 Found 54 hits of ic50 for UniProtKB: P24008
Found 54 hits of ic50 for UniProtKB: P24008 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50334788

((17beta-(N-tert-butylcarbamoyl)-4-aza-5alpha-andro...)Show SMILES CC(C)(C)NC(=O)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4NC(=O)C=C[C@]4(C)[C@H]3CC[C@]12C |r,c:18| Show InChI InChI=1S/C23H36N2O2/c1-21(2,3)25-20(27)17-8-7-15-14-6-9-18-23(5,13-11-19(26)24-18)16(14)10-12-22(15,17)4/h11,13-18H,6-10,12H2,1-5H3,(H,24,26)(H,25,27)/t14-,15-,16-,17+,18+,22-,23+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Kingston University
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibitory activity against Steroid 5-alpha-reductase type 1 in rat |
Bioorg Med Chem Lett 8: 409-14 (1999)
BindingDB Entry DOI: 10.7270/Q2JW8FDB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50334788

((17beta-(N-tert-butylcarbamoyl)-4-aza-5alpha-andro...)Show SMILES CC(C)(C)NC(=O)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4NC(=O)C=C[C@]4(C)[C@H]3CC[C@]12C |r,c:18| Show InChI InChI=1S/C23H36N2O2/c1-21(2,3)25-20(27)17-8-7-15-14-6-9-18-23(5,13-11-19(26)24-18)16(14)10-12-22(15,17)4/h11,13-18H,6-10,12H2,1-5H3,(H,24,26)(H,25,27)/t14-,15-,16-,17+,18+,22-,23+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of Steroid 5-alpha-reductase type I from rat ventral prostate (RVP) |
J Med Chem 45: 3406-17 (2002)
BindingDB Entry DOI: 10.7270/Q2T43TT8 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50517901

(CHEMBL4540042)Show SMILES [H][C@@]12CC[C@H](C(=O)NC(C)(c3ccccc3)C(F)(F)F)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])NC(=O)C=C[C@]12C |r,c:38| Show InChI InChI=1S/C28H35F3N2O2/c1-25-15-13-20-18(9-12-22-26(20,2)16-14-23(34)32-22)19(25)10-11-21(25)24(35)33-27(3,28(29,30)31)17-7-5-4-6-8-17/h4-8,14,16,18-22H,9-13,15H2,1-3H3,(H,32,34)(H,33,35)/t18-,19-,20-,21+,22+,25-,26+,27?/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of type 1 5alpha reductase in rat prostate |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50334788

((17beta-(N-tert-butylcarbamoyl)-4-aza-5alpha-andro...)Show SMILES CC(C)(C)NC(=O)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4NC(=O)C=C[C@]4(C)[C@H]3CC[C@]12C |r,c:18| Show InChI InChI=1S/C23H36N2O2/c1-21(2,3)25-20(27)17-8-7-15-14-6-9-18-23(5,13-11-19(26)24-18)16(14)10-12-22(15,17)4/h11,13-18H,6-10,12H2,1-5H3,(H,24,26)(H,25,27)/t14-,15-,16-,17+,18+,22-,23+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of type 1 5alpha reductase in rat prostate |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50517880
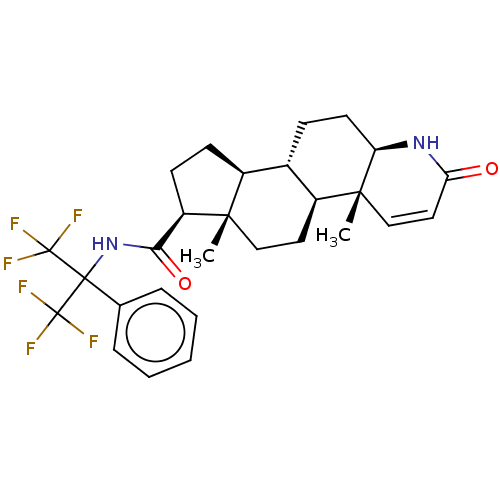
(CHEMBL4569322)Show SMILES [H][C@@]12CC[C@H](C(=O)NC(c3ccccc3)(C(F)(F)F)C(F)(F)F)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])NC(=O)C=C[C@]12C |r,c:41| Show InChI InChI=1S/C28H32F6N2O2/c1-24-14-12-19-17(8-11-21-25(19,2)15-13-22(37)35-21)18(24)9-10-20(24)23(38)36-26(27(29,30)31,28(32,33)34)16-6-4-3-5-7-16/h3-7,13,15,17-21H,8-12,14H2,1-2H3,(H,35,37)(H,36,38)/t17-,18-,19-,20+,21+,24-,25+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of type 1 5alpha reductase in rat prostate |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50043604
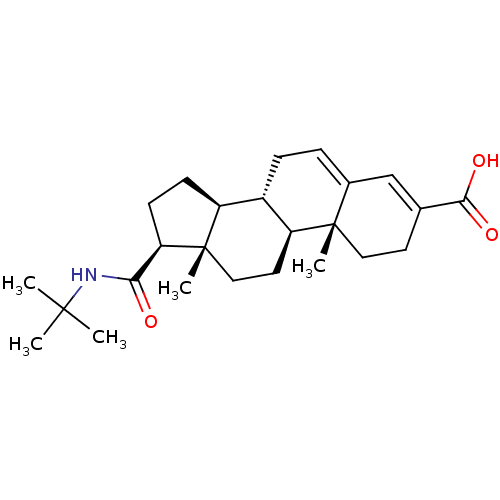
((8S,9S,10R,13S,14S,17S)-17-(tert-butylcarbamoyl)-1...)Show SMILES CC(C)(C)NC(=O)[C@H]1CC[C@H]2[C@@H]3CC=C4C=C(CC[C@]4(C)[C@H]3CC[C@]12C)C(O)=O |c:15,t:13| Show InChI InChI=1S/C25H37NO3/c1-23(2,3)26-21(27)20-9-8-18-17-7-6-16-14-15(22(28)29)10-12-24(16,4)19(17)11-13-25(18,20)5/h6,14,17-20H,7-13H2,1-5H3,(H,26,27)(H,28,29)/t17-,18-,19-,20+,24-,25-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität des Saarlandes
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat Steroid 5-alpha-reductase type I using 18213 3H testosterone 210 nM as substrate |
Bioorg Med Chem Lett 9: 1601-6 (1999)
BindingDB Entry DOI: 10.7270/Q2P84B24 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50462940

(CHEMBL4244937)Show SMILES [H][C@@]12CC(C=O)=C(n3cnc4ccccc34)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2C[C@@H](O)CC[C@]12C |r,t:5,29| Show InChI InChI=1S/C27H32N2O2/c1-26-11-9-19(31)14-18(26)7-8-20-21(26)10-12-27(2)22(20)13-17(15-30)25(27)29-16-28-23-5-3-4-6-24(23)29/h3-7,15-16,19-22,31H,8-14H2,1-2H3/t19-,20+,21-,22-,26-,27-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116882
BindingDB Entry DOI: 10.7270/Q2FR01JT |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50462940

(CHEMBL4244937)Show SMILES [H][C@@]12CC(C=O)=C(n3cnc4ccccc34)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2C[C@@H](O)CC[C@]12C |r,t:5,29| Show InChI InChI=1S/C27H32N2O2/c1-26-11-9-19(31)14-18(26)7-8-20-21(26)10-12-27(2)22(20)13-17(15-30)25(27)29-16-28-23-5-3-4-6-24(23)29/h3-7,15-16,19-22,31H,8-14H2,1-2H3/t19-,20+,21-,22-,26-,27-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 291 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Aut£noma de M£xico
Curated by ChEMBL
| Assay Description
Inhibition of rat liver microsomes 5alpha-reductase1 using [1,2,6,7-3H]T as substrate measured after 60 mins by chromatographic method |
Bioorg Med Chem 26: 4058-4064 (2018)
Article DOI: 10.1016/j.bmc.2018.06.030
BindingDB Entry DOI: 10.7270/Q20K2C7X |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50589275

