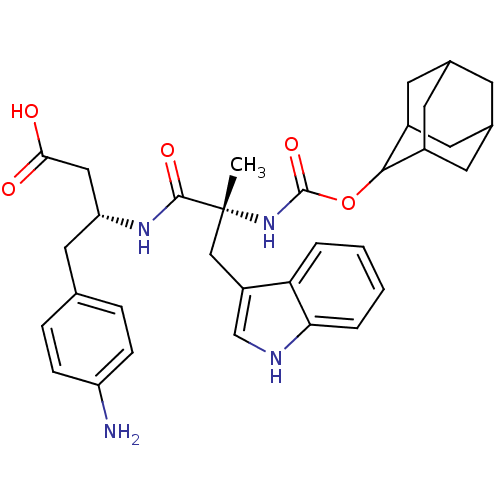
((R)-3-[(R)-2-(Adamantan-2-yloxycarbonylamino)-3-(1...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N[C@@H](CC(O)=O)Cc1ccc(N)cc1 |wU:1.0,wD:1.13,29.33,TLB:22:17:25:21.23.20,22:21:16.17.18:25,15:16:21.23.22:19.18.25,THB:20:21:16:19.18.25,20:19:16:21.23.22,15:16:25:21.23.20,(3.41,-9.92,;4.74,-10.71,;6.07,-9.95,;6.09,-8.41,;5.19,-7.17,;6.1,-5.93,;7.57,-6.4,;8.89,-5.63,;10.23,-6.4,;10.23,-7.94,;8.89,-8.71,;7.56,-7.94,;3.39,-11.46,;2.06,-10.69,;2.08,-9.15,;.73,-11.44,;-.81,-11.44,;-.62,-13,;-2,-13.53,;-3.42,-13.21,;-4.47,-14.66,;-3,-14.03,;-1.49,-14.43,;-3.21,-12.42,;-2.3,-11.04,;-3.61,-11.69,;6.07,-11.48,;6.05,-13.02,;7.4,-10.71,;8.73,-11.48,;8.73,-13.02,;10.06,-13.79,;10.06,-15.33,;8.73,-14.54,;10.06,-10.71,;11.39,-11.48,;11.39,-13.02,;12.72,-13.79,;14.05,-13.02,;15.38,-13.79,;14.05,-11.46,;12.72,-10.71,)| Show InChI InChI=1S/C33H40N4O5/c1-33(17-24-18-35-28-5-3-2-4-27(24)28,31(40)36-26(16-29(38)39)15-19-6-8-25(34)9-7-19)37-32(41)42-30-22-11-20-10-21(13-22)14-23(30)12-20/h2-9,18,20-23,26,30,35H,10-17,34H2,1H3,(H,36,40)(H,37,41)(H,38,39)/t20?,21?,22?,23?,26-,30?,33-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was evaluated for the inhibition of binding of [3H]-PD 140376 to Cholecystokinin type B receptor in mouse cortex. |
Bioorg Med Chem Lett 3: 885-888 (1993)
Article DOI: 10.1016/S0960-894X(00)80686-1
BindingDB Entry DOI: 10.7270/Q2D79BWZ |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50230677

(CHEMBL3351022)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NC[C@H](NC(=O)\C=C\C(O)=O)c1ccccc1 |wD:1.1,30.43,1.0,TLB:18:19:16.17.22:23,15:16:23:19.25.20,THB:20:21:16:19.18.25,20:19:16:21.22.23,18:17:23:19.25.20,(13.63,-4.94,;12.13,-5.3,;10.64,-5.67,;9.57,-4.55,;9.85,-3.04,;8.49,-2.31,;7.38,-3.37,;5.84,-3.25,;4.98,-4.53,;5.64,-5.91,;7.18,-6.03,;8.05,-4.76,;11.77,-3.81,;12.88,-2.74,;14.36,-3.18,;12.52,-1.25,;13.63,-.18,;14.47,1.29,;14.47,2.69,;15.85,3.38,;17.22,2.69,;17.22,1.29,;15.85,.59,;16.38,-.18,;15.01,.51,;15.01,1.91,;12.5,-6.8,;13.97,-7.23,;11.38,-7.86,;11.75,-9.36,;10.63,-10.42,;10.99,-11.92,;9.88,-12.98,;8.4,-12.55,;10.24,-14.48,;9.13,-15.54,;9.49,-17.04,;10.97,-17.47,;8.38,-18.1,;9.15,-9.99,;8.04,-11.05,;6.56,-10.62,;6.2,-9.12,;7.31,-8.06,;8.79,-8.49,)| Show InChI InChI=1S/C35H40N4O6/c1-35(18-26-19-36-28-10-6-5-9-27(26)28,39-34(44)45-32-24-14-21-13-22(16-24)17-25(32)15-21)33(43)37-20-29(23-7-3-2-4-8-23)38-30(40)11-12-31(41)42/h2-12,19,21-22,24-25,29,32,36H,13-18,20H2,1H3,(H,37,43)(H,38,40)(H,39,44)(H,41,42)/b12-11+/t21?,22?,24?,25?,29-,32?,35+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.513 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Cholecystokinin type B receptor binding assay performed on homogenized cerebral cortex from male mouse |
J Med Chem 35: 1572-7 (1992)
BindingDB Entry DOI: 10.7270/Q26H4KM8 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50285623
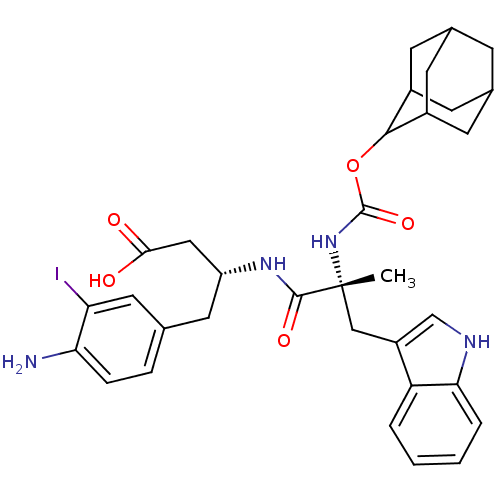
((R)-3-[(R)-2-(Adamantan-2-yloxycarbonylamino)-3-(1...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N[C@@H](CC(O)=O)Cc1ccc(N)c(I)c1 |wU:1.0,wD:29.33,1.13,TLB:22:21:25:18.17.16,22:17:20.21.23:25,THB:16:17:20:23.24.25,16:24:20:18.22.17,15:16:20.21.23:25,(8.02,-5.46,;9.37,-6.2,;10.7,-5.42,;10.68,-3.88,;11.96,-4.76,;13.17,-3.83,;12.66,-2.38,;13.41,-1.04,;12.62,.28,;11.08,.25,;10.33,-1.09,;11.13,-2.4,;9.4,-7.74,;8.09,-8.54,;8.12,-10.08,;6.74,-7.8,;5.46,-8.64,;5.45,-10.17,;4.05,-10.52,;2.72,-10.03,;1.53,-11.3,;3.03,-10.88,;4.43,-11.45,;3.02,-9.29,;4.06,-8.06,;2.72,-8.54,;10.47,-7.28,;12.01,-7.27,;10.51,-8.82,;11.86,-9.56,;11.89,-11.1,;10.57,-11.9,;10.61,-13.44,;9.22,-11.17,;13.17,-8.76,;14.52,-9.5,;14.55,-11.04,;15.9,-11.78,;17.22,-10.99,;18.57,-11.73,;17.18,-9.44,;18.5,-8.64,;15.83,-8.7,)| Show InChI InChI=1S/C33H39IN4O5/c1-33(16-23-17-36-28-5-3-2-4-25(23)28,38-32(42)43-30-21-9-19-8-20(11-21)12-22(30)10-19)31(41)37-24(15-29(39)40)13-18-6-7-27(35)26(34)14-18/h2-7,14,17,19-22,24,30,36H,8-13,15-16,35H2,1H3,(H,37,41)(H,38,42)(H,39,40)/t19?,20?,21?,22?,24-,30?,33-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity towards CCK-B receptor in mouse cerebral cortex membrane using [125I]bolton assay |
Bioorg Med Chem Lett 5: 2501-2506 (1995)
Article DOI: 10.1016/0960-894X(95)00435-V
BindingDB Entry DOI: 10.7270/Q2DZ08SM |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50230683

(CHEMBL3351024)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N[C@H](CNC(=O)\C=C\C(O)=O)Cc1ccccc1 |wU:29.43,wD:1.1,1.0,TLB:22:17:25:21.23.20,22:21:16.17.18:25,THB:15:16:25:21.23.20,20:21:16:19.18.25,20:19:16:21.23.22,(12.45,-4.16,;10.95,-4.52,;9.45,-4.88,;8.39,-3.77,;8.67,-2.25,;7.31,-1.52,;6.2,-2.59,;4.66,-2.47,;3.79,-3.74,;4.46,-5.13,;6,-5.25,;6.86,-3.97,;10.59,-3.02,;11.7,-1.96,;13.18,-2.39,;11.34,-.46,;12.45,.6,;13.29,2.07,;14.66,1.38,;16.04,2.07,;16.04,3.47,;14.66,4.17,;13.29,3.47,;13.82,2.69,;13.82,1.3,;15.2,.6,;11.31,-6.02,;12.79,-6.45,;10.2,-7.08,;10.56,-8.58,;9.45,-9.64,;9.81,-11.14,;8.7,-12.2,;7.22,-11.76,;9.06,-13.69,;7.95,-14.76,;8.31,-16.25,;9.79,-16.69,;7.2,-17.32,;12.04,-9.01,;12.4,-10.51,;13.88,-10.94,;14.25,-12.44,;13.13,-13.5,;11.65,-13.06,;11.29,-11.57,)| Show InChI InChI=1S/C36H42N4O6/c1-36(19-27-20-37-30-10-6-5-9-29(27)30,40-35(45)46-33-25-14-23-13-24(16-25)17-26(33)15-23)34(44)39-28(18-22-7-3-2-4-8-22)21-38-31(41)11-12-32(42)43/h2-12,20,23-26,28,33,37H,13-19,21H2,1H3,(H,38,41)(H,39,44)(H,40,45)(H,42,43)/b12-11+/t23?,24?,25?,26?,28-,33?,36+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.708 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Cholecystokinin type B receptor binding assay performed on homogenized cerebral cortex from male mouse |
J Med Chem 35: 1572-7 (1992)
BindingDB Entry DOI: 10.7270/Q26H4KM8 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50471066
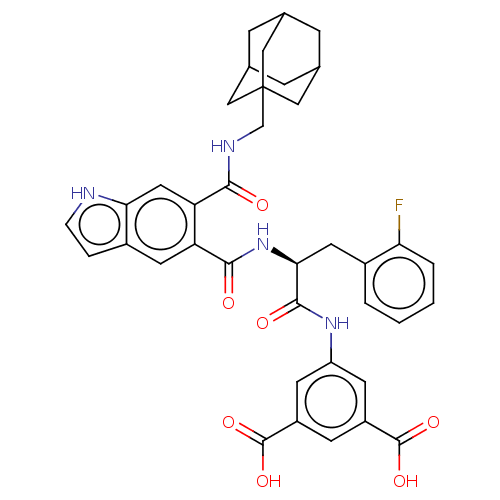
(CHEMBL415936)Show SMILES OC(=O)c1cc(NC(=O)[C@H](Cc2ccccc2F)NC(=O)c2cc3cc[nH]c3cc2C(=O)NCC23CC4CC(CC(C4)C2)C3)cc(c1)C(O)=O |TLB:41:36:43:40.42.39,41:40:43:36.35.37,THB:39:38:35:40.42.41,39:40:35:38.43.37| Show InChI InChI=1S/C38H37FN4O7/c39-30-4-2-1-3-23(30)14-32(35(46)42-27-11-25(36(47)48)10-26(12-27)37(49)50)43-34(45)28-13-24-5-6-40-31(24)15-29(28)33(44)41-19-38-16-20-7-21(17-38)9-22(8-20)18-38/h1-6,10-13,15,20-22,32,40H,7-9,14,16-19H2,(H,41,44)(H,42,46)(H,43,45)(H,47,48)(H,49,50)/t20?,21?,22?,32-,38?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.741 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Cholecystokinin type B receptor using [125I]BH-CCK-8S as radioligand in mouse cortical membranes |
J Med Chem 39: 1806-15 (1996)
Article DOI: 10.1021/jm9508907
BindingDB Entry DOI: 10.7270/Q2571FR6 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50285625
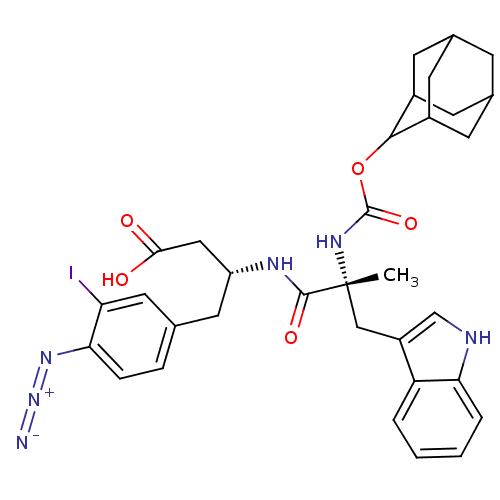
((R)-3-[(R)-2-(Adamantan-2-yloxycarbonylamino)-3-(1...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N[C@@H](CC(O)=O)Cc1ccc(N=[N+]=[N-])c(I)c1 |wU:1.0,wD:29.33,1.13,TLB:22:21:25:18.17.16,22:17:20.21.23:25,THB:16:17:20:23.24.25,16:24:20:18.22.17,15:16:20.21.23:25,(7.11,-5.46,;8.46,-6.2,;9.79,-5.42,;9.77,-3.88,;11.05,-4.76,;12.26,-3.83,;11.76,-2.38,;12.5,-1.04,;11.71,.28,;10.17,.25,;9.42,-1.09,;10.22,-2.4,;8.5,-7.74,;7.18,-8.54,;7.21,-10.08,;5.83,-7.8,;4.55,-8.64,;4.54,-10.17,;3.14,-10.52,;1.81,-10.03,;.62,-11.31,;2.12,-10.89,;3.52,-11.45,;2.11,-9.29,;3.15,-8.07,;1.81,-8.54,;9.57,-7.28,;11.1,-7.28,;9.6,-8.82,;10.95,-9.56,;10.98,-11.1,;9.67,-11.9,;9.7,-13.44,;8.31,-11.17,;12.27,-8.76,;13.62,-9.5,;13.64,-11.04,;14.99,-11.79,;16.31,-10.99,;17.66,-11.73,;18.98,-10.93,;20.3,-10.13,;16.28,-9.44,;17.59,-8.64,;14.93,-8.7,)| Show InChI InChI=1S/C33H37IN6O5/c1-33(16-23-17-36-27-5-3-2-4-25(23)27,38-32(44)45-30-21-9-19-8-20(11-21)12-22(30)10-19)31(43)37-24(15-29(41)42)13-18-6-7-28(39-40-35)26(34)14-18/h2-7,14,17,19-22,24,30,36H,8-13,15-16H2,1H3,(H,37,43)(H,38,44)(H,41,42)/t19?,20?,21?,22?,24-,30?,33-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity towards CCK-B receptor in mouse cerebral cortex membrane using [125I]bolton assay |
Bioorg Med Chem Lett 5: 2501-2506 (1995)
Article DOI: 10.1016/0960-894X(95)00435-V
BindingDB Entry DOI: 10.7270/Q2DZ08SM |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50213845
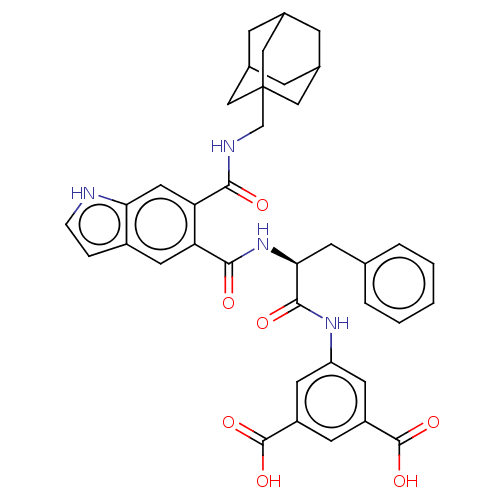
(CHEMBL14557)Show SMILES OC(=O)c1cc(NC(=O)[C@H](Cc2ccccc2)NC(=O)c2cc3cc[nH]c3cc2C(=O)NCC23CC4CC(CC(C4)C2)C3)cc(c1)C(O)=O |TLB:40:35:42:39.41.38,40:39:42:35.34.36,THB:38:37:34:39.41.40,38:39:34:37.42.36| Show InChI InChI=1S/C38H38N4O7/c43-33(40-20-38-17-22-8-23(18-38)10-24(9-22)19-38)30-16-31-25(6-7-39-31)15-29(30)34(44)42-32(11-21-4-2-1-3-5-21)35(45)41-28-13-26(36(46)47)12-27(14-28)37(48)49/h1-7,12-16,22-24,32,39H,8-11,17-20H2,(H,40,43)(H,41,45)(H,42,44)(H,46,47)(H,48,49)/t22?,23?,24?,32-,38?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Inhibition of 20 pM [125I]BH-CCK-8S binding to mouse cortical membrane Cholecystokinin 2 receptor |
J Med Chem 48: 6790-802 (2005)
Article DOI: 10.1021/jm049069y
BindingDB Entry DOI: 10.7270/Q2BC428F |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50230676
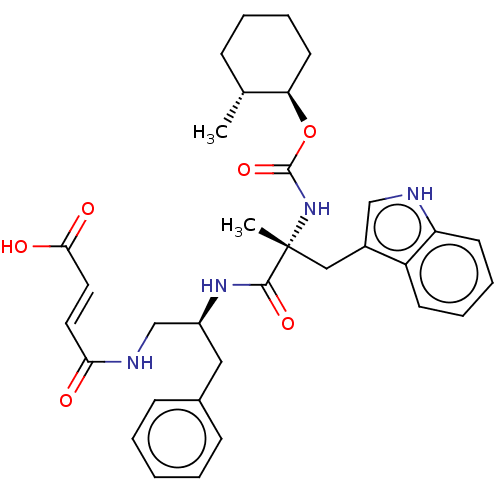
(CHEMBL3351013)Show SMILES C[C@@H]1CCCC[C@H]1OC(=O)N[C@](C)(Cc1c[nH]c2ccccc12)C(=O)N[C@H](CNC(=O)\C=C\C(O)=O)Cc1ccccc1 Show InChI InChI=1S/C33H40N4O6/c1-22-10-6-9-15-28(22)43-32(42)37-33(2,19-24-20-34-27-14-8-7-13-26(24)27)31(41)36-25(18-23-11-4-3-5-12-23)21-35-29(38)16-17-30(39)40/h3-5,7-8,11-14,16-17,20,22,25,28,34H,6,9-10,15,18-19,21H2,1-2H3,(H,35,38)(H,36,41)(H,37,42)(H,39,40)/b17-16+/t22-,25+,28-,33-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Cholecystokinin type B receptor binding assay performed on homogenized cerebral cortex from male mouse |
J Med Chem 35: 1572-7 (1992)
BindingDB Entry DOI: 10.7270/Q26H4KM8 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50213845