(CHEMBL5190333)Show SMILES [H][C@@]12CC(C=O)=C(n3cnc4ccccc34)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2C[C@H](CC[C@]12C)OC(=O)C1CC1 |r,t:5,29| | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116882
BindingDB Entry DOI: 10.7270/Q2FR01JT |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50287684

((4aR,6aS,7S)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7...)Show SMILES CCN(CCCCNC(=O)[C@H]1CCC2C3CCC4NC(=O)C=C[C@]4(C)C3CC[C@]12C)c1ccc2c(c1)c(=O)[nH][nH]c2=O |c:21| Show InChI InChI=1S/C33H45N5O4/c1-4-38(20-7-8-21-23(19-20)30(41)37-36-29(21)40)18-6-5-17-34-31(42)26-11-10-24-22-9-12-27-33(3,16-14-28(39)35-27)25(22)13-15-32(24,26)2/h7-8,14,16,19,22,24-27H,4-6,9-13,15,17-18H2,1-3H3,(H,34,42)(H,35,39)(H,36,40)(H,37,41)/t22?,24?,25?,26-,27?,32+,33-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibitory potency against rat Steroid 5-alpha-reductase type 1 expressed in transformed yeast Saccharomyces cerevesiae |
Bioorg Med Chem Lett 6: 1997-2002 (1996)
Article DOI: 10.1016/0960-894X(96)00360-5
BindingDB Entry DOI: 10.7270/Q2K0748S |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50116417
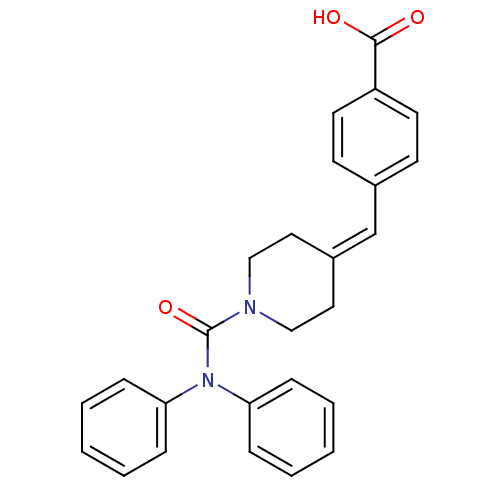
(4-(1-Diphenylcarbamoyl-piperidin-4-ylidenemethyl)-...)Show SMILES [#8]-[#6](=O)-c1ccc(\[#6]=[#6]-2\[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#7](-c2ccccc2)-c2ccccc2)cc1 Show InChI InChI=1S/C26H24N2O3/c29-25(30)22-13-11-20(12-14-22)19-21-15-17-27(18-16-21)26(31)28(23-7-3-1-4-8-23)24-9-5-2-6-10-24/h1-14,19H,15-18H2,(H,29,30) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of Steroid 5-alpha-reductase type I from rat ventral prostate (RVP) |
J Med Chem 45: 3406-17 (2002)
BindingDB Entry DOI: 10.7270/Q2T43TT8 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50116432

(4-(1-Diphenylacetyl-piperidin-4-ylidenemethyl)-3-f...)Show SMILES [#8]-[#6](=O)-c1ccc(\[#6]=[#6]-2\[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#6](-c2ccccc2)-c2ccccc2)c(F)c1 Show InChI InChI=1S/C27H24FNO3/c28-24-18-23(27(31)32)12-11-22(24)17-19-13-15-29(16-14-19)26(30)25(20-7-3-1-4-8-20)21-9-5-2-6-10-21/h1-12,17-18,25H,13-16H2,(H,31,32) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of Steroid 5-alpha-reductase type I from rat ventral prostate (RVP) |
J Med Chem 45: 3406-17 (2002)
BindingDB Entry DOI: 10.7270/Q2T43TT8 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50180893

((3-methyl-4-(4-phenoxybenzoyl)phenyl)acetic acid |...)Show InChI InChI=1S/C22H18O4/c1-15-13-16(14-21(23)24)7-12-20(15)22(25)17-8-10-19(11-9-17)26-18-5-3-2-4-6-18/h2-13H,14H2,1H3,(H,23,24) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.45E+3 | n/a | n/a | n/a | n/a | 6.6 | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-androstenedione from 5alpha reductase1 in rat ventral prostrate homogenate at pH 6.6 |
J Med Chem 49: 748-59 (2006)
Article DOI: 10.1021/jm050728w
BindingDB Entry DOI: 10.7270/Q2VQ3285 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50116422

(4-[1-(3,3-Diphenyl-propionyl)-piperidin-4-ylidenem...)Show SMILES [#8]-[#6](=O)-c1ccc(\[#6]=[#6]-2/[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#6]-[#6](-c2ccccc2)-c2ccccc2)cc1 Show InChI InChI=1S/C28H27NO3/c30-27(20-26(23-7-3-1-4-8-23)24-9-5-2-6-10-24)29-17-15-22(16-18-29)19-21-11-13-25(14-12-21)28(31)32/h1-14,19,26H,15-18,20H2,(H,31,32) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of Steroid 5-alpha-reductase type I from rat ventral prostate (RVP) |
J Med Chem 45: 3406-17 (2002)
BindingDB Entry DOI: 10.7270/Q2T43TT8 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50116419

(4-[1-(2,2-Dicyclohexyl-acetyl)-piperidin-4-ylidene...)Show SMILES [#8]-[#6](=O)-c1ccc(\[#6]=[#6]-2\[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#6](-[#6]-2-[#6]-[#6]-[#6]-[#6]-[#6]-2)-[#6]-2-[#6]-[#6]-[#6]-[#6]-[#6]-2)c(F)c1 Show InChI InChI=1S/C27H36FNO3/c28-24-18-23(27(31)32)12-11-22(24)17-19-13-15-29(16-14-19)26(30)25(20-7-3-1-4-8-20)21-9-5-2-6-10-21/h11-12,17-18,20-21,25H,1-10,13-16H2,(H,31,32) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against steroid Steroid 5-alpha-reductase type I of rat ventral prostate (RVP) |
J Med Chem 45: 3406-17 (2002)
BindingDB Entry DOI: 10.7270/Q2T43TT8 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50116428