(CHEMBL14557)Show SMILES OC(=O)c1cc(NC(=O)[C@H](Cc2ccccc2)NC(=O)c2cc3cc[nH]c3cc2C(=O)NCC23CC4CC(CC(C4)C2)C3)cc(c1)C(O)=O |TLB:40:35:42:39.41.38,40:39:42:35.34.36,THB:38:37:34:39.41.40,38:39:34:37.42.36| Show InChI InChI=1S/C38H38N4O7/c43-33(40-20-38-17-22-8-23(18-38)10-24(9-22)19-38)30-16-31-25(6-7-39-31)15-29(30)34(44)42-32(11-21-4-2-1-3-5-21)35(45)41-28-13-26(36(46)47)12-27(14-28)37(48)49/h1-7,12-16,22-24,32,39H,8-11,17-20H2,(H,40,43)(H,41,45)(H,42,44)(H,46,47)(H,48,49)/t22?,23?,24?,32-,38?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Cholecystokinin type B receptor using [125I]BH-CCK-8S as radioligand in mouse cortical membranes |
J Med Chem 39: 1806-15 (1996)
Article DOI: 10.1021/jm9508907
BindingDB Entry DOI: 10.7270/Q2571FR6 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50213845

(CHEMBL14557)Show SMILES OC(=O)c1cc(NC(=O)[C@H](Cc2ccccc2)NC(=O)c2cc3cc[nH]c3cc2C(=O)NCC23CC4CC(CC(C4)C2)C3)cc(c1)C(O)=O |TLB:40:35:42:39.41.38,40:39:42:35.34.36,THB:38:37:34:39.41.40,38:39:34:37.42.36| Show InChI InChI=1S/C38H38N4O7/c43-33(40-20-38-17-22-8-23(18-38)10-24(9-22)19-38)30-16-31-25(6-7-39-31)15-29(30)34(44)42-32(11-21-4-2-1-3-5-21)35(45)41-28-13-26(36(46)47)12-27(14-28)37(48)49/h1-7,12-16,22-24,32,39H,8-11,17-20H2,(H,40,43)(H,41,45)(H,42,44)(H,46,47)(H,48,49)/t22?,23?,24?,32-,38?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Displacement of binding of [125I]CCK-8S to Cholecystokinin type B receptor in mouse cerebral cortex homogenates |
J Med Chem 43: 3518-29 (2000)
Article DOI: 10.1021/jm000960w
BindingDB Entry DOI: 10.7270/Q2KS6V9G |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50471075
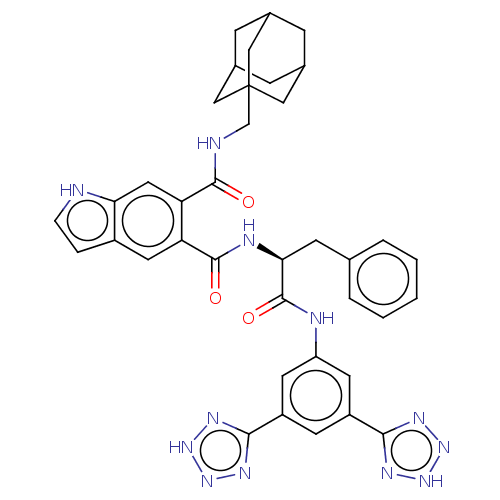
(CHEMBL299540)Show SMILES O=C(Nc1cc(cc(c1)-c1nn[nH]n1)-c1nn[nH]n1)[C@H](Cc1ccccc1)NC(=O)c1cc2cc[nH]c2cc1C(=O)NCC12CC3CC(CC(C3)C1)C2 |TLB:46:47:51:45.44.50,50:49:52:45.44.46,50:45:52:49.51.48,THB:46:45:51:47.52.48| Show InChI InChI=1S/C38H38N12O3/c51-35(40-20-38-17-22-8-23(18-38)10-24(9-22)19-38)30-16-31-25(6-7-39-31)15-29(30)36(52)42-32(11-21-4-2-1-3-5-21)37(53)41-28-13-26(33-43-47-48-44-33)12-27(14-28)34-45-49-50-46-34/h1-7,12-16,22-24,32,39H,8-11,17-20H2,(H,40,51)(H,41,53)(H,42,52)(H,43,44,47,48)(H,45,46,49,50)/t22?,23?,24?,32-,38?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Cholecystokinin type B receptor using [125I]BH-CCK-8S as radioligand in mouse cortical membranes |
J Med Chem 39: 1806-15 (1996)
Article DOI: 10.1021/jm9508907
BindingDB Entry DOI: 10.7270/Q2571FR6 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50471067
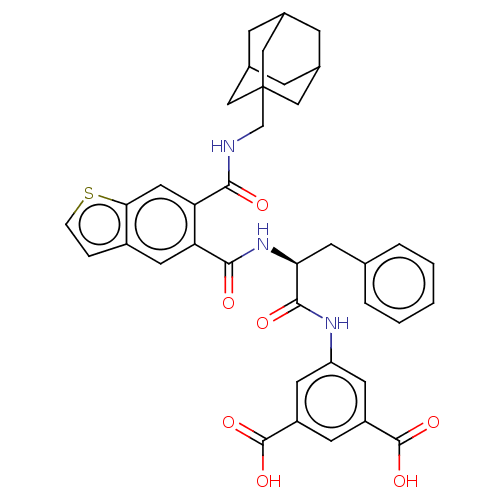
(CHEMBL298521)Show SMILES OC(=O)c1cc(NC(=O)[C@H](Cc2ccccc2)NC(=O)c2cc3ccsc3cc2C(=O)NCC23CC4CC(CC(C4)C2)C3)cc(c1)C(O)=O |TLB:36:37:41:35.34.40,THB:36:35:41:37.42.38,38:37:34:39.41.40,38:39:34:37.42.36| Show InChI InChI=1S/C38H37N3O7S/c42-33(39-20-38-17-22-8-23(18-38)10-24(9-22)19-38)30-16-32-25(6-7-49-32)15-29(30)34(43)41-31(11-21-4-2-1-3-5-21)35(44)40-28-13-26(36(45)46)12-27(14-28)37(47)48/h1-7,12-16,22-24,31H,8-11,17-20H2,(H,39,42)(H,40,44)(H,41,43)(H,45,46)(H,47,48)/t22?,23?,24?,31-,38?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Cholecystokinin type B receptor using [125I]BH-CCK-8S as radioligand in mouse cortical membranes |
J Med Chem 39: 1806-15 (1996)
Article DOI: 10.1021/jm9508907
BindingDB Entry DOI: 10.7270/Q2571FR6 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50230678
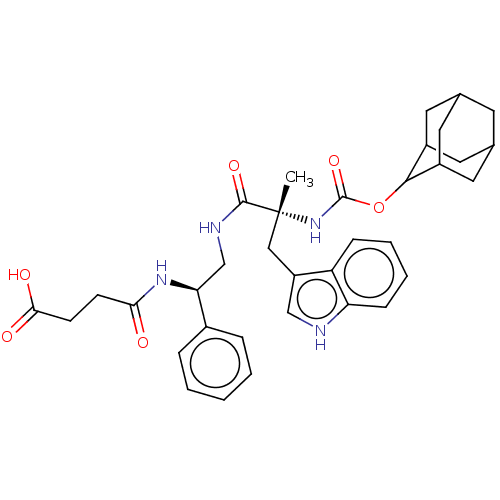
(CHEMBL287735)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NC[C@H](NC(=O)CCC(O)=O)c1ccccc1 |wU:1.1,30.35,wD:1.0,TLB:18:19:16.17.22:23,15:16:23:19.25.20,THB:18:17:23:19.25.20,20:19:16:21.22.23,20:21:16:19.18.25,(10.04,-3.61,;10.44,-2.12,;11.77,-2.89,;11.76,-4.44,;10.49,-5.31,;10.97,-6.79,;12.51,-6.79,;13.53,-7.95,;15.04,-7.63,;15.52,-6.17,;14.49,-5.02,;12.99,-5.35,;8.93,-1.81,;7.92,-2.97,;8.4,-4.44,;6.4,-2.68,;5.38,-3.84,;3.89,-3.61,;2.73,-4.41,;3.13,-5.83,;2.32,-7.36,;3.64,-6.59,;3.22,-5.06,;5.15,-6.76,;5.78,-5.3,;4.53,-5.98,;11.45,-.96,;10.97,.5,;12.96,-1.26,;13.99,-.11,;15.5,-.4,;16,-1.87,;17.52,-2.19,;18.01,-3.64,;18.52,-1.03,;20.03,-1.33,;21.06,-.17,;22.57,-.46,;20.57,1.31,;16.53,.74,;16.02,2.21,;17.04,3.36,;18.55,3.05,;19.06,1.6,;18.04,.44,)| Show InChI InChI=1S/C35H42N4O6/c1-35(18-26-19-36-28-10-6-5-9-27(26)28,39-34(44)45-32-24-14-21-13-22(16-24)17-25(32)15-21)33(43)37-20-29(23-7-3-2-4-8-23)38-30(40)11-12-31(41)42/h2-10,19,21-22,24-25,29,32,36H,11-18,20H2,1H3,(H,37,43)(H,38,40)(H,39,44)(H,41,42)/t21?,22?,24?,25?,29-,32?,35+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Cholecystokinin type B receptor binding assay performed on homogenized cerebral cortex from male mouse |
J Med Chem 35: 1572-7 (1992)
BindingDB Entry DOI: 10.7270/Q26H4KM8 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50470629
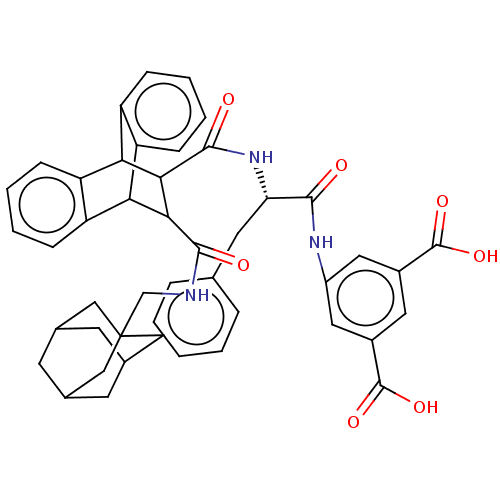
(CHEMBL342616)Show SMILES OC(=O)c1cc(NC(=O)[C@H](Cc2ccccc2)NC(=O)C2C(C3c4ccccc4C2c2ccccc32)C(=O)NCC23CC4CC(CC(C4)C2)C3)cc(c1)C(O)=O |wD:9.9,TLB:43:44:48:42.41.47,47:46:49:42.41.43,47:42:49:46.48.45,24:23:20.21:35.30,THB:18:20:35.30:28.23,43:42:48:44.49.45,34:35:20.21:28.23,27:28:20.21:35.30,31:30:20.21:28.23,(16.62,-7.02,;16.63,-5.48,;15.29,-4.71,;17.97,-4.72,;17.97,-3.18,;19.29,-2.43,;19.31,-.88,;20.64,-.12,;21.97,-.9,;20.64,1.42,;19.31,2.19,;17.98,2.97,;16.63,2.21,;15.31,2.97,;15.33,4.52,;16.65,5.3,;17.99,4.5,;21.97,2.19,;21.97,3.73,;20.64,4.51,;23.44,4.41,;24.65,3.71,;25.05,5.21,;27.84,6.65,;29.36,6.44,;30.3,7.66,;29.71,9.1,;28.18,9.3,;27.25,8.09,;23.52,5.95,;22.2,7.73,;21.67,9.18,;22.67,10.37,;24.18,10.1,;24.71,8.66,;23.73,7.47,;24.66,2.18,;25.98,2.96,;25.99,1.41,;27.32,2.18,;28.66,1.41,;30.24,1.7,;31.62,.9,;31.41,-.6,;29.78,-.84,;30.79,.2,;31.02,1.59,;32.73,1.99,;29.75,2.35,;28.46,-.09,;20.63,-3.19,;20.63,-4.73,;19.3,-5.51,;21.96,-5.51,;21.97,-7.04,;23.29,-4.74,)| Show InChI InChI=1S/C46H45N3O7/c50-41(48-31-19-29(44(53)54)18-30(20-31)45(55)56)36(17-25-8-2-1-3-9-25)49-43(52)40-38-34-12-6-4-10-32(34)37(33-11-5-7-13-35(33)38)39(40)42(51)47-24-46-21-26-14-27(22-46)16-28(15-26)23-46/h1-13,18-20,26-28,36-40H,14-17,21-24H2,(H,47,51)(H,48,50)(H,49,52)(H,53,54)(H,55,56)/t26?,27?,28?,36-,37?,38?,39?,40?,46?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Inhibition of 20 pM [125I]BH-CCK-8S binding to mouse cortical membrane Cholecystokinin 2 receptor |
J Med Chem 48: 6790-802 (2005)
Article DOI: 10.1021/jm049069y
BindingDB Entry DOI: 10.7270/Q2BC428F |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50471065
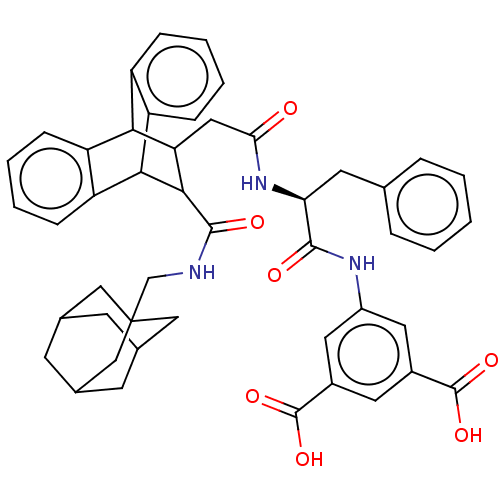
(CHEMBL296167)Show SMILES OC(=O)c1cc(NC(=O)[C@H](Cc2ccccc2)NC(=O)CC2C(C3c4ccccc4C2c2ccccc32)C(=O)NCC23CC4CC(CC(C4)C2)C3)cc(c1)C(O)=O |wD:9.9,TLB:44:45:49:43.42.48,37:22:29.24:36.31,28:29:22.21:36.31,35:36:22.21:29.24,25:24:22.21:36.31,THB:20:21:29.24:36.31,44:43:49:45.50.46,46:45:42:47.49.48,46:47:42:45.50.44,32:31:22.21:29.24,(8.01,-6.63,;9.37,-5.87,;10.71,-6.64,;9.37,-4.31,;8.05,-3.54,;8.05,-1.98,;6.95,-.88,;5.47,-.48,;5.23,1.05,;4.2,-1.35,;4.05,-2.9,;5.21,-3.96,;6.55,-3.18,;7.89,-3.96,;7.89,-5.51,;6.52,-6.29,;5.21,-5.51,;2.83,-.57,;1.51,-1.35,;1.51,-2.9,;1.18,-1.33,;-.3,-.93,;.88,-.85,;.17,-1.58,;-1.64,-1.11,;-1.36,.19,;-2.44,1.1,;-3.8,.71,;-4.09,-.59,;-3,-1.49,;-1.14,-1.86,;-.53,-3.49,;-.89,-4.71,;.13,-5.65,;1.47,-5.34,;1.8,-4.1,;.81,-3.2,;1.28,.65,;.76,2.13,;2.78,.35,;3.8,1.53,;5.02,2.46,;6.24,1.69,;7.4,2.63,;9.16,2.49,;7.89,3.4,;7.47,4.87,;5.82,4.86,;6.97,3.98,;4.6,3.92,;6.64,2.4,;9.39,-1.21,;10.74,-1.98,;10.74,-3.54,;12.08,-1.22,;12.1,.33,;13.42,-2.01,)| Show InChI InChI=1S/C47H47N3O7/c51-39(50-38(17-26-8-2-1-3-9-26)43(52)49-32-19-30(45(54)55)18-31(20-32)46(56)57)21-37-40-33-10-4-6-12-35(33)41(36-13-7-5-11-34(36)40)42(37)44(53)48-25-47-22-27-14-28(23-47)16-29(15-27)24-47/h1-13,18-20,27-29,37-38,40-42H,14-17,21-25H2,(H,48,53)(H,49,52)(H,50,51)(H,54,55)(H,56,57)/t27?,28?,29?,37?,38-,40?,41?,42?,47?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Cholecystokinin type B receptor using [125I]BH-CCK-8S as radioligand in mouse cortical membranes |
J Med Chem 39: 1806-15 (1996)
Article DOI: 10.1021/jm9508907
BindingDB Entry DOI: 10.7270/Q2571FR6 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50061220
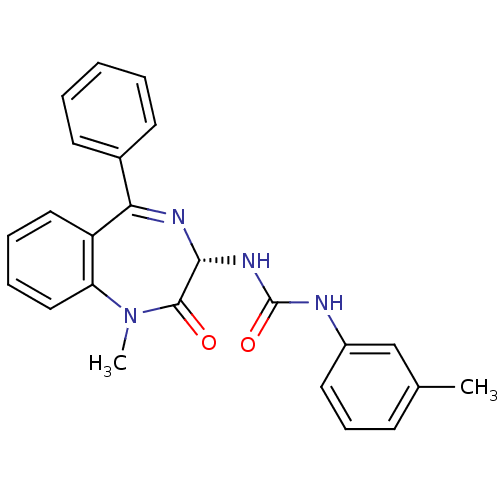
(1-((S)-1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...)Show SMILES CN1c2ccccc2C(=N[C@H](NC(=O)Nc2cccc(C)c2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C24H22N4O2/c1-16-9-8-12-18(15-16)25-24(30)27-22-23(29)28(2)20-14-7-6-13-19(20)21(26-22)17-10-4-3-5-11-17/h3-15,22H,1-2H3,(H2,25,27,30)/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 162: 273-80 (1989)
Article DOI: 10.1016/0014-2999(89)90290-2
BindingDB Entry DOI: 10.7270/Q2QF8RB7 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50230680
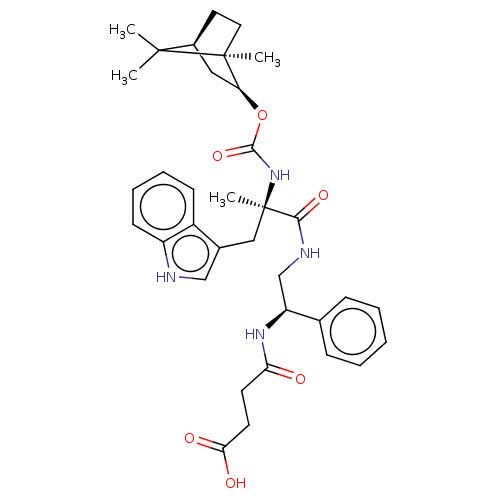
(CHEMBL3350364)Show SMILES [H][C@]12CC[C@@](C)([C@H](C1)OC(=O)N[C@](C)(Cc1c[nH]c3ccccc13)C(=O)NC[C@H](NC(=O)CCC(O)=O)c1ccccc1)C2(C)C |TLB:8:6:3.2:43| Show InChI InChI=1S/C35H44N4O6/c1-33(2)24-16-17-34(33,3)28(18-24)45-32(44)39-35(4,19-23-20-36-26-13-9-8-12-25(23)26)31(43)37-21-27(22-10-6-5-7-11-22)38-29(40)14-15-30(41)42/h5-13,20,24,27-28,36H,14-19,21H2,1-4H3,(H,37,43)(H,38,40)(H,39,44)(H,41,42)/t24-,27-,28-,34-,35+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Cholecystokinin type B receptor binding assay performed on homogenized cerebral cortex from male mouse |
J Med Chem 35: 1572-7 (1992)
BindingDB Entry DOI: 10.7270/Q26H4KM8 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50230681
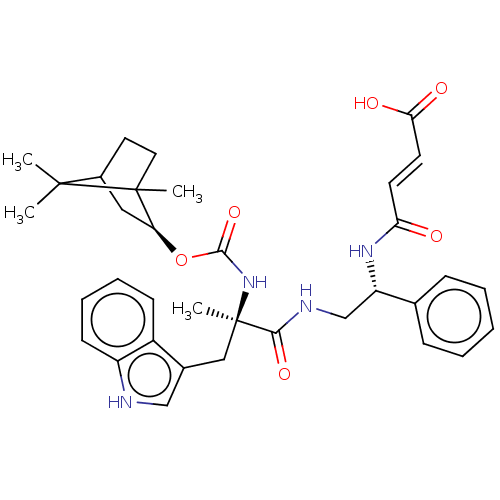
(CHEMBL3351023)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)O[C@H]1CC2CCC1(C)C2(C)C)C(=O)NC[C@H](NC(=O)\C=C\C(O)=O)c1ccccc1 |THB:15:16:23:20.19| Show InChI InChI=1S/C35H42N4O6/c1-33(2)24-16-17-34(33,3)28(18-24)45-32(44)39-35(4,19-23-20-36-26-13-9-8-12-25(23)26)31(43)37-21-27(22-10-6-5-7-11-22)38-29(40)14-15-30(41)42/h5-15,20,24,27-28,36H,16-19,21H2,1-4H3,(H,37,43)(H,38,40)(H,39,44)(H,41,42)/b15-14+/t24?,27-,28-,34?,35+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Cholecystokinin type B receptor binding assay performed on homogenized cerebral cortex from male mouse |
J Med Chem 35: 1572-7 (1992)
BindingDB Entry DOI: 10.7270/Q26H4KM8 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50470612
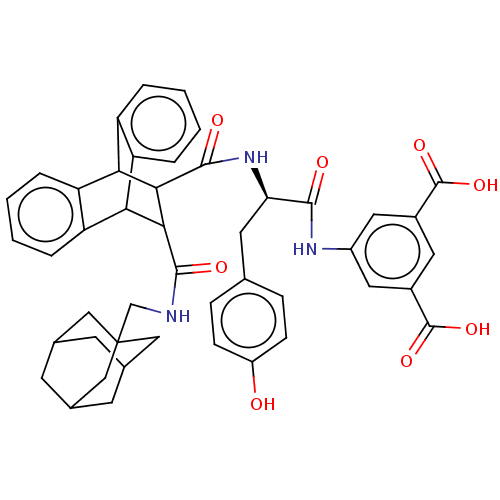
(CHEMBL436209)Show SMILES OC(=O)c1cc(NC(=O)[C@@H](Cc2ccc(O)cc2)NC(=O)C2C(C3c4ccccc4C2c2ccccc32)C(=O)NCC23CC4CC(CC(C4)C2)C3)cc(c1)C(O)=O |wU:9.9,TLB:19:21:31.36:24.29,44:45:49:43.42.48,48:43:50:47.49.46,48:47:50:43.42.44,37:22:31.36:24.29,25:24:21.22:31.36,35:36:21.22:24.29,THB:44:43:49:45.50.46,28:29:21.22:31.36,(19.63,-7.77,;18.28,-7,;18.28,-5.44,;16.94,-7.79,;15.59,-7.01,;14.26,-7.77,;12.94,-7,;11.38,-7.01,;10.98,-5.49,;10.28,-8.1,;10.69,-9.6,;11.77,-10.69,;13.25,-10.3,;14.33,-11.38,;13.94,-12.87,;15.04,-13.97,;12.45,-13.26,;11.37,-12.18,;8.79,-7.71,;7.29,-7.3,;7.75,-5.77,;2.67,-8.1,;3.67,-7.07,;3.67,-8.95,;5.65,-9.56,;7.45,-9.56,;8.72,-10.82,;8.07,-12.08,;6.09,-12.08,;4.93,-10.77,;2.69,-10.25,;.79,-10.83,;-.77,-11.41,;-2.3,-10.93,;-1.97,-9.88,;-.32,-9.21,;1.37,-9.5,;4.32,-5.93,;3.37,-5.02,;5.45,-5.26,;5.42,-3.95,;6.55,-3.28,;7.75,-3.57,;8.89,-3.15,;9.91,-4.25,;8.63,-3.89,;8.63,-2.53,;7.75,-1.49,;8.91,-1.89,;6.55,-1.98,;7.43,-4.37,;14.26,-9.33,;15.59,-10.11,;16.94,-9.34,;16.36,-11.44,;15.65,-13,;17.91,-11.45,)| Show InChI InChI=1S/C46H45N3O8/c50-31-11-9-24(10-12-31)16-36(41(51)48-30-18-28(44(54)55)17-29(19-30)45(56)57)49-43(53)40-38-34-7-3-1-5-32(34)37(33-6-2-4-8-35(33)38)39(40)42(52)47-23-46-20-25-13-26(21-46)15-27(14-25)22-46/h1-12,17-19,25-27,36-40,50H,13-16,20-23H2,(H,47,52)(H,48,51)(H,49,53)(H,54,55)(H,56,57)/t25?,26?,27?,36-,37?,38?,39?,40?,46?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Competition with 20 pM [125I]BH-CCK-8S for Cholecystokinin type B receptor binding sites in mouse cortical homogenates |