(4-(1-Diphenylacetyl-piperidin-4-ylidenemethyl)-3-m...)Show SMILES [#6]-[#8]-c1cc(ccc1\[#6]=[#6]-1\[#6]-[#6]-[#7](-[#6]-[#6]-1)-[#6](=O)-[#6](-c1ccccc1)-c1ccccc1)-[#6](-[#8])=O Show InChI InChI=1S/C28H27NO4/c1-33-25-19-24(28(31)32)13-12-23(25)18-20-14-16-29(17-15-20)27(30)26(21-8-4-2-5-9-21)22-10-6-3-7-11-22/h2-13,18-19,26H,14-17H2,1H3,(H,31,32) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of Steroid 5-alpha-reductase type I from rat ventral prostate (RVP) |
J Med Chem 45: 3406-17 (2002)
BindingDB Entry DOI: 10.7270/Q2T43TT8 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50032282
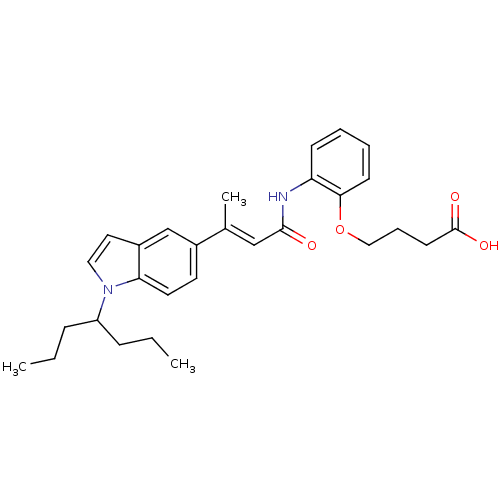
(4-(2-{(E)-3-[1-(1-Propyl-butyl)-1H-indol-5-yl]-but...)Show SMILES CCCC(CCC)n1ccc2cc(ccc12)C(\C)=C\C(=O)Nc1ccccc1OCCCC(O)=O Show InChI InChI=1S/C29H36N2O4/c1-4-9-24(10-5-2)31-17-16-23-20-22(14-15-26(23)31)21(3)19-28(32)30-25-11-6-7-12-27(25)35-18-8-13-29(33)34/h6-7,11-12,14-17,19-20,24H,4-5,8-10,13,18H2,1-3H3,(H,30,32)(H,33,34)/b21-19+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by ChEMBL
| Assay Description
5 alpha- reductase inhibitory activity |
J Med Chem 39: 5047-52 (1997)
Article DOI: 10.1021/jm9601819
BindingDB Entry DOI: 10.7270/Q2TQ6263 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50055147
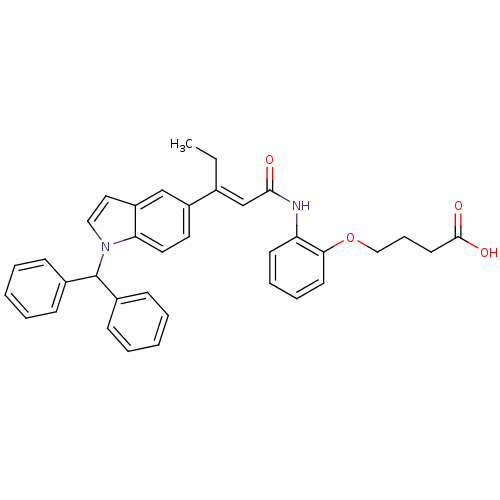
(4-{2-[(E)-3-(1-Benzhydryl-1H-indol-5-yl)-pent-2-en...)Show SMILES CC\C(=C/C(=O)Nc1ccccc1OCCCC(O)=O)c1ccc2n(ccc2c1)C(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C36H34N2O4/c1-2-26(25-34(39)37-31-16-9-10-17-33(31)42-23-11-18-35(40)41)29-19-20-32-30(24-29)21-22-38(32)36(27-12-5-3-6-13-27)28-14-7-4-8-15-28/h3-10,12-17,19-22,24-25,36H,2,11,18,23H2,1H3,(H,37,39)(H,40,41)/b26-25+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by ChEMBL
| Assay Description
5 alpha- reductase inhibitory activity |
J Med Chem 39: 5047-52 (1997)
Article DOI: 10.1021/jm9601819
BindingDB Entry DOI: 10.7270/Q2TQ6263 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50116416

(4-[1-(Adamantane-1-carbonyl)-piperidin-4-ylideneme...)Show SMILES [#8]-[#6](=O)-c1ccc(\[#6]=[#6]-2/[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)C23[#6]-[#6]-4-[#6]-[#6](-[#6]-[#6](-[#6]-4)-[#6]2)-[#6]3)c(F)c1 |TLB:23:18:25:24.22.21,23:22:25:17.18.19,THB:21:20:17:24.22.23,21:22:17:25.20.19| Show InChI InChI=1S/C24H28FNO3/c25-21-11-20(22(27)28)2-1-19(21)10-15-3-5-26(6-4-15)23(29)24-12-16-7-17(13-24)9-18(8-16)14-24/h1-2,10-11,16-18H,3-9,12-14H2,(H,27,28) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of Steroid 5-alpha-reductase type I from rat ventral prostate (RVP) |
J Med Chem 45: 3406-17 (2002)
BindingDB Entry DOI: 10.7270/Q2T43TT8 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50116424

(CHEMBL115438 | {4-[1-(2,2-Dicyclohexyl-acetyl)-pip...)Show SMILES [#8]-[#6](=O)-[#6]-c1ccc(\[#6]=[#6]-2\[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#6](-[#6]-2-[#6]-[#6]-[#6]-[#6]-[#6]-2)-[#6]-2-[#6]-[#6]-[#6]-[#6]-[#6]-2)c(F)c1 Show InChI InChI=1S/C28H38FNO3/c29-25-18-21(19-26(31)32)11-12-24(25)17-20-13-15-30(16-14-20)28(33)27(22-7-3-1-4-8-22)23-9-5-2-6-10-23/h11-12,17-18,22-23,27H,1-10,13-16,19H2,(H,31,32) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of Steroid 5-alpha-reductase type I from rat ventral prostate (RVP) |
J Med Chem 45: 3406-17 (2002)
BindingDB Entry DOI: 10.7270/Q2T43TT8 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50055149
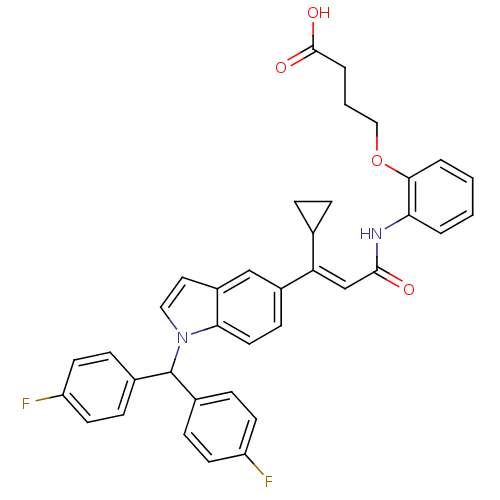
(4-[2-((E)-3-{1-[Bis-(4-fluoro-phenyl)-methyl]-1H-i...)Show SMILES OC(=O)CCCOc1ccccc1NC(=O)\C=C(/C1CC1)c1ccc2n(ccc2c1)C(c1ccc(F)cc1)c1ccc(F)cc1 Show InChI InChI=1S/C37H32F2N2O4/c38-29-14-9-25(10-15-29)37(26-11-16-30(39)17-12-26)41-20-19-28-22-27(13-18-33(28)41)31(24-7-8-24)23-35(42)40-32-4-1-2-5-34(32)45-21-3-6-36(43)44/h1-2,4-5,9-20,22-24,37H,3,6-8,21H2,(H,40,42)(H,43,44)/b31-23+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by ChEMBL
| Assay Description
5 alpha- reductase inhibitory activity |
J Med Chem 39: 5047-52 (1997)
Article DOI: 10.1021/jm9601819
BindingDB Entry DOI: 10.7270/Q2TQ6263 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50116423
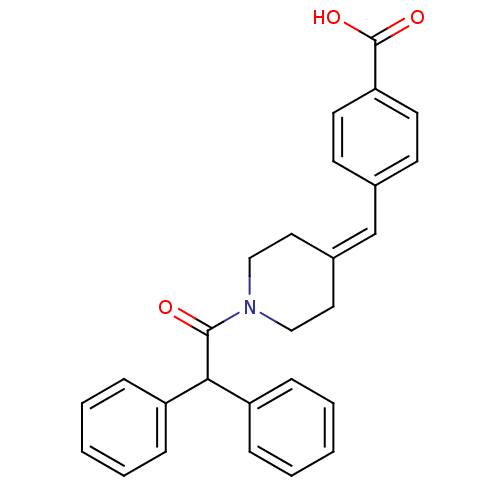
(4-(1-Diphenylacetyl-piperidin-4-ylidenemethyl)-ben...)Show SMILES [#8]-[#6](=O)-c1ccc(\[#6]=[#6]-2\[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#6](-c2ccccc2)-c2ccccc2)cc1 Show InChI InChI=1S/C27H25NO3/c29-26(25(22-7-3-1-4-8-22)23-9-5-2-6-10-23)28-17-15-21(16-18-28)19-20-11-13-24(14-12-20)27(30)31/h1-14,19,25H,15-18H2,(H,30,31) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of Steroid 5-alpha-reductase type I from rat ventral prostate (RVP) |
J Med Chem 45: 3406-17 (2002)
BindingDB Entry DOI: 10.7270/Q2T43TT8 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50055154
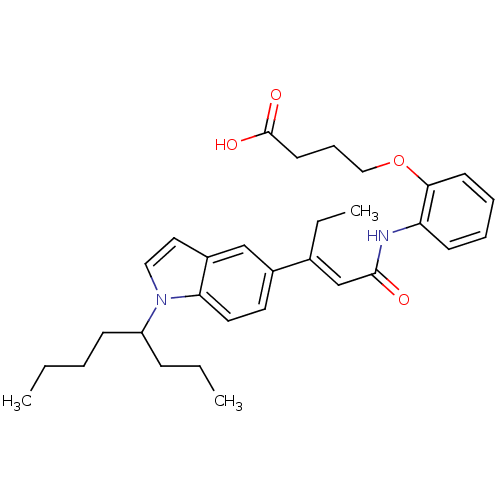
(4-(2-{(E)-3-[1-(1-Propyl-pentyl)-1H-indol-5-yl]-pe...)Show SMILES CCCCC(CCC)n1ccc2cc(ccc12)C(\CC)=C\C(=O)Nc1ccccc1OCCCC(O)=O Show InChI InChI=1S/C31H40N2O4/c1-4-7-12-26(11-5-2)33-19-18-25-21-24(16-17-28(25)33)23(6-3)22-30(34)32-27-13-8-9-14-29(27)37-20-10-15-31(35)36/h8-9,13-14,16-19,21-22,26H,4-7,10-12,15,20H2,1-3H3,(H,32,34)(H,35,36)/b23-22+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by ChEMBL
| Assay Description
5 alpha- reductase inhibitory activity |
J Med Chem 39: 5047-52 (1997)
Article DOI: 10.1021/jm9601819
BindingDB Entry DOI: 10.7270/Q2TQ6263 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50032281