J Med Chem 38: 4294-302 (1995)
Article DOI: 10.1021/jm00021a019
BindingDB Entry DOI: 10.7270/Q2930WWV |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50470623
(CHEMBL140179)Show SMILES OC(=O)c1cc(NC(=O)[C@@H](Cc2ccsc2)NC(=O)C2C(C3c4ccccc4C2c2ccccc32)C(=O)NCC23CC4CC(CC(C4)C2)C3)cc(c1)C(O)=O |wU:9.9,TLB:33:34:19.20:27.22,23:22:19.20:34.29,46:41:48:45.47.44,46:45:48:41.40.42,THB:30:29:19.20:27.22,17:19:27.22:34.29,35:20:27.22:34.29,44:43:40:45.47.46,44:45:40:43.48.42,(14.85,-1.43,;13.52,-.66,;13.5,.89,;12.18,-1.43,;10.84,-.66,;9.52,-1.43,;8.17,-.66,;6.63,-.66,;6.24,.84,;5.55,-1.75,;5.95,-3.26,;7.27,-4.03,;7.43,-5.56,;8.94,-5.88,;9.71,-4.55,;8.68,-3.4,;4.04,-1.37,;2.56,-.97,;3.01,.57,;-2.05,-1.75,;-1.03,-.74,;-1.05,-2.59,;-3.32,-3.16,;-5.02,-2.87,;-6.67,-3.53,;-6.99,-4.56,;-5.45,-5.07,;-3.93,-4.46,;-2.02,-3.9,;.2,-4.4,;1.37,-5.72,;3.33,-5.72,;4,-4.46,;2.72,-3.21,;.91,-3.21,;-.41,.41,;-1.34,1.31,;.72,1.06,;.71,2.38,;1.84,3.05,;2.71,1.96,;3.88,2.44,;3.9,3.8,;3.01,4.83,;4.17,4.44,;4.16,3.16,;5.17,2.09,;3.03,2.74,;1.82,4.35,;9.5,-2.98,;10.84,-3.75,;12.18,-2.98,;11.6,-5.08,;10.89,-6.63,;13.14,-5.08,)| Show InChI InChI=1S/C44H43N3O7S/c48-39(46-29-16-27(42(51)52)15-28(17-29)43(53)54)34(14-23-9-10-55-21-23)47-41(50)38-36-32-7-3-1-5-30(32)35(31-6-2-4-8-33(31)36)37(38)40(49)45-22-44-18-24-11-25(19-44)13-26(12-24)20-44/h1-10,15-17,21,24-26,34-38H,11-14,18-20,22H2,(H,45,49)(H,46,48)(H,47,50)(H,51,52)(H,53,54)/t24?,25?,26?,34-,35?,36?,37?,38?,44?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Competition with 20 pM [125I]BH-CCK-8S for Cholecystokinin type B receptor binding sites in mouse cortical homogenates |
J Med Chem 38: 4294-302 (1995)
Article DOI: 10.1021/jm00021a019
BindingDB Entry DOI: 10.7270/Q2930WWV |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50470617
(CHEMBL137180)Show SMILES OC(=O)c1cc(NC(=O)[C@H](Cc2ccccc2)NC(=O)C2C(C3c4ccccc4C2c2ccccc32)C(=O)NCc2ccc3ccccc3c2)cc(c1)C(O)=O |wD:9.9,TLB:18:20:30.35:23.28,34:35:20.21:23.28,24:23:20.21:30.35,36:21:30.35:23.28,THB:27:28:20.21:30.35,(18.12,-9.94,;16.79,-9.16,;16.76,-7.61,;15.44,-9.96,;14.1,-9.19,;12.78,-9.96,;11.43,-9.19,;9.88,-9.19,;9.49,-7.68,;8.8,-10.28,;9.2,-11.78,;10.52,-12.56,;10.51,-14.1,;11.85,-14.88,;13.17,-14.11,;13.17,-12.56,;11.85,-11.78,;7.29,-9.88,;5.8,-9.49,;6.25,-7.93,;1.18,-10.28,;2.2,-9.26,;2.18,-11.13,;4.15,-11.72,;5.96,-11.72,;7.24,-13,;6.58,-14.24,;4.6,-14.24,;3.44,-12.94,;1.21,-12.43,;-.7,-13,;-2.23,-13.59,;-3.77,-13.1,;-3.45,-12.06,;-1.8,-11.4,;-.1,-11.69,;2.82,-8.12,;1.89,-7.19,;3.96,-7.45,;3.95,-6.12,;5.08,-5.47,;6.21,-6.12,;7.34,-5.47,;7.34,-4.15,;8.47,-3.49,;8.45,-2.18,;7.31,-1.53,;6.19,-2.2,;6.19,-3.5,;5.06,-4.15,;12.75,-11.49,;14.1,-12.27,;15.44,-11.49,;14.86,-13.62,;14.14,-15.17,;16.41,-13.62,)| Show InChI InChI=1S/C46H37N3O7/c50-42(48-32-23-30(45(53)54)22-31(24-32)46(55)56)37(21-26-10-2-1-3-11-26)49-44(52)41-39-35-16-8-6-14-33(35)38(34-15-7-9-17-36(34)39)40(41)43(51)47-25-27-18-19-28-12-4-5-13-29(28)20-27/h1-20,22-24,37-41H,21,25H2,(H,47,51)(H,48,50)(H,49,52)(H,53,54)(H,55,56)/t37-,38?,39?,40?,41?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Competition with 20 pM [125I]BH-CCK-8S for Cholecystokinin type B receptor binding sites in mouse cortical homogenates |
J Med Chem 38: 4294-302 (1995)
Article DOI: 10.1021/jm00021a019
BindingDB Entry DOI: 10.7270/Q2930WWV |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50470636
(CHEMBL343367)Show SMILES OC(=O)c1cc(NC(=O)[C@@H](Cc2ccccc2F)NC(=O)C2C(C3c4ccccc4C2c2ccccc32)C(=O)NCC23CC4CC(CC(C4)C2)C3)cc(c1)C(O)=O |wU:9.9,TLB:35:36:21.22:29.24,48:43:50:47.49.46,48:47:50:43.42.44,25:24:21.22:36.31,THB:19:21:29.24:36.31,46:45:42:47.49.48,46:47:42:45.50.44,37:22:29.24:36.31,32:31:21.22:29.24,(10.91,-6.64,;11.62,-5.09,;13.17,-5.09,;10.87,-3.76,;9.52,-2.99,;9.54,-1.43,;8.19,-.66,;6.64,-.66,;6.25,.84,;5.56,-1.76,;5.96,-3.27,;7.05,-4.34,;6.64,-5.83,;7.71,-6.92,;9.2,-6.53,;9.61,-5.02,;8.52,-3.96,;8.39,-2.37,;4.05,-1.37,;2.57,-.97,;3.02,.57,;-2.06,-1.76,;-1.03,-.74,;-1.06,-2.6,;-3.33,-3.17,;-5.03,-2.88,;-6.68,-3.54,;-7.01,-4.57,;-5.47,-5.08,;-3.93,-4.47,;-2.03,-3.91,;.2,-4.41,;1.37,-5.74,;3.34,-5.74,;4.01,-4.47,;2.73,-3.21,;.92,-3.21,;-.41,.41,;-1.35,1.32,;.72,1.07,;.71,2.38,;1.84,3.06,;2.72,1.96,;3.89,2.45,;3.91,3.81,;3.02,4.84,;4.18,4.45,;4.17,3.17,;5.19,2.09,;3.04,2.75,;1.82,4.36,;10.87,-.66,;12.2,-1.43,;12.2,-2.99,;13.55,-.66,;14.89,-1.43,;13.53,.89,)| Show InChI InChI=1S/C46H44FN3O7/c47-35-12-6-1-7-27(35)19-36(41(51)49-30-17-28(44(54)55)16-29(18-30)45(56)57)50-43(53)40-38-33-10-4-2-8-31(33)37(32-9-3-5-11-34(32)38)39(40)42(52)48-23-46-20-24-13-25(21-46)15-26(14-24)22-46/h1-12,16-18,24-26,36-40H,13-15,19-23H2,(H,48,52)(H,49,51)(H,50,53)(H,54,55)(H,56,57)/t24?,25?,26?,36-,37?,38?,39?,40?,46?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Competition with 20 pM [125I]BH-CCK-8S for Cholecystokinin type B receptor binding sites in mouse cortical homogenates |
J Med Chem 38: 4294-302 (1995)
Article DOI: 10.1021/jm00021a019
BindingDB Entry DOI: 10.7270/Q2930WWV |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50230689

(CHEMBL3351018)Show SMILES C[C@@H]1CCCC[C@H]1OC(=O)N[C@](C)(Cc1c[nH]c2ccccc12)C(=O)NC[C@H](NC(=O)CCC(O)=O)c1ccccc1 Show InChI InChI=1S/C32H40N4O6/c1-21-10-6-9-15-27(21)42-31(41)36-32(2,18-23-19-33-25-14-8-7-13-24(23)25)30(40)34-20-26(22-11-4-3-5-12-22)35-28(37)16-17-29(38)39/h3-5,7-8,11-14,19,21,26-27,33H,6,9-10,15-18,20H2,1-2H3,(H,34,40)(H,35,37)(H,36,41)(H,38,39)/t21-,26+,27-,32-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Cholecystokinin type B receptor binding assay performed on homogenized cerebral cortex from male mouse |
J Med Chem 35: 1572-7 (1992)
BindingDB Entry DOI: 10.7270/Q26H4KM8 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM81962

(S-L-365,260)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2cccc(C)c2)C1=O)c1ccccc1 |r,c:9| Show InChI InChI=1S/C24H22N4O2/c1-16-9-8-12-18(15-16)25-24(30)27-22-23(29)28(2)20-14-7-6-13-19(20)21(26-22)17-10-4-3-5-11-17/h3-15,22H,1-2H3,(H2,25,27,30)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Tested for its receptor affinity from competition with 20 pM [125I]BH-CCK-8S for Cholecystokinin type B receptor binding sites in mouse cortical homo... |
J Med Chem 37: 3671-3 (1994)
Article DOI: 10.1021/jm00048a001
BindingDB Entry DOI: 10.7270/Q28918MZ |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50230687
(CHEMBL3351021)Show SMILES C[C@@H]1CCCC[C@H]1OC(=O)N[C@](C)(Cc1c[nH]c2ccccc12)C(=O)NC[C@H](NC(=O)\C=C\C(O)=O)c1ccccc1 Show InChI InChI=1S/C32H38N4O6/c1-21-10-6-9-15-27(21)42-31(41)36-32(2,18-23-19-33-25-14-8-7-13-24(23)25)30(40)34-20-26(22-11-4-3-5-12-22)35-28(37)16-17-29(38)39/h3-5,7-8,11-14,16-17,19,21,26-27,33H,6,9-10,15,18,20H2,1-2H3,(H,34,40)(H,35,37)(H,36,41)(H,38,39)/b17-16+/t21-,26+,27-,32-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Cholecystokinin type B receptor binding assay performed on homogenized cerebral cortex from male mouse |
J Med Chem 35: 1572-7 (1992)
BindingDB Entry DOI: 10.7270/Q26H4KM8 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50471076
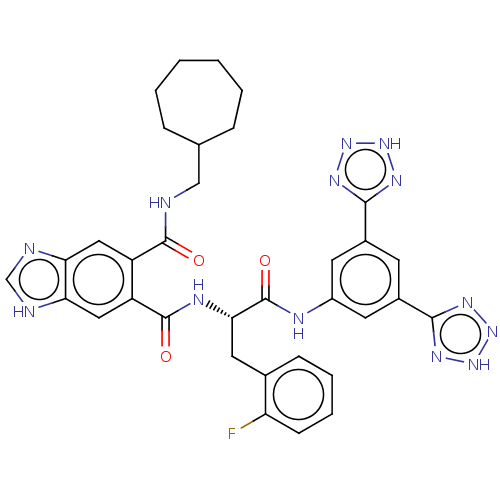
(CHEMBL299387)Show SMILES Fc1ccccc1C[C@H](NC(=O)c1cc2[nH]cnc2cc1C(=O)NCC1CCCCCC1)C(=O)Nc1cc(cc(c1)-c1nn[nH]n1)-c1nn[nH]n1 Show InChI InChI=1S/C34H34FN13O3/c35-26-10-6-5-9-20(26)14-29(34(51)39-23-12-21(30-41-45-46-42-30)11-22(13-23)31-43-47-48-44-31)40-33(50)25-16-28-27(37-18-38-28)15-24(25)32(49)36-17-19-7-3-1-2-4-8-19/h5-6,9-13,15-16,18-19,29H,1-4,7-8,14,17H2,(H,36,49)(H,37,38)(H,39,51)(H,40,50)(H,41,42,45,46)(H,43,44,47,48)/t29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Cholecystokinin type B receptor using [125I]BH-CCK-8S as radioligand in mouse cortical membranes |
J Med Chem 39: 1806-15 (1996)
Article DOI: 10.1021/jm9508907
BindingDB Entry DOI: 10.7270/Q2571FR6 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM81962

(S-L-365,260)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2cccc(C)c2)C1=O)c1ccccc1 |r,c:9| Show InChI InChI=1S/C24H22N4O2/c1-16-9-8-12-18(15-16)25-24(30)27-22-23(29)28(2)20-14-7-6-13-19(20)21(26-22)17-10-4-3-5-11-17/h3-15,22H,1-2H3,(H2,25,27,30)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Displacement of binding of [125I]CCK-8S to Cholecystokinin type B receptor in mouse cerebral cortex homogenates |
J Med Chem 43: 3518-29 (2000)
Article DOI: 10.1021/jm000960w
BindingDB Entry DOI: 10.7270/Q2KS6V9G |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50470639
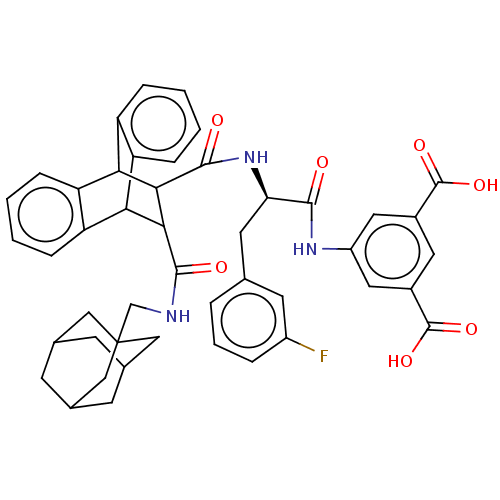
(CHEMBL433656)Show SMILES OC(=O)c1cc(NC(=O)[C@@H](Cc2cccc(F)c2)NC(=O)C2C(C3c4ccccc4C2c2ccccc32)C(=O)NCC23CC4CC(CC(C4)C2)C3)cc(c1)C(O)=O |wU:9.9,TLB:19:21:31.36:24.29,44:45:49:43.42.48,37:22:31.36:24.29,25:24:21.22:31.36,35:36:21.22:24.29,THB:44:43:49:45.50.46,46:47:42:45.50.44,46:45:42:47.49.48,28:29:21.22:31.36,(10.91,-6.64,;11.62,-5.09,;13.17,-5.09,;10.87,-3.76,;9.52,-2.99,;9.54,-1.43,;8.19,-.66,;6.64,-.66,;6.25,.84,;5.56,-1.76,;5.96,-3.27,;7.05,-4.34,;8.52,-3.96,;9.61,-5.02,;9.2,-6.53,;7.71,-6.92,;7.32,-8.42,;6.64,-5.83,;4.05,-1.37,;2.57,-.97,;3.02,.57,;-2.06,-1.76,;-1.03,-.74,;-1.06,-2.6,;.92,-3.21,;2.73,-3.21,;4.01,-4.47,;3.34,-5.74,;1.37,-5.74,;.2,-4.41,;-2.03,-3.91,;-3.93,-4.47,;-5.47,-5.08,;-7.01,-4.57,;-6.68,-3.54,;-5.03,-2.88,;-3.33,-3.17,;-.41,.41,;-1.35,1.32,;.72,1.07,;.71,2.38,;1.84,3.06,;1.82,4.36,;3.02,4.84,;4.18,4.45,;4.17,3.17,;5.19,2.09,;3.89,2.45,;3.91,3.81,;2.72,1.96,;3.04,2.75,;10.87,-.66,;12.2,-1.43,;12.2,-2.99,;13.55,-.66,;14.89,-1.43,;13.53,.89,)| Show InChI InChI=1S/C46H44FN3O7/c47-30-7-5-6-24(15-30)16-36(41(51)49-31-18-28(44(54)55)17-29(19-31)45(56)57)50-43(53)40-38-34-10-3-1-8-32(34)37(33-9-2-4-11-35(33)38)39(40)42(52)48-23-46-20-25-12-26(21-46)14-27(13-25)22-46/h1-11,15,17-19,25-27,36-40H,12-14,16,20-23H2,(H,48,52)(H,49,51)(H,50,53)(H,54,55)(H,56,57)/t25?,26?,27?,36-,37?,38?,39?,40?,46?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Competition with 20 pM [125I]BH-CCK-8S for Cholecystokinin type B receptor binding sites in mouse cortical homogenates |
J Med Chem 38: 4294-302 (1995)
Article DOI: 10.1021/jm00021a019
BindingDB Entry DOI: 10.7270/Q2930WWV |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50230684
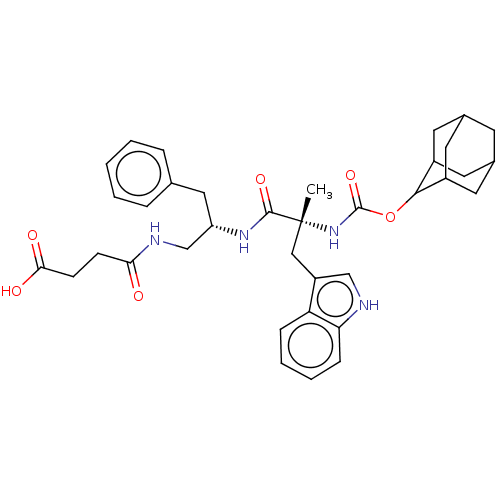
(CHEMBL353157 | PD-135118)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N[C@H](CNC(=O)CCC(O)=O)Cc1ccccc1 |wU:29.34,1.0,TLB:18:19:23:16.17.22,15:16:23:19.25.20,THB:18:17:23:19.25.20,15:16:21.23.22:19.18.25,20:21:16:19.18.25,20:19:16:21.23.22,(3.3,-9.92,;4.62,-10.71,;5.96,-9.95,;5.97,-8.41,;5.07,-7.17,;5.98,-5.93,;7.45,-6.4,;8.77,-5.63,;10.11,-6.4,;10.11,-7.94,;8.77,-8.71,;7.44,-7.94,;3.27,-11.46,;1.94,-10.69,;1.97,-9.15,;.61,-11.44,;-.93,-11.44,;-.74,-13,;-1.6,-14.43,;-3.12,-14.03,;-4.59,-14.66,;-3.54,-13.21,;-2.12,-13.53,;-3.73,-11.69,;-2.42,-11.04,;-3.33,-12.42,;5.95,-11.48,;5.93,-13.02,;7.28,-10.71,;8.61,-11.48,;8.61,-13.02,;7.28,-13.79,;7.26,-15.33,;5.93,-16.1,;8.61,-16.1,;8.59,-17.64,;9.94,-18.41,;9.92,-19.95,;11.27,-17.64,;9.95,-10.71,;11.28,-11.48,;11.28,-13.02,;12.61,-13.79,;13.94,-13.02,;13.94,-11.46,;12.61,-10.71,)| Show InChI InChI=1S/C36H44N4O6/c1-36(19-27-20-37-30-10-6-5-9-29(27)30,40-35(45)46-33-25-14-23-13-24(16-25)17-26(33)15-23)34(44)39-28(18-22-7-3-2-4-8-22)21-38-31(41)11-12-32(42)43/h2-10,20,23-26,28,33,37H,11-19,21H2,1H3,(H,38,41)(H,39,44)(H,40,45)(H,42,43)/t23?,24?,25?,26?,28-,33?,36+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Cholecystokinin type B receptor binding assay performed on homogenized cerebral cortex from male mouse |
J Med Chem 35: 1572-7 (1992)
BindingDB Entry DOI: 10.7270/Q26H4KM8 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50471072
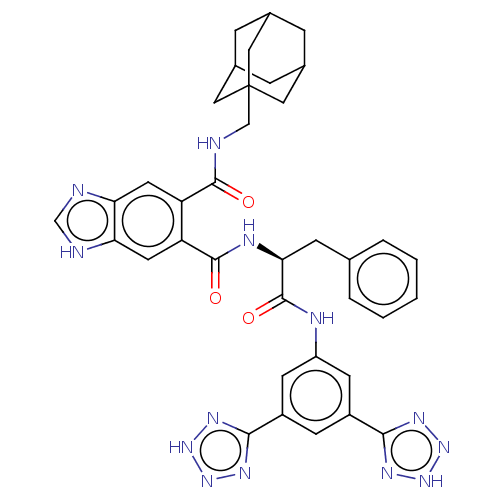
(CHEMBL53237)Show SMILES O=C(Nc1cc(cc(c1)-c1nn[nH]n1)-c1nn[nH]n1)[C@H](Cc1ccccc1)NC(=O)c1cc2[nH]cnc2cc1C(=O)NCC12CC3CC(CC(C3)C1)C2 |TLB:46:47:51:45.44.50,50:49:52:45.44.46,50:45:52:49.51.48,THB:46:45:51:47.52.48| Show InChI InChI=1S/C37H37N13O3/c51-34(38-18-37-15-21-6-22(16-37)8-23(7-21)17-37)27-13-29-30(40-19-39-29)14-28(27)35(52)42-31(9-20-4-2-1-3-5-20)36(53)41-26-11-24(32-43-47-48-44-32)10-25(12-26)33-45-49-50-46-33/h1-5,10-14,19,21-23,31H,6-9,15-18H2,(H,38,51)(H,39,40)(H,41,53)(H,42,52)(H,43,44,47,48)(H,45,46,49,50)/t21?,22?,23?,31-,37?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Cholecystokinin type B receptor using [125I]BH-CCK-8S as radioligand in mouse cortical membranes |
J Med Chem 39: 1806-15 (1996)
Article DOI: 10.1021/jm9508907
BindingDB Entry DOI: 10.7270/Q2571FR6 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50470624
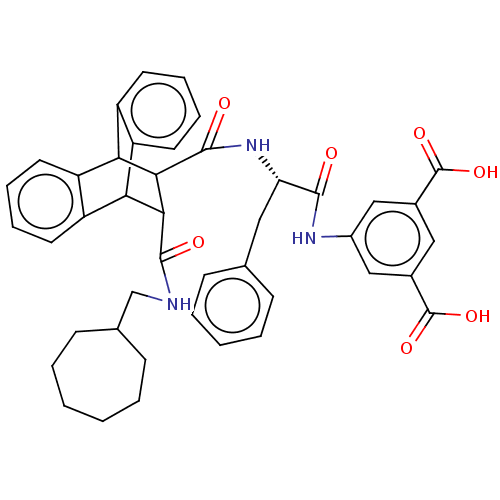
(CHEMBL337673)Show SMILES OC(=O)c1cc(NC(=O)[C@H](Cc2ccccc2)NC(=O)C2C(C3c4ccccc4C2c2ccccc32)C(=O)NCC2CCCCCC2)cc(c1)C(O)=O |wD:9.9,TLB:24:23:20.21:35.30,34:35:20.21:28.23,THB:31:30:20.21:28.23,18:20:28.23:35.30,36:21:28.23:35.30,(18.07,-9.91,;16.74,-9.14,;16.72,-7.59,;15.39,-9.93,;14.06,-9.16,;12.74,-9.93,;11.39,-9.16,;9.85,-9.16,;9.46,-7.65,;8.77,-10.26,;9.17,-11.75,;10.49,-12.52,;11.81,-11.75,;13.14,-12.52,;13.14,-14.07,;11.81,-14.83,;10.48,-14.06,;7.27,-9.85,;5.78,-9.46,;6.23,-7.91,;1.17,-10.26,;2.19,-9.23,;2.17,-11.09,;-.1,-11.65,;-1.8,-11.36,;-3.44,-12.03,;-3.76,-13.06,;-2.23,-13.56,;-.7,-12.96,;1.2,-12.39,;3.43,-12.9,;4.59,-14.2,;6.56,-14.2,;7.22,-12.96,;5.94,-11.68,;4.14,-11.68,;2.82,-8.09,;1.88,-7.17,;3.95,-7.43,;3.93,-6.11,;5.06,-5.45,;4.94,-4.14,;5.9,-3.26,;7.2,-3.43,;7.85,-4.56,;7.38,-5.78,;6.14,-6.17,;12.72,-11.46,;14.06,-12.23,;15.39,-11.46,;14.81,-13.58,;14.1,-15.12,;16.36,-13.58,)| Show InChI InChI=1S/C43H43N3O7/c47-39(45-29-22-27(42(50)51)21-28(23-29)43(52)53)34(20-25-12-6-3-7-13-25)46-41(49)38-36-32-18-10-8-16-30(32)35(31-17-9-11-19-33(31)36)37(38)40(48)44-24-26-14-4-1-2-5-15-26/h3,6-13,16-19,21-23,26,34-38H,1-2,4-5,14-15,20,24H2,(H,44,48)(H,45,47)(H,46,49)(H,50,51)(H,52,53)/t34-,35?,36?,37?,38?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Competition with 20 pM [125I]BH-CCK-8S for Cholecystokinin type B receptor binding sites in mouse cortical homogenates |
J Med Chem 38: 4294-302 (1995)
Article DOI: 10.1021/jm00021a019
BindingDB Entry DOI: 10.7270/Q2930WWV |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50471068
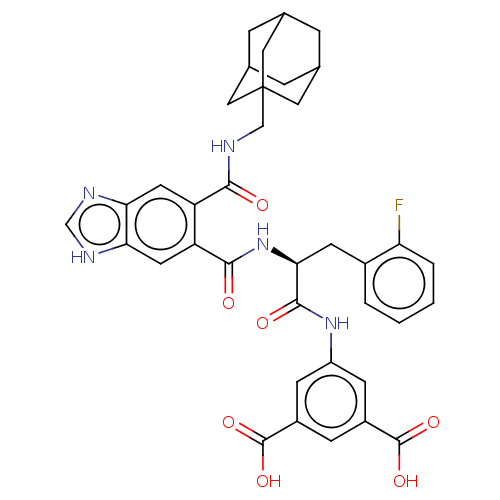
(CHEMBL298551)Show SMILES OC(=O)c1cc(NC(=O)[C@H](Cc2ccccc2F)NC(=O)c2cc3[nH]cnc3cc2C(=O)NCC23CC4CC(CC(C4)C2)C3)cc(c1)C(O)=O |TLB:41:40:43:36.35.37,41:36:43:40.42.39,THB:39:38:35:40.42.41,39:40:35:38.43.37| Show InChI InChI=1S/C37H36FN5O7/c38-28-4-2-1-3-22(28)11-31(34(46)42-25-9-23(35(47)48)8-24(10-25)36(49)50)43-33(45)27-13-30-29(40-18-41-30)12-26(27)32(44)39-17-37-14-19-5-20(15-37)7-21(6-19)16-37/h1-4,8-10,12-13,18-21,31H,5-7,11,14-17H2,(H,39,44)(H,40,41)(H,42,46)(H,43,45)(H,47,48)(H,49,50)/t19?,20?,21?,31-,37?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Cholecystokinin type B receptor using [125I]BH-CCK-8S as radioligand in mouse cortical membranes |
J Med Chem 39: 1806-15 (1996)
Article DOI: 10.1021/jm9508907
BindingDB Entry DOI: 10.7270/Q2571FR6 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50421390