(4-(2-{(E)-3-[1-(1-Propyl-pentyl)-1H-indol-5-yl]-bu...)Show SMILES CCCCC(CCC)n1ccc2cc(ccc12)C(\C)=C\C(=O)Nc1ccccc1OCCCC(O)=O Show InChI InChI=1S/C30H38N2O4/c1-4-6-11-25(10-5-2)32-18-17-24-21-23(15-16-27(24)32)22(3)20-29(33)31-26-12-7-8-13-28(26)36-19-9-14-30(34)35/h7-8,12-13,15-18,20-21,25H,4-6,9-11,14,19H2,1-3H3,(H,31,33)(H,34,35)/b22-20+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by ChEMBL
| Assay Description
5 alpha- reductase inhibitory activity |
J Med Chem 39: 5047-52 (1997)
Article DOI: 10.1021/jm9601819
BindingDB Entry DOI: 10.7270/Q2TQ6263 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50116418
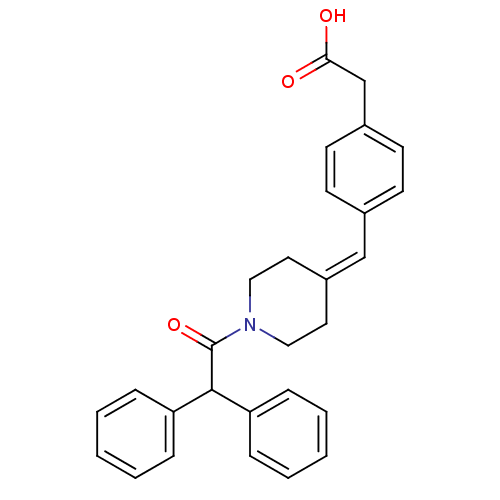
(CHEMBL323856 | [4-(1-Diphenylacetyl-piperidin-4-yl...)Show SMILES [#8]-[#6](=O)-[#6]-c1ccc(\[#6]=[#6]-2\[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#6](-c2ccccc2)-c2ccccc2)cc1 Show InChI InChI=1S/C28H27NO3/c30-26(31)20-22-13-11-21(12-14-22)19-23-15-17-29(18-16-23)28(32)27(24-7-3-1-4-8-24)25-9-5-2-6-10-25/h1-14,19,27H,15-18,20H2,(H,30,31) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of Steroid 5-alpha-reductase type I from rat ventral prostate (RVP) |
J Med Chem 45: 3406-17 (2002)
BindingDB Entry DOI: 10.7270/Q2T43TT8 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50055146
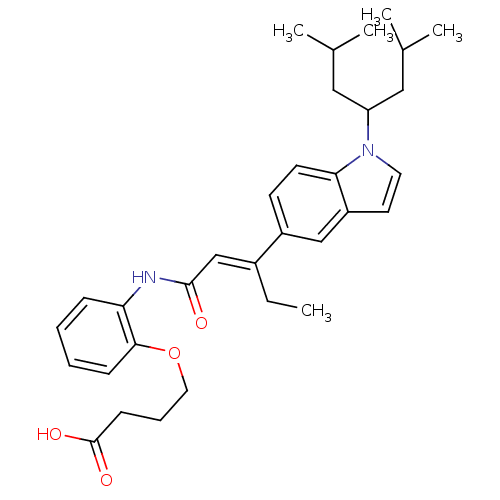
(4-(2-{(E)-3-[1-(1-Isobutyl-3-methyl-butyl)-1H-indo...)Show SMILES CC\C(=C/C(=O)Nc1ccccc1OCCCC(O)=O)c1ccc2n(ccc2c1)C(CC(C)C)CC(C)C Show InChI InChI=1S/C32H42N2O4/c1-6-24(21-31(35)33-28-10-7-8-11-30(28)38-17-9-12-32(36)37)25-13-14-29-26(20-25)15-16-34(29)27(18-22(2)3)19-23(4)5/h7-8,10-11,13-16,20-23,27H,6,9,12,17-19H2,1-5H3,(H,33,35)(H,36,37)/b24-21+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by ChEMBL
| Assay Description
5 alpha- reductase inhibitory activity |
J Med Chem 39: 5047-52 (1997)
Article DOI: 10.1021/jm9601819
BindingDB Entry DOI: 10.7270/Q2TQ6263 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50055152
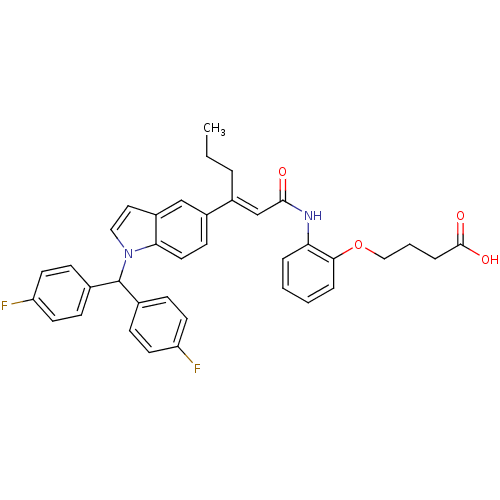
(4-[2-((E)-3-{1-[Bis-(4-fluoro-phenyl)-methyl]-1H-i...)Show SMILES CCC\C(=C/C(=O)Nc1ccccc1OCCCC(O)=O)c1ccc2n(ccc2c1)C(c1ccc(F)cc1)c1ccc(F)cc1 Show InChI InChI=1S/C37H34F2N2O4/c1-2-6-27(24-35(42)40-32-7-3-4-8-34(32)45-22-5-9-36(43)44)28-14-19-33-29(23-28)20-21-41(33)37(25-10-15-30(38)16-11-25)26-12-17-31(39)18-13-26/h3-4,7-8,10-21,23-24,37H,2,5-6,9,22H2,1H3,(H,40,42)(H,43,44)/b27-24+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by ChEMBL
| Assay Description
5 alpha- reductase inhibitory activity |
J Med Chem 39: 5047-52 (1997)
Article DOI: 10.1021/jm9601819
BindingDB Entry DOI: 10.7270/Q2TQ6263 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50032288
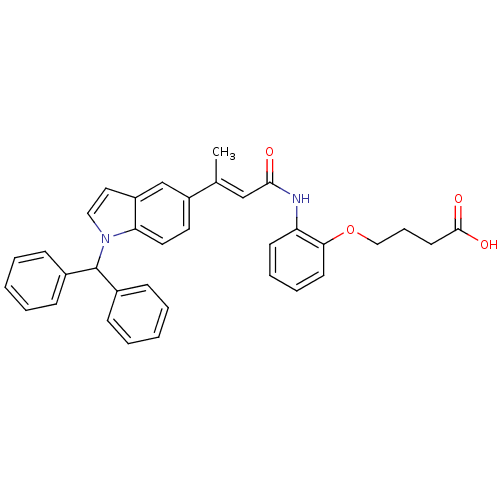
(4-{2-[(E)-3-(1-Benzhydryl-1H-indol-5-yl)-but-2-eno...)Show SMILES C\C(=C/C(=O)Nc1ccccc1OCCCC(O)=O)c1ccc2n(ccc2c1)C(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C35H32N2O4/c1-25(23-33(38)36-30-15-8-9-16-32(30)41-22-10-17-34(39)40)28-18-19-31-29(24-28)20-21-37(31)35(26-11-4-2-5-12-26)27-13-6-3-7-14-27/h2-9,11-16,18-21,23-24,35H,10,17,22H2,1H3,(H,36,38)(H,39,40)/b25-23+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by ChEMBL
| Assay Description
5 alpha- reductase inhibitory activity |
J Med Chem 39: 5047-52 (1997)
Article DOI: 10.1021/jm9601819
BindingDB Entry DOI: 10.7270/Q2TQ6263 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50116426