(CHEMBL24313)Show SMILES OC(=O)c1cc(NC(=O)[C@H](Cc2ccccc2)NC(=O)c2cc3[nH]cnc3cc2C(=O)NCC23CC4CC(CC(C4)C2)C3)cc(c1)C(O)=O |TLB:36:37:41:35.34.40,THB:36:35:41:37.42.38,38:37:34:39.41.40,38:39:34:37.42.36| Show InChI InChI=1S/C37H37N5O7/c43-32(38-18-37-15-21-6-22(16-37)8-23(7-21)17-37)27-13-29-30(40-19-39-29)14-28(27)33(44)42-31(9-20-4-2-1-3-5-20)34(45)41-26-11-24(35(46)47)10-25(12-26)36(48)49/h1-5,10-14,19,21-23,31H,6-9,15-18H2,(H,38,43)(H,39,40)(H,41,45)(H,42,44)(H,46,47)(H,48,49)/t21?,22?,23?,31-,37?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Inhibition of 20 pM [125I]BH-CCK-8S binding to mouse cortical membrane Cholecystokinin 2 receptor |
J Med Chem 48: 6790-802 (2005)
Article DOI: 10.1021/jm049069y
BindingDB Entry DOI: 10.7270/Q2BC428F |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50421390

(CHEMBL24313)Show SMILES OC(=O)c1cc(NC(=O)[C@H](Cc2ccccc2)NC(=O)c2cc3[nH]cnc3cc2C(=O)NCC23CC4CC(CC(C4)C2)C3)cc(c1)C(O)=O |TLB:36:37:41:35.34.40,THB:36:35:41:37.42.38,38:37:34:39.41.40,38:39:34:37.42.36| Show InChI InChI=1S/C37H37N5O7/c43-32(38-18-37-15-21-6-22(16-37)8-23(7-21)17-37)27-13-29-30(40-19-39-29)14-28(27)33(44)42-31(9-20-4-2-1-3-5-20)34(45)41-26-11-24(35(46)47)10-25(12-26)36(48)49/h1-5,10-14,19,21-23,31H,6-9,15-18H2,(H,38,43)(H,39,40)(H,41,45)(H,42,44)(H,46,47)(H,48,49)/t21?,22?,23?,31-,37?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Cholecystokinin type B receptor using [125I]BH-CCK-8S as radioligand in mouse cortical membranes |
J Med Chem 39: 1806-15 (1996)
Article DOI: 10.1021/jm9508907
BindingDB Entry DOI: 10.7270/Q2571FR6 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50471071
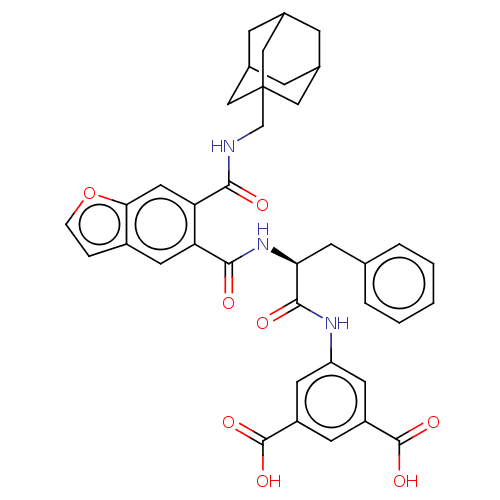
(CHEMBL297480)Show SMILES OC(=O)c1cc(NC(=O)[C@H](Cc2ccccc2)NC(=O)c2cc3ccoc3cc2C(=O)NCC23CC4CC(CC(C4)C2)C3)cc(c1)C(O)=O |TLB:36:37:41:35.34.40,THB:36:35:41:37.42.38,38:37:34:39.41.40,38:39:34:37.42.36| Show InChI InChI=1S/C38H37N3O8/c42-33(39-20-38-17-22-8-23(18-38)10-24(9-22)19-38)30-16-32-25(6-7-49-32)15-29(30)34(43)41-31(11-21-4-2-1-3-5-21)35(44)40-28-13-26(36(45)46)12-27(14-28)37(47)48/h1-7,12-16,22-24,31H,8-11,17-20H2,(H,39,42)(H,40,44)(H,41,43)(H,45,46)(H,47,48)/t22?,23?,24?,31-,38?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Cholecystokinin type B receptor using [125I]BH-CCK-8S as radioligand in mouse cortical membranes |
J Med Chem 39: 1806-15 (1996)
Article DOI: 10.1021/jm9508907
BindingDB Entry DOI: 10.7270/Q2571FR6 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50061988
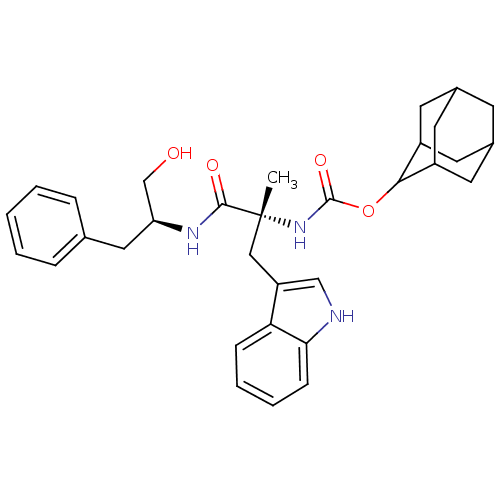
(CHEMBL138657 | [(R)-1-((S)-1-Hydroxymethyl-2-pheny...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N[C@H](CO)Cc1ccccc1 |wU:1.13,wD:29.33,1.0,TLB:25:24:22:19.18.20,THB:25:19:16.24.23:22,20:19:16:21.23.22,20:21:16:19.18.25,15:16:22:19.18.20,(-.04,-8.98,;-.02,-10.34,;.05,-11.88,;1.42,-12.58,;.17,-13.49,;.65,-14.96,;2.19,-14.95,;3.2,-16.09,;4.71,-15.77,;5.18,-14.3,;4.16,-13.18,;2.67,-13.49,;-1.41,-9.83,;-2.7,-10.68,;-2.67,-12.13,;-4.07,-9.99,;-5.36,-10.84,;-5.36,-12.37,;-6.76,-12.72,;-8.08,-12.23,;-9.28,-13.5,;-7.78,-13.08,;-6.38,-13.65,;-7.79,-11.5,;-6.75,-10.27,;-8.11,-10.75,;1.24,-9.66,;1.24,-8.29,;2.62,-10.35,;3.9,-9.5,;3.81,-7.96,;4.58,-6.63,;5.28,-10.19,;6.56,-9.34,;6.48,-7.96,;7.74,-6.96,;9.13,-7.63,;9.22,-9.17,;7.94,-10.03,)| Show InChI InChI=1S/C32H39N3O4/c1-32(17-25-18-33-28-10-6-5-9-27(25)28,30(37)34-26(19-36)16-20-7-3-2-4-8-20)35-31(38)39-29-23-12-21-11-22(14-23)15-24(29)13-21/h2-10,18,21-24,26,29,33,36H,11-17,19H2,1H3,(H,34,37)(H,35,38)/t21?,22?,23?,24?,26-,29?,32+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Cholecystokinin type B receptor binding assay performed on homogenized cerebral cortex from male mouse |
J Med Chem 35: 1572-7 (1992)
BindingDB Entry DOI: 10.7270/Q26H4KM8 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50471073

(CHEMBL301777)Show SMILES OC(=O)c1cc(NC(=O)[C@H](Cc2ccccc2F)NC(=O)c2cc3[nH]cnc3cc2C(=O)NCC2CCCCCC2)cc(c1)C(O)=O Show InChI InChI=1S/C34H34FN5O7/c35-26-10-6-5-9-20(26)14-29(32(43)39-23-12-21(33(44)45)11-22(13-23)34(46)47)40-31(42)25-16-28-27(37-18-38-28)15-24(25)30(41)36-17-19-7-3-1-2-4-8-19/h5-6,9-13,15-16,18-19,29H,1-4,7-8,14,17H2,(H,36,41)(H,37,38)(H,39,43)(H,40,42)(H,44,45)(H,46,47)/t29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Cholecystokinin type B receptor using [125I]BH-CCK-8S as radioligand in mouse cortical membranes |
J Med Chem 39: 1806-15 (1996)
Article DOI: 10.1021/jm9508907
BindingDB Entry DOI: 10.7270/Q2571FR6 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50470625
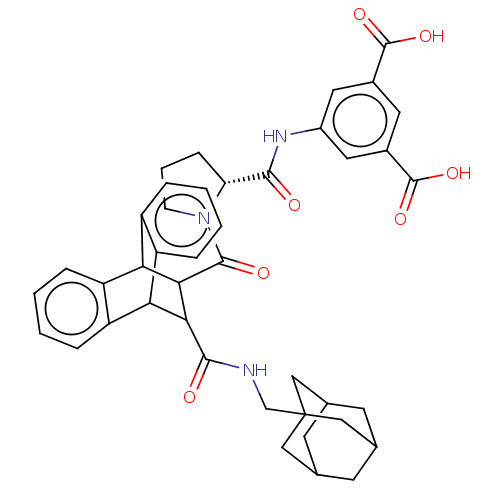
(CHEMBL335587)Show SMILES OC(=O)c1cc(NC(=O)[C@H]2CCCN2C(=O)C2C(C3c4ccccc4C2c2ccccc32)C(=O)NCC23CC4CC(CC(C4)C2)C3)cc(c1)C(O)=O |wU:9.8,TLB:14:16:19.24:31.26,43:38:45:42.44.41,43:42:45:38.37.39,30:31:16.17:19.24,32:17:19.24:31.26,20:19:16.17:31.26,THB:41:42:37:40.45.39,41:40:37:42.44.43,23:24:16.17:31.26,(21.09,-9.48,;21.07,-7.94,;22.4,-7.17,;19.74,-7.19,;18.4,-7.97,;17.08,-7.2,;15.74,-7.99,;14.41,-7.23,;14.39,-5.69,;13.07,-8.02,;14.38,-8.86,;13.97,-10.35,;12.43,-10.41,;11.89,-9,;10.4,-8.6,;10.86,-7.07,;5.82,-9.38,;6.82,-8.38,;6.82,-10.22,;8.77,-10.83,;10.56,-10.83,;11.82,-12.08,;11.17,-13.33,;9.21,-13.33,;8.07,-12.02,;5.84,-11.52,;3.96,-12.08,;2.42,-12.68,;.9,-12.18,;1.23,-11.15,;2.86,-10.5,;4.54,-10.76,;7.46,-7.23,;6.52,-6.33,;8.58,-6.58,;8.55,-5.27,;9.67,-4.6,;10.54,-5.69,;11.73,-5.21,;11.73,-3.86,;10.86,-2.84,;12,-3.22,;11.98,-4.49,;13,-5.56,;10.86,-4.91,;9.67,-3.32,;17.06,-5.68,;18.37,-4.89,;19.72,-5.65,;18.34,-3.35,;19.68,-2.57,;16.99,-2.6,)| Show InChI InChI=1S/C42H43N3O7/c46-37(44-27-16-25(40(49)50)15-26(17-27)41(51)52)32-10-5-11-45(32)39(48)36-34-30-8-3-1-6-28(30)33(29-7-2-4-9-31(29)34)35(36)38(47)43-21-42-18-22-12-23(19-42)14-24(13-22)20-42/h1-4,6-9,15-17,22-24,32-36H,5,10-14,18-21H2,(H,43,47)(H,44,46)(H,49,50)(H,51,52)/t22?,23?,24?,32-,33?,34?,35?,36?,42?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Competition with 20 pM [125I]BH-CCK-8S for Cholecystokinin type B receptor binding sites in mouse cortical homogenates |
J Med Chem 38: 4294-302 (1995)
Article DOI: 10.1021/jm00021a019
BindingDB Entry DOI: 10.7270/Q2930WWV |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50470629

(CHEMBL342616)Show SMILES OC(=O)c1cc(NC(=O)[C@H](Cc2ccccc2)NC(=O)C2C(C3c4ccccc4C2c2ccccc32)C(=O)NCC23CC4CC(CC(C4)C2)C3)cc(c1)C(O)=O |wD:9.9,TLB:43:44:48:42.41.47,47:46:49:42.41.43,47:42:49:46.48.45,24:23:20.21:35.30,THB:18:20:35.30:28.23,43:42:48:44.49.45,34:35:20.21:28.23,27:28:20.21:35.30,31:30:20.21:28.23,(16.62,-7.02,;16.63,-5.48,;15.29,-4.71,;17.97,-4.72,;17.97,-3.18,;19.29,-2.43,;19.31,-.88,;20.64,-.12,;21.97,-.9,;20.64,1.42,;19.31,2.19,;17.98,2.97,;16.63,2.21,;15.31,2.97,;15.33,4.52,;16.65,5.3,;17.99,4.5,;21.97,2.19,;21.97,3.73,;20.64,4.51,;23.44,4.41,;24.65,3.71,;25.05,5.21,;27.84,6.65,;29.36,6.44,;30.3,7.66,;29.71,9.1,;28.18,9.3,;27.25,8.09,;23.52,5.95,;22.2,7.73,;21.67,9.18,;22.67,10.37,;24.18,10.1,;24.71,8.66,;23.73,7.47,;24.66,2.18,;25.98,2.96,;25.99,1.41,;27.32,2.18,;28.66,1.41,;30.24,1.7,;31.62,.9,;31.41,-.6,;29.78,-.84,;30.79,.2,;31.02,1.59,;32.73,1.99,;29.75,2.35,;28.46,-.09,;20.63,-3.19,;20.63,-4.73,;19.3,-5.51,;21.96,-5.51,;21.97,-7.04,;23.29,-4.74,)| Show InChI InChI=1S/C46H45N3O7/c50-41(48-31-19-29(44(53)54)18-30(20-31)45(55)56)36(17-25-8-2-1-3-9-25)49-43(52)40-38-34-12-6-4-10-32(34)37(33-11-5-7-13-35(33)38)39(40)42(51)47-24-46-21-26-14-27(22-46)16-28(15-26)23-46/h1-13,18-20,26-28,36-40H,14-17,21-24H2,(H,47,51)(H,48,50)(H,49,52)(H,53,54)(H,55,56)/t26?,27?,28?,36-,37?,38?,39?,40?,46?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Competition with 20 pM [125I]BH-CCK-8S for Cholecystokinin type B receptor binding sites in mouse cortical homogenates |
J Med Chem 38: 4294-302 (1995)
Article DOI: 10.1021/jm00021a019
BindingDB Entry DOI: 10.7270/Q2930WWV |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50470629

(CHEMBL342616)Show SMILES OC(=O)c1cc(NC(=O)[C@H](Cc2ccccc2)NC(=O)C2C(C3c4ccccc4C2c2ccccc32)C(=O)NCC23CC4CC(CC(C4)C2)C3)cc(c1)C(O)=O |wD:9.9,TLB:43:44:48:42.41.47,47:46:49:42.41.43,47:42:49:46.48.45,24:23:20.21:35.30,THB:18:20:35.30:28.23,43:42:48:44.49.45,34:35:20.21:28.23,27:28:20.21:35.30,31:30:20.21:28.23,(16.62,-7.02,;16.63,-5.48,;15.29,-4.71,;17.97,-4.72,;17.97,-3.18,;19.29,-2.43,;19.31,-.88,;20.64,-.12,;21.97,-.9,;20.64,1.42,;19.31,2.19,;17.98,2.97,;16.63,2.21,;15.31,2.97,;15.33,4.52,;16.65,5.3,;17.99,4.5,;21.97,2.19,;21.97,3.73,;20.64,4.51,;23.44,4.41,;24.65,3.71,;25.05,5.21,;27.84,6.65,;29.36,6.44,;30.3,7.66,;29.71,9.1,;28.18,9.3,;27.25,8.09,;23.52,5.95,;22.2,7.73,;21.67,9.18,;22.67,10.37,;24.18,10.1,;24.71,8.66,;23.73,7.47,;24.66,2.18,;25.98,2.96,;25.99,1.41,;27.32,2.18,;28.66,1.41,;30.24,1.7,;31.62,.9,;31.41,-.6,;29.78,-.84,;30.79,.2,;31.02,1.59,;32.73,1.99,;29.75,2.35,;28.46,-.09,;20.63,-3.19,;20.63,-4.73,;19.3,-5.51,;21.96,-5.51,;21.97,-7.04,;23.29,-4.74,)| Show InChI InChI=1S/C46H45N3O7/c50-41(48-31-19-29(44(53)54)18-30(20-31)45(55)56)36(17-25-8-2-1-3-9-25)49-43(52)40-38-34-12-6-4-10-32(34)37(33-11-5-7-13-35(33)38)39(40)42(51)47-24-46-21-26-14-27(22-46)16-28(15-26)23-46/h1-13,18-20,26-28,36-40H,14-17,21-24H2,(H,47,51)(H,48,50)(H,49,52)(H,53,54)(H,55,56)/t26?,27?,28?,36-,37?,38?,39?,40?,46?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Competition with 20 pM [125I]BH-CCK-8S for Cholecystokinin type B receptor binding sites in mouse cortical homogenates |
J Med Chem 38: 4294-302 (1995)
Article DOI: 10.1021/jm00021a019
BindingDB Entry DOI: 10.7270/Q2930WWV |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50061990
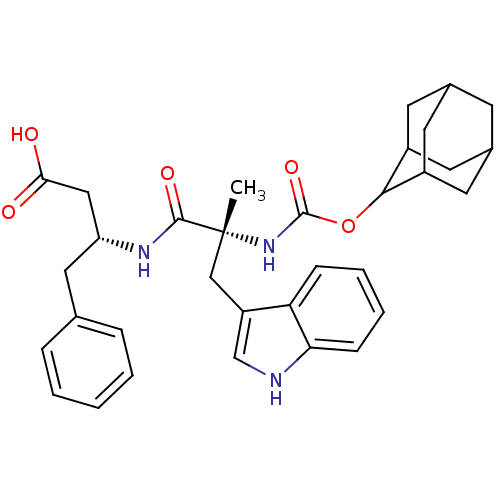
((R)-3-[(R)-2-(Adamantan-2-yloxycarbonylamino)-3-(1...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N[C@@H](CC(O)=O)Cc1ccccc1 |wU:1.13,29.33,wD:1.0,TLB:15:16:18:21.22.20,THB:23:24:18:21.22.20,23:21:18:16.24.25,20:21:16:19.18.25,20:19:16:21.23.22,(10.8,-2.25,;10.81,-3.63,;10.9,-5.18,;12.26,-5.88,;11.02,-6.79,;11.49,-8.25,;13.03,-8.24,;14.06,-9.39,;15.56,-9.07,;16.04,-7.61,;15.01,-6.46,;13.52,-6.79,;9.43,-3.12,;8.15,-3.96,;8.17,-5.43,;6.76,-3.28,;5.47,-4.12,;5.46,-5.67,;4.44,-6.95,;3.04,-6.37,;1.53,-6.79,;2.74,-5.52,;4.05,-6.01,;2.72,-4.03,;4.08,-3.55,;3.02,-4.78,;12.1,-2.95,;12.07,-1.57,;13.48,-3.63,;14.76,-2.78,;14.67,-1.25,;15.43,.09,;14.65,1.43,;16.98,.11,;16.14,-3.47,;17.43,-2.62,;17.34,-1.25,;18.6,-.23,;20,-.92,;20.1,-2.46,;18.81,-3.31,)| Show InChI InChI=1S/C33H39N3O5/c1-33(18-25-19-34-28-10-6-5-9-27(25)28,31(39)35-26(17-29(37)38)16-20-7-3-2-4-8-20)36-32(40)41-30-23-12-21-11-22(14-23)15-24(30)13-21/h2-10,19,21-24,26,30,34H,11-18H2,1H3,(H,35,39)(H,36,40)(H,37,38)/t21?,22?,23?,24?,26-,30?,33-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Cholecystokinin type B receptor binding assay performed on homogenized cerebral cortex from male mouse |
J Med Chem 35: 1572-7 (1992)
BindingDB Entry DOI: 10.7270/Q26H4KM8 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50470634
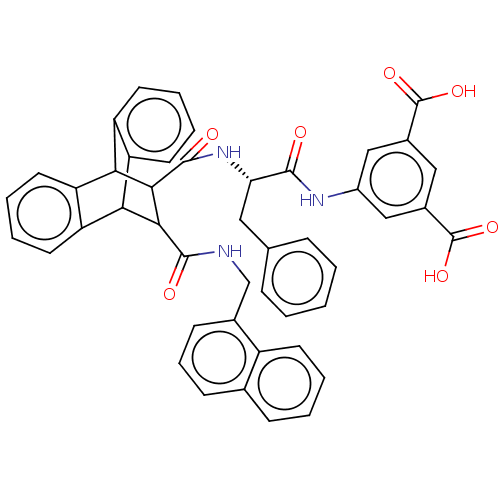
(CHEMBL135350)Show SMILES OC(=O)c1cc(NC(=O)[C@H](Cc2ccccc2)NC(=O)C2C(C3c4ccccc4C2c2ccccc32)C(=O)NCc2cccc3ccccc23)cc(c1)C(O)=O |wD:9.9,TLB:24:23:20.21:30.35,18:20:30.35:23.28,34:35:20.21:23.28,36:21:30.35:23.28,THB:27:28:20.21:30.35,(18.12,-9.94,;16.79,-9.16,;16.76,-7.61,;15.44,-9.96,;14.1,-9.19,;12.78,-9.96,;11.43,-9.19,;9.88,-9.19,;9.49,-7.68,;8.8,-10.28,;9.2,-11.78,;10.52,-12.56,;10.51,-14.1,;11.85,-14.88,;13.17,-14.11,;13.17,-12.56,;11.85,-11.78,;7.29,-9.88,;5.8,-9.49,;6.25,-7.93,;1.18,-10.28,;2.2,-9.26,;2.18,-11.13,;4.15,-11.72,;5.96,-11.72,;7.24,-13,;6.58,-14.24,;4.6,-14.24,;3.44,-12.94,;1.21,-12.43,;-.7,-13,;-2.23,-13.59,;-3.77,-13.1,;-3.45,-12.06,;-1.8,-11.4,;-.1,-11.69,;2.82,-8.12,;1.89,-7.19,;3.96,-7.45,;3.95,-6.12,;5.08,-5.47,;6.21,-6.12,;7.34,-5.47,;7.34,-4.15,;6.19,-3.5,;6.19,-2.21,;5.06,-1.56,;3.92,-2.23,;3.95,-3.53,;5.06,-4.15,;12.75,-11.49,;14.1,-12.27,;15.44,-11.49,;14.86,-13.62,;14.14,-15.17,;16.41,-13.62,)| Show InChI InChI=1S/C46H37N3O7/c50-42(48-31-23-29(45(53)54)22-30(24-31)46(55)56)37(21-26-11-2-1-3-12-26)49-44(52)41-39-35-19-8-6-17-33(35)38(34-18-7-9-20-36(34)39)40(41)43(51)47-25-28-15-10-14-27-13-4-5-16-32(27)28/h1-20,22-24,37-41H,21,25H2,(H,47,51)(H,48,50)(H,49,52)(H,53,54)(H,55,56)/t37-,38?,39?,40?,41?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Competition with 20 pM [125I]BH-CCK-8S for Cholecystokinin type B receptor binding sites in mouse cortical homogenates |
J Med Chem 38: 4294-302 (1995)
Article DOI: 10.1021/jm00021a019
BindingDB Entry DOI: 10.7270/Q2930WWV |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50470642