(4-[1-(Adamantane-1-carbonyl)-piperidin-4-ylideneme...)Show SMILES [#8]-[#6](=O)-c1ccc(\[#6]=[#6]-2/[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)C23[#6]-[#6]-4-[#6]-[#6](-[#6]-[#6](-[#6]-4)-[#6]2)-[#6]3)cc1 |TLB:19:20:24:17.18.23,23:22:25:17.18.19,23:18:25:24.22.21,THB:19:18:24:25.20.21| Show InChI InChI=1S/C24H29NO3/c26-22(27)21-3-1-16(2-4-21)9-17-5-7-25(8-6-17)23(28)24-13-18-10-19(14-24)12-20(11-18)15-24/h1-4,9,18-20H,5-8,10-15H2,(H,26,27) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of Steroid 5-alpha-reductase type I from rat ventral prostate (RVP) |
J Med Chem 45: 3406-17 (2002)
BindingDB Entry DOI: 10.7270/Q2T43TT8 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50032264
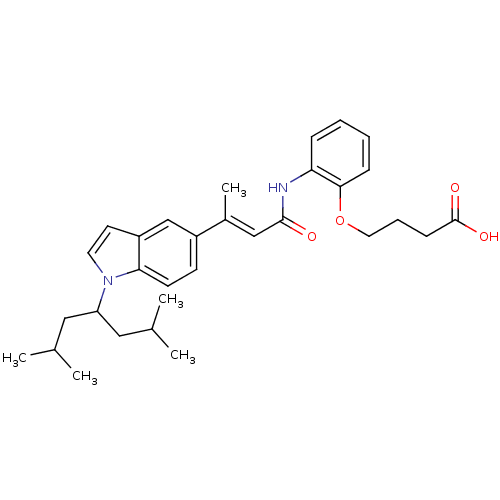
(4-(2-{(E)-3-[1-(1-Isobutyl-3-methyl-butyl)-1H-indo...)Show SMILES CC(C)CC(CC(C)C)n1ccc2cc(ccc12)C(\C)=C\C(=O)Nc1ccccc1OCCCC(O)=O Show InChI InChI=1S/C31H40N2O4/c1-21(2)17-26(18-22(3)4)33-15-14-25-20-24(12-13-28(25)33)23(5)19-30(34)32-27-9-6-7-10-29(27)37-16-8-11-31(35)36/h6-7,9-10,12-15,19-22,26H,8,11,16-18H2,1-5H3,(H,32,34)(H,35,36)/b23-19+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by ChEMBL
| Assay Description
5 alpha- reductase inhibitory activity |
J Med Chem 39: 5047-52 (1997)
Article DOI: 10.1021/jm9601819
BindingDB Entry DOI: 10.7270/Q2TQ6263 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50025356
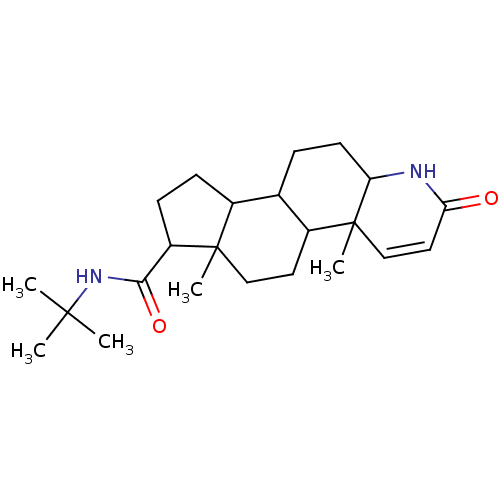
(4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10...)Show SMILES CC(C)(C)NC(=O)C1CCC2C3CCC4NC(=O)C=CC4(C)C3CCC12C |c:18| Show InChI InChI=1S/C23H36N2O2/c1-21(2,3)25-20(27)17-8-7-15-14-6-9-18-23(5,13-11-19(26)24-18)16(14)10-12-22(15,17)4/h11,13-18H,6-10,12H2,1-5H3,(H,24,26)(H,25,27) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibitory potency against rat Steroid 5-alpha-reductase type 1 expressed in transformed yeast Saccharomyces cerevesiae |
Bioorg Med Chem Lett 6: 1997-2002 (1996)
Article DOI: 10.1016/0960-894X(96)00360-5
BindingDB Entry DOI: 10.7270/Q2K0748S |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50462939
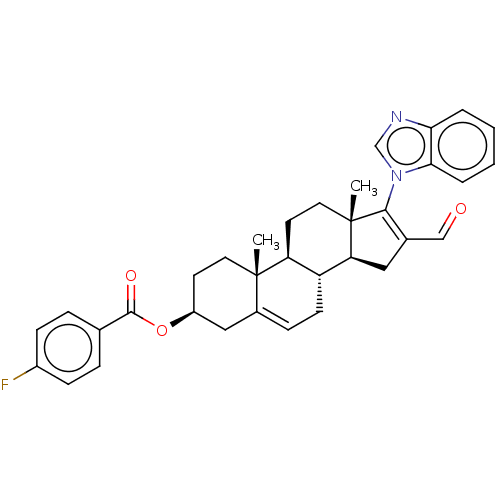
(CHEMBL4247033)Show SMILES [H][C@@]12CC(C=O)=C(n3cnc4ccccc34)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2C[C@H](CC[C@]12C)OC(=O)c1ccc(F)cc1 |r,t:5,29| Show InChI InChI=1S/C34H35FN2O3/c1-33-15-13-25(40-32(39)21-7-10-24(35)11-8-21)18-23(33)9-12-26-27(33)14-16-34(2)28(26)17-22(19-38)31(34)37-20-36-29-5-3-4-6-30(29)37/h3-11,19-20,25-28H,12-18H2,1-2H3/t25-,26+,27-,28-,33-,34-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Aut£noma de M£xico
Curated by ChEMBL
| Assay Description
Inhibition of rat liver microsomes 5alpha-reductase1 using [1,2,6,7-3H]T as substrate measured after 60 mins by chromatographic method |
Bioorg Med Chem 26: 4058-4064 (2018)
Article DOI: 10.1016/j.bmc.2018.06.030
BindingDB Entry DOI: 10.7270/Q20K2C7X |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50032315
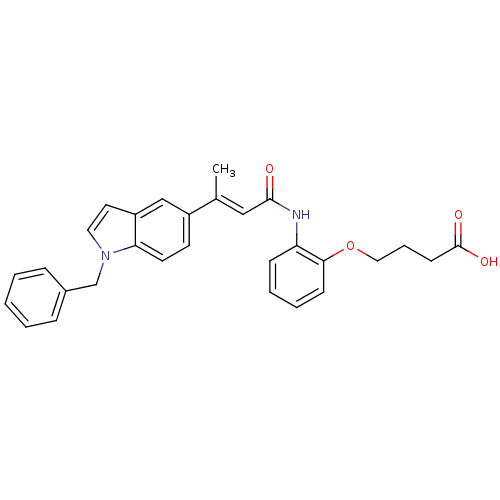
(4-{2-[(E)-3-(1-Benzyl-1H-indol-5-yl)-but-2-enoylam...)Show SMILES C\C(=C/C(=O)Nc1ccccc1OCCCC(O)=O)c1ccc2n(Cc3ccccc3)ccc2c1 Show InChI InChI=1S/C29H28N2O4/c1-21(18-28(32)30-25-10-5-6-11-27(25)35-17-7-12-29(33)34)23-13-14-26-24(19-23)15-16-31(26)20-22-8-3-2-4-9-22/h2-6,8-11,13-16,18-19H,7,12,17,20H2,1H3,(H,30,32)(H,33,34)/b21-18+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by ChEMBL
| Assay Description
5 alpha- reductase inhibitory activity |
J Med Chem 39: 5047-52 (1997)
Article DOI: 10.1021/jm9601819
BindingDB Entry DOI: 10.7270/Q2TQ6263 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50462938

(CHEMBL4238710)Show SMILES [H][C@@]12CC(C=O)=C(n3cnc4ccccc34)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2C[C@H](CC[C@]12C)OC(=O)c1ccc(Cl)cc1 |r,t:5,29| Show InChI InChI=1S/C34H35ClN2O3/c1-33-15-13-25(40-32(39)21-7-10-24(35)11-8-21)18-23(33)9-12-26-27(33)14-16-34(2)28(26)17-22(19-38)31(34)37-20-36-29-5-3-4-6-30(29)37/h3-11,19-20,25-28H,12-18H2,1-2H3/t25-,26+,27-,28-,33-,34-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Aut£noma de M£xico
Curated by ChEMBL
| Assay Description
Inhibition of rat liver microsomes 5alpha-reductase1 using [1,2,6,7-3H]T as substrate measured after 60 mins by chromatographic method |
Bioorg Med Chem 26: 4058-4064 (2018)
Article DOI: 10.1016/j.bmc.2018.06.030
BindingDB Entry DOI: 10.7270/Q20K2C7X |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50135045

(CHEMBL3735672)Show SMILES [H][C@@]12CC=C(C(=O)Cn3cncn3)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2C[C@H](CC[C@]12C)OC(=O)CC |r,t:3,25| Show InChI InChI=1S/C26H35N3O3/c1-4-24(31)32-18-9-11-25(2)17(13-18)5-6-19-20-7-8-22(26(20,3)12-10-21(19)25)23(30)14-29-16-27-15-28-29/h5,8,15-16,18-21H,4,6-7,9-14H2,1-3H3/t18-,19-,20-,21-,25-,26-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Aut£noma de M£xico
Curated by ChEMBL
| Assay Description
Inhibition of rat liver type 1 5alpha-reductase assessed as transformation of testosterone to dihydrotestosterone |
Bioorg Med Chem 23: 7535-42 (2015)
Article DOI: 10.1016/j.bmc.2015.10.047
BindingDB Entry DOI: 10.7270/Q2G44S45 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50102597
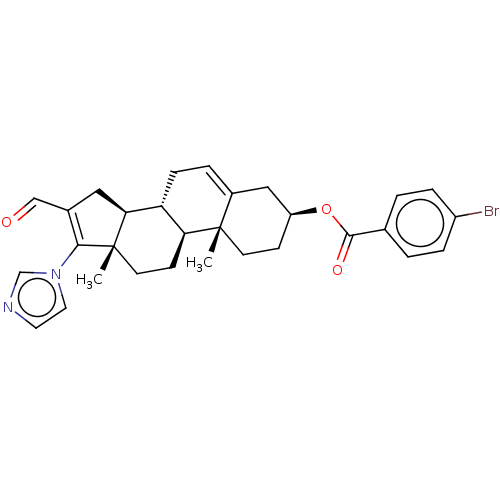
(CHEMBL3342904)Show SMILES [H][C@@]12CC(C=O)=C(n3ccnc3)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2C[C@H](CC[C@]12C)OC(=O)c1ccc(Br)cc1 |r,t:5,24| Show InChI InChI=1S/C30H33BrN2O3/c1-29-11-9-23(36-28(35)19-3-6-22(31)7-4-19)16-21(29)5-8-24-25(29)10-12-30(2)26(24)15-20(17-34)27(30)33-14-13-32-18-33/h3-7,13-14,17-18,23-26H,8-12,15-16H2,1-2H3/t23-,24+,25-,26-,29-,30-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Mexico
Curated by ChEMBL
| Assay Description
Inhibition of rat liver 5alpha-reductase type 1 assessed as conversion of [3H]testosterone to dihydrotestosterone |
Bioorg Med Chem 22: 6233-41 (2014)
Article DOI: 10.1016/j.bmc.2014.08.019
BindingDB Entry DOI: 10.7270/Q2K35WFD |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50135092
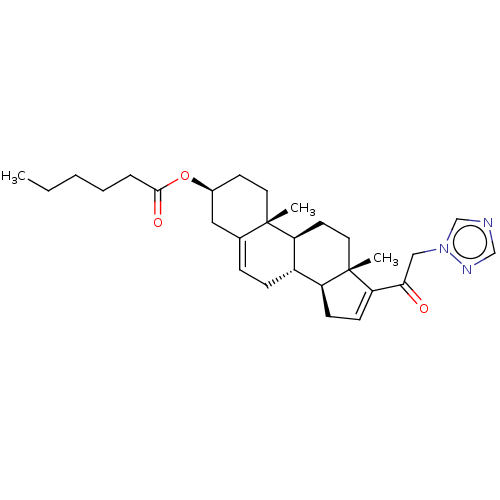
(CHEMBL3735149)Show SMILES [H][C@@]12CC=C(C(=O)Cn3cncn3)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2C[C@H](CC[C@]12C)OC(=O)CCCCC |r,t:3,25| Show InChI InChI=1S/C29H41N3O3/c1-4-5-6-7-27(34)35-21-12-14-28(2)20(16-21)8-9-22-23-10-11-25(29(23,3)15-13-24(22)28)26(33)17-32-19-30-18-31-32/h8,11,18-19,21-24H,4-7,9-10,12-17H2,1-3H3/t21-,22-,23-,24-,28-,29-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Aut£noma de M£xico
Curated by ChEMBL
| Assay Description
Inhibition of rat liver type 1 5alpha-reductase assessed as transformation of testosterone to dihydrotestosterone |
Bioorg Med Chem 23: 7535-42 (2015)
Article DOI: 10.1016/j.bmc.2015.10.047
BindingDB Entry DOI: 10.7270/Q2G44S45 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50102596
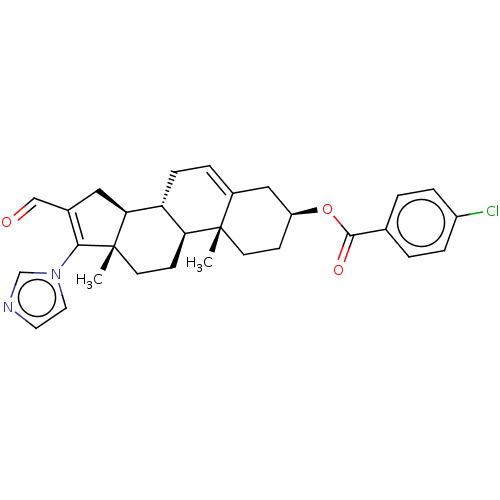
(CHEMBL3342903)Show SMILES [H][C@@]12CC(C=O)=C(n3ccnc3)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2C[C@H](CC[C@]12C)OC(=O)c1ccc(Cl)cc1 |r,t:5,24| Show InChI InChI=1S/C30H33ClN2O3/c1-29-11-9-23(36-28(35)19-3-6-22(31)7-4-19)16-21(29)5-8-24-25(29)10-12-30(2)26(24)15-20(17-34)27(30)33-14-13-32-18-33/h3-7,13-14,17-18,23-26H,8-12,15-16H2,1-2H3/t23-,24+,25-,26-,29-,30-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Mexico
Curated by ChEMBL
| Assay Description
Inhibition of rat liver 5alpha-reductase type 1 assessed as conversion of [3H]testosterone to dihydrotestosterone |
Bioorg Med Chem 22: 6233-41 (2014)
Article DOI: 10.1016/j.bmc.2014.08.019
BindingDB Entry DOI: 10.7270/Q2K35WFD |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50055145