(CHEMBL421921)Show SMILES OC(=O)c1cc(NC(=O)[C@@H](Cc2ccccc2Cl)NC(=O)C2C(C3c4ccccc4C2c2ccccc32)C(=O)NCC23CC4CC(CC(C4)C2)C3)cc(c1)C(O)=O |wU:9.9,TLB:35:36:21.22:29.24,48:43:50:47.49.46,48:47:50:43.42.44,25:24:21.22:36.31,THB:19:21:29.24:36.31,46:45:42:47.49.48,46:47:42:45.50.44,37:22:29.24:36.31,32:31:21.22:29.24,(10.91,-6.64,;11.62,-5.09,;13.17,-5.09,;10.87,-3.76,;9.52,-2.99,;9.54,-1.43,;8.19,-.66,;6.64,-.66,;6.25,.84,;5.56,-1.76,;5.96,-3.27,;7.05,-4.34,;6.64,-5.83,;7.71,-6.92,;9.2,-6.53,;9.61,-5.02,;8.52,-3.96,;8.39,-2.37,;4.05,-1.37,;2.57,-.97,;3.02,.57,;-2.06,-1.76,;-1.03,-.74,;-1.06,-2.6,;-3.33,-3.17,;-5.03,-2.88,;-6.68,-3.54,;-7.01,-4.57,;-5.47,-5.08,;-3.93,-4.47,;-2.03,-3.91,;.2,-4.41,;1.37,-5.74,;3.34,-5.74,;4.01,-4.47,;2.73,-3.21,;.92,-3.21,;-.41,.41,;-1.35,1.32,;.72,1.07,;.71,2.38,;1.84,3.06,;2.72,1.96,;3.89,2.45,;3.91,3.81,;3.02,4.84,;4.18,4.45,;4.17,3.17,;5.19,2.09,;3.04,2.75,;1.82,4.36,;10.87,-.66,;12.2,-1.43,;12.2,-2.99,;13.55,-.66,;14.89,-1.43,;13.53,.89,)| Show InChI InChI=1S/C46H44ClN3O7/c47-35-12-6-1-7-27(35)19-36(41(51)49-30-17-28(44(54)55)16-29(18-30)45(56)57)50-43(53)40-38-33-10-4-2-8-31(33)37(32-9-3-5-11-34(32)38)39(40)42(52)48-23-46-20-24-13-25(21-46)15-26(14-24)22-46/h1-12,16-18,24-26,36-40H,13-15,19-23H2,(H,48,52)(H,49,51)(H,50,53)(H,54,55)(H,56,57)/t24?,25?,26?,36-,37?,38?,39?,40?,46?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Competition with 20 pM [125I]BH-CCK-8S for Cholecystokinin type B receptor binding sites in mouse cortical homogenates |
J Med Chem 38: 4294-302 (1995)
Article DOI: 10.1021/jm00021a019
BindingDB Entry DOI: 10.7270/Q2930WWV |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50230666

(CHEMBL3351025)Show SMILES C[C@@H]1CCCC[C@H]1OC(=O)N[C@](C)(Cc1c[nH]c2ccccc12)C(=O)N[C@H](CNC(=O)CCC(O)=O)Cc1ccccc1 Show InChI InChI=1S/C33H42N4O6/c1-22-10-6-9-15-28(22)43-32(42)37-33(2,19-24-20-34-27-14-8-7-13-26(24)27)31(41)36-25(18-23-11-4-3-5-12-23)21-35-29(38)16-17-30(39)40/h3-5,7-8,11-14,20,22,25,28,34H,6,9-10,15-19,21H2,1-2H3,(H,35,38)(H,36,41)(H,37,42)(H,39,40)/t22-,25+,28-,33-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Cholecystokinin type B receptor binding assay performed on homogenized cerebral cortex from male mouse |
J Med Chem 35: 1572-7 (1992)
BindingDB Entry DOI: 10.7270/Q26H4KM8 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
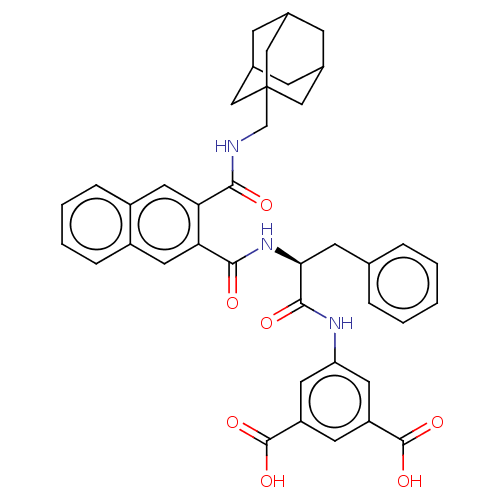
(MOUSE) | BDBM50471064
(CHEMBL301810)Show SMILES OC(=O)c1cc(NC(=O)[C@H](Cc2ccccc2)NC(=O)c2cc3ccccc3cc2C(=O)NCC23CC4CC(CC(C4)C2)C3)cc(c1)C(O)=O |TLB:41:40:43:36.35.37,41:36:43:40.42.39,THB:39:38:35:40.42.41,39:40:35:38.43.37| Show InChI InChI=1S/C40H39N3O7/c44-35(41-22-40-19-24-10-25(20-40)12-26(11-24)21-40)32-17-27-8-4-5-9-28(27)18-33(32)36(45)43-34(13-23-6-2-1-3-7-23)37(46)42-31-15-29(38(47)48)14-30(16-31)39(49)50/h1-9,14-18,24-26,34H,10-13,19-22H2,(H,41,44)(H,42,46)(H,43,45)(H,47,48)(H,49,50)/t24?,25?,26?,34-,40?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Inhibition of 20 pM [125I]BH-CCK-8S binding to mouse cortical membrane Cholecystokinin 2 receptor |
J Med Chem 48: 6790-802 (2005)
Article DOI: 10.1021/jm049069y
BindingDB Entry DOI: 10.7270/Q2BC428F |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50073727

((S)-3-[(S)-2-(Adamantan-2-yloxycarbonylamino)-3-(1...)Show SMILES C[C@@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N[C@H](CC(O)=O)Cc1ccccc1 |wU:1.1,29.33,wD:1.0,TLB:25:24:22:19.18.20,15:16:19.18.25:21.22.23,THB:25:19:22:16.24.23,20:19:16:21.22.23,20:21:16:19.18.25,15:16:22:19.18.20,(11.44,-4.79,;12.24,-6.15,;10.69,-6.15,;9.93,-7.51,;10.42,-8.97,;9.17,-9.86,;7.93,-8.97,;6.43,-9.3,;5.41,-8.14,;5.87,-6.68,;7.37,-6.35,;8.4,-7.51,;13.8,-9.46,;15.33,-9.46,;16.12,-8.1,;16.12,-10.79,;17.98,-11.52,;17.89,-13.08,;19.44,-13.68,;21,-13.15,;21.89,-14.47,;20.27,-13.74,;18.81,-14.24,;20.3,-12.28,;19.48,-11.02,;21.07,-11.59,;13,-4.82,;12.24,-3.49,;14.54,-4.82,;15.3,-3.46,;16.85,-3.46,;17.61,-4.79,;19.14,-4.79,;16.85,-6.11,;14.53,-2.13,;15.29,-.81,;14.49,.52,;15.26,1.85,;16.79,1.85,;17.58,.52,;16.82,-.81,)| Show InChI InChI=1S/C33H39N3O5/c1-33(18-25-19-34-28-10-6-5-9-27(25)28,31(39)35-26(17-29(37)38)16-20-7-3-2-4-8-20)36-32(40)41-30-23-12-21-11-22(14-23)15-24(30)13-21/h2-10,19,21-24,26,30,34H,11-18H2,1H3,(H,35,39)(H,36,40)(H,37,38)/t21?,22?,23?,24?,26-,30?,33-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Cholecystokinin type B receptor binding assay performed on homogenized cerebral cortex from male mouse |
J Med Chem 35: 1572-7 (1992)
BindingDB Entry DOI: 10.7270/Q26H4KM8 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50471077
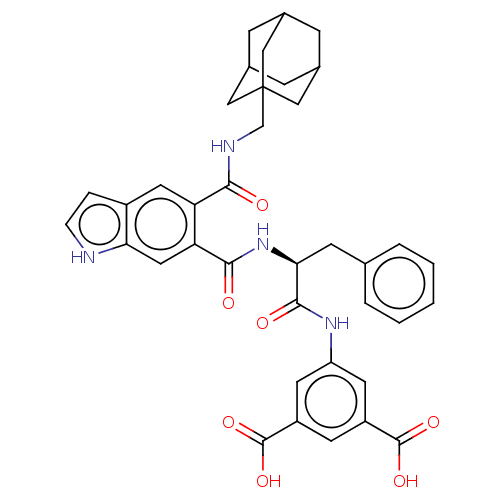
(CHEMBL280248)Show SMILES OC(=O)c1cc(NC(=O)[C@H](Cc2ccccc2)NC(=O)c2cc3[nH]ccc3cc2C(=O)NCC23CC4CC(CC(C4)C2)C3)cc(c1)C(O)=O |TLB:36:37:41:35.34.40,40:39:42:35.34.36,40:35:42:39.41.38,THB:36:35:41:37.42.38| Show InChI InChI=1S/C38H38N4O7/c43-33(40-20-38-17-22-8-23(18-38)10-24(9-22)19-38)29-15-25-6-7-39-31(25)16-30(29)34(44)42-32(11-21-4-2-1-3-5-21)35(45)41-28-13-26(36(46)47)12-27(14-28)37(48)49/h1-7,12-16,22-24,32,39H,8-11,17-20H2,(H,40,43)(H,41,45)(H,42,44)(H,46,47)(H,48,49)/t22?,23?,24?,32-,38?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Cholecystokinin type B receptor using [125I]BH-CCK-8S as radioligand in mouse cortical membranes |
J Med Chem 39: 1806-15 (1996)
Article DOI: 10.1021/jm9508907
BindingDB Entry DOI: 10.7270/Q2571FR6 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50471064

(CHEMBL301810)Show SMILES OC(=O)c1cc(NC(=O)[C@H](Cc2ccccc2)NC(=O)c2cc3ccccc3cc2C(=O)NCC23CC4CC(CC(C4)C2)C3)cc(c1)C(O)=O |TLB:41:40:43:36.35.37,41:36:43:40.42.39,THB:39:38:35:40.42.41,39:40:35:38.43.37| Show InChI InChI=1S/C40H39N3O7/c44-35(41-22-40-19-24-10-25(20-40)12-26(11-24)21-40)32-17-27-8-4-5-9-28(27)18-33(32)36(45)43-34(13-23-6-2-1-3-7-23)37(46)42-31-15-29(38(47)48)14-30(16-31)39(49)50/h1-9,14-18,24-26,34H,10-13,19-22H2,(H,41,44)(H,42,46)(H,43,45)(H,47,48)(H,49,50)/t24?,25?,26?,34-,40?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Cholecystokinin type B receptor using [125I]BH-CCK-8S as radioligand in mouse cortical membranes |
J Med Chem 39: 1806-15 (1996)
Article DOI: 10.1021/jm9508907
BindingDB Entry DOI: 10.7270/Q2571FR6 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50470641
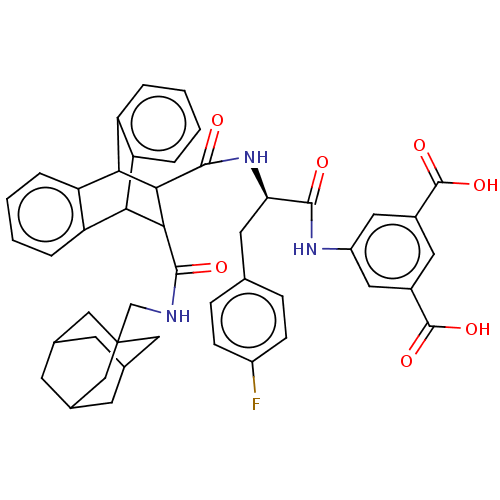
(CHEMBL334714)Show SMILES OC(=O)c1cc(NC(=O)[C@@H](Cc2ccc(F)cc2)NC(=O)C2C(C3c4ccccc4C2c2ccccc32)C(=O)NCC23CC4CC(CC(C4)C2)C3)cc(c1)C(O)=O |wU:9.9,TLB:19:21:31.36:24.29,44:45:49:43.42.48,48:43:50:47.49.46,48:47:50:43.42.44,37:22:31.36:24.29,25:24:21.22:31.36,35:36:21.22:24.29,THB:44:43:49:45.50.46,28:29:21.22:31.36,(14.89,-1.43,;13.55,-.66,;13.53,.89,;12.2,-1.43,;10.87,-.66,;9.54,-1.43,;8.19,-.66,;6.64,-.66,;6.25,.84,;5.56,-1.76,;5.96,-3.27,;7.05,-4.34,;6.64,-5.83,;7.71,-6.92,;9.2,-6.53,;10.3,-7.61,;9.61,-5.02,;8.52,-3.96,;4.05,-1.37,;2.57,-.97,;3.02,.57,;-2.06,-1.76,;-1.03,-.74,;-1.06,-2.6,;.92,-3.21,;2.73,-3.21,;4.01,-4.47,;3.34,-5.74,;1.37,-5.74,;.2,-4.41,;-2.03,-3.91,;-3.93,-4.47,;-5.47,-5.08,;-7.01,-4.57,;-6.68,-3.54,;-5.03,-2.88,;-3.33,-3.17,;-.41,.41,;-1.35,1.32,;.72,1.07,;.71,2.38,;1.84,3.06,;3.04,2.75,;4.17,3.17,;5.19,2.09,;3.89,2.45,;3.91,3.81,;3.02,4.84,;4.18,4.45,;1.82,4.36,;2.72,1.96,;9.52,-2.99,;10.87,-3.76,;12.2,-2.99,;11.62,-5.09,;10.91,-6.64,;13.17,-5.09,)| Show InChI InChI=1S/C46H44FN3O7/c47-30-11-9-24(10-12-30)16-36(41(51)49-31-18-28(44(54)55)17-29(19-31)45(56)57)50-43(53)40-38-34-7-3-1-5-32(34)37(33-6-2-4-8-35(33)38)39(40)42(52)48-23-46-20-25-13-26(21-46)15-27(14-25)22-46/h1-12,17-19,25-27,36-40H,13-16,20-23H2,(H,48,52)(H,49,51)(H,50,53)(H,54,55)(H,56,57)/t25?,26?,27?,36-,37?,38?,39?,40?,46?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Competition with 20 pM [125I]BH-CCK-8S for Cholecystokinin type B receptor binding sites in mouse cortical homogenates |
J Med Chem 38: 4294-302 (1995)
Article DOI: 10.1021/jm00021a019
BindingDB Entry DOI: 10.7270/Q2930WWV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

















