(4-[2-((Z)-3-{1-[Bis-(4-fluoro-phenyl)-methyl]-1H-i...)Show SMILES CC\C(=C\C(=O)Nc1ccccc1OCCCC(O)=O)c1ccc2n(ccc2c1)C(c1ccc(F)cc1)c1ccc(F)cc1 Show InChI InChI=1S/C36H32F2N2O4/c1-2-24(23-34(41)39-31-6-3-4-7-33(31)44-21-5-8-35(42)43)27-13-18-32-28(22-27)19-20-40(32)36(25-9-14-29(37)15-10-25)26-11-16-30(38)17-12-26/h3-4,6-7,9-20,22-23,36H,2,5,8,21H2,1H3,(H,39,41)(H,42,43)/b24-23- | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by ChEMBL
| Assay Description
5 alpha- reductase inhibitory activity |
J Med Chem 39: 5047-52 (1997)
Article DOI: 10.1021/jm9601819
BindingDB Entry DOI: 10.7270/Q2TQ6263 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50055153
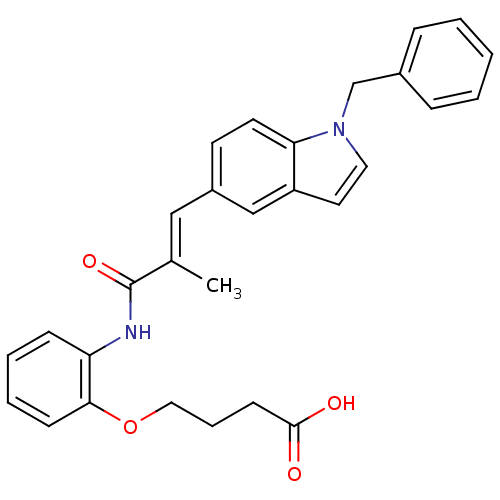
(4-{2-[(E)-3-(1-Benzyl-1H-indol-5-yl)-2-methyl-acry...)Show SMILES C\C(=C/c1ccc2n(Cc3ccccc3)ccc2c1)C(=O)Nc1ccccc1OCCCC(O)=O Show InChI InChI=1S/C29H28N2O4/c1-21(29(34)30-25-10-5-6-11-27(25)35-17-7-12-28(32)33)18-23-13-14-26-24(19-23)15-16-31(26)20-22-8-3-2-4-9-22/h2-6,8-11,13-16,18-19H,7,12,17,20H2,1H3,(H,30,34)(H,32,33)/b21-18+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by ChEMBL
| Assay Description
5 alpha- reductase inhibitory activity |
J Med Chem 39: 5047-52 (1997)
Article DOI: 10.1021/jm9601819
BindingDB Entry DOI: 10.7270/Q2TQ6263 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50046527

(CHEMBL3309454)Show InChI InChI=1S/C12H20O/c1-10-4-3-5-11(2)7-9-12(13)8-6-10/h4,7,12-13H,3,5-6,8-9H2,1-2H3/b10-4+,11-7+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Naresuan University
Curated by ChEMBL
| Assay Description
Inhibition of Sprague-Dawley rat liver steroid 5-alpha-reductase assessed as inhibition of testosterone conversion to dihydrotestosterone incubated f... |
Bioorg Med Chem Lett 24: 3526-9 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.057
BindingDB Entry DOI: 10.7270/Q2PG1TCW |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50102570

(CHEMBL3342897)Show SMILES [H][C@@]12CC(C=O)=C(n3ccnc3)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2C[C@H](CC[C@]12C)OC(=O)C1CC1 |r,t:5,24| Show InChI InChI=1S/C27H34N2O3/c1-26-9-7-20(32-25(31)17-3-4-17)14-19(26)5-6-21-22(26)8-10-27(2)23(21)13-18(15-30)24(27)29-12-11-28-16-29/h5,11-12,15-17,20-23H,3-4,6-10,13-14H2,1-2H3/t20-,21+,22-,23-,26-,27-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Mexico
Curated by ChEMBL
| Assay Description
Inhibition of rat liver 5alpha-reductase type 1 assessed as conversion of [3H]testosterone to dihydrotestosterone |
Bioorg Med Chem 22: 6233-41 (2014)
Article DOI: 10.1016/j.bmc.2014.08.019
BindingDB Entry DOI: 10.7270/Q2K35WFD |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50102573
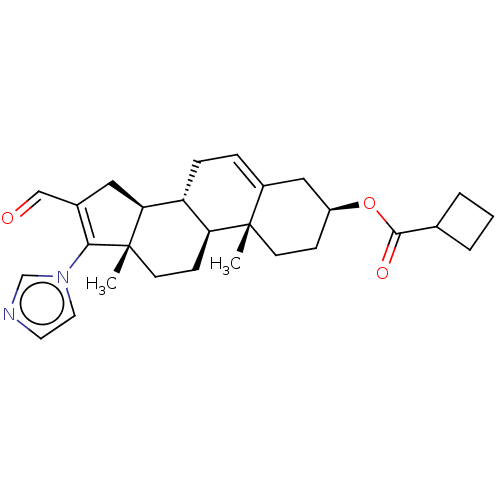
(CHEMBL3342898)Show SMILES [H][C@@]12CC(C=O)=C(n3ccnc3)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2C[C@H](CC[C@]12C)OC(=O)C1CCC1 |r,t:5,24| Show InChI InChI=1S/C28H36N2O3/c1-27-10-8-21(33-26(32)18-4-3-5-18)15-20(27)6-7-22-23(27)9-11-28(2)24(22)14-19(16-31)25(28)30-13-12-29-17-30/h6,12-13,16-18,21-24H,3-5,7-11,14-15H2,1-2H3/t21-,22+,23-,24-,27-,28-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Mexico
Curated by ChEMBL
| Assay Description
Inhibition of rat liver 5alpha-reductase type 1 assessed as conversion of [3H]testosterone to dihydrotestosterone |
Bioorg Med Chem 22: 6233-41 (2014)
Article DOI: 10.1016/j.bmc.2014.08.019
BindingDB Entry DOI: 10.7270/Q2K35WFD |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50102584

(CHEMBL3342899)Show SMILES [H][C@@]12CC(C=O)=C(n3ccnc3)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2C[C@H](CC[C@]12C)OC(=O)C1CCCC1 |r,t:5,24| Show InChI InChI=1S/C29H38N2O3/c1-28-11-9-22(34-27(33)19-5-3-4-6-19)16-21(28)7-8-23-24(28)10-12-29(2)25(23)15-20(17-32)26(29)31-14-13-30-18-31/h7,13-14,17-19,22-25H,3-6,8-12,15-16H2,1-2H3/t22-,23+,24-,25-,28-,29-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.65E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Mexico
Curated by ChEMBL
| Assay Description
Inhibition of rat liver 5alpha-reductase type 1 assessed as conversion of [3H]testosterone to dihydrotestosterone |
Bioorg Med Chem 22: 6233-41 (2014)
Article DOI: 10.1016/j.bmc.2014.08.019
BindingDB Entry DOI: 10.7270/Q2K35WFD |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50102592
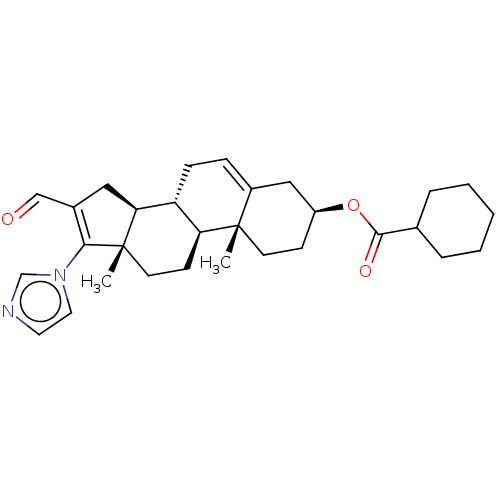
(CHEMBL3342900)Show SMILES [H][C@@]12CC(C=O)=C(n3ccnc3)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2C[C@H](CC[C@]12C)OC(=O)C1CCCCC1 |r,t:5,24| Show InChI InChI=1S/C30H40N2O3/c1-29-12-10-23(35-28(34)20-6-4-3-5-7-20)17-22(29)8-9-24-25(29)11-13-30(2)26(24)16-21(18-33)27(30)32-15-14-31-19-32/h8,14-15,18-20,23-26H,3-7,9-13,16-17H2,1-2H3/t23-,24+,25-,26-,29-,30-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Mexico
Curated by ChEMBL
| Assay Description
Inhibition of rat liver 5alpha-reductase type 1 assessed as conversion of [3H]testosterone to dihydrotestosterone |
Bioorg Med Chem 22: 6233-41 (2014)
Article DOI: 10.1016/j.bmc.2014.08.019
BindingDB Entry DOI: 10.7270/Q2K35WFD |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50102598
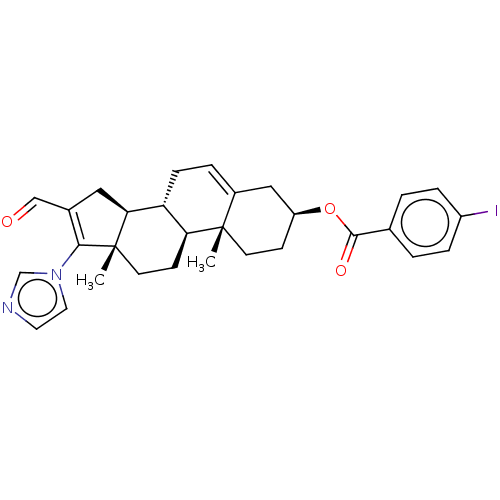
(CHEMBL3342905)Show SMILES [H][C@@]12CC(C=O)=C(n3ccnc3)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2C[C@H](CC[C@]12C)OC(=O)c1ccc(I)cc1 |r,t:5,24| Show InChI InChI=1S/C30H33IN2O3/c1-29-11-9-23(36-28(35)19-3-6-22(31)7-4-19)16-21(29)5-8-24-25(29)10-12-30(2)26(24)15-20(17-34)27(30)33-14-13-32-18-33/h3-7,13-14,17-18,23-26H,8-12,15-16H2,1-2H3/t23-,24+,25-,26-,29-,30-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Mexico
Curated by ChEMBL
| Assay Description
Inhibition of rat liver 5alpha-reductase type 1 assessed as conversion of [3H]testosterone to dihydrotestosterone |
Bioorg Med Chem 22: 6233-41 (2014)
Article DOI: 10.1016/j.bmc.2014.08.019
BindingDB Entry DOI: 10.7270/Q2K35WFD |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50102595
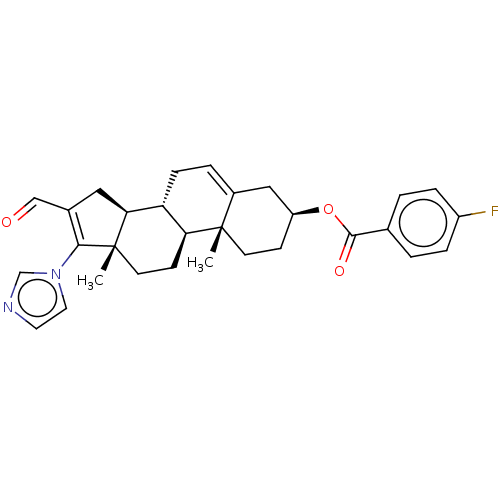
(CHEMBL3342902)Show SMILES [H][C@@]12CC(C=O)=C(n3ccnc3)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2C[C@H](CC[C@]12C)OC(=O)c1ccc(F)cc1 |r,t:5,24| Show InChI InChI=1S/C30H33FN2O3/c1-29-11-9-23(36-28(35)19-3-6-22(31)7-4-19)16-21(29)5-8-24-25(29)10-12-30(2)26(24)15-20(17-34)27(30)33-14-13-32-18-33/h3-7,13-14,17-18,23-26H,8-12,15-16H2,1-2H3/t23-,24+,25-,26-,29-,30-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Mexico
Curated by ChEMBL
| Assay Description
Inhibition of rat liver 5alpha-reductase type 1 assessed as conversion of [3H]testosterone to dihydrotestosterone |
Bioorg Med Chem 22: 6233-41 (2014)
Article DOI: 10.1016/j.bmc.2014.08.019
BindingDB Entry DOI: 10.7270/Q2K35WFD |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50135090

(CHEMBL3735678)Show SMILES [H][C@@]12CC=C(C(=O)Cn3cncn3)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2C[C@H](CC[C@]12C)OC(=O)CCCC |r,t:3,25| Show InChI InChI=1S/C28H39N3O3/c1-4-5-6-26(33)34-20-11-13-27(2)19(15-20)7-8-21-22-9-10-24(28(22,3)14-12-23(21)27)25(32)16-31-18-29-17-30-31/h7,10,17-18,20-23H,4-6,8-9,11-16H2,1-3H3/t20-,21-,22-,23-,27-,28-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Aut£noma de M£xico
Curated by ChEMBL
| Assay Description
Inhibition of rat liver type 1 5alpha-reductase assessed as transformation of testosterone to dihydrotestosterone |
Bioorg Med Chem 23: 7535-42 (2015)
Article DOI: 10.1016/j.bmc.2015.10.047
BindingDB Entry DOI: 10.7270/Q2G44S45 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50102593
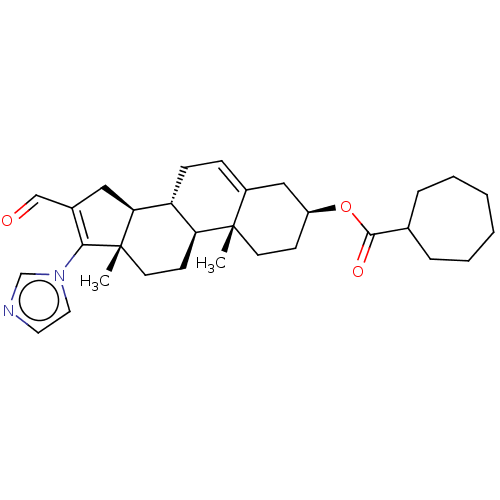
(CHEMBL3342901)Show SMILES [H][C@@]12CC(C=O)=C(n3ccnc3)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2C[C@H](CC[C@]12C)OC(=O)C1CCCCCC1 |r,t:5,24| Show InChI InChI=1S/C31H42N2O3/c1-30-13-11-24(36-29(35)21-7-5-3-4-6-8-21)18-23(30)9-10-25-26(30)12-14-31(2)27(25)17-22(19-34)28(31)33-16-15-32-20-33/h9,15-16,19-21,24-27H,3-8,10-14,17-18H2,1-2H3/t24-,25+,26-,27-,30-,31-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Mexico
Curated by ChEMBL
| Assay Description
Inhibition of rat liver 5alpha-reductase type 1 assessed as conversion of [3H]testosterone to dihydrotestosterone |
Bioorg Med Chem 22: 6233-41 (2014)
Article DOI: 10.1016/j.bmc.2014.08.019
BindingDB Entry DOI: 10.7270/Q2K35WFD |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50334788

((17beta-(N-tert-butylcarbamoyl)-4-aza-5alpha-andro...)Show SMILES CC(C)(C)NC(=O)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4NC(=O)C=C[C@]4(C)[C@H]3CC[C@]12C |r,c:18| Show InChI InChI=1S/C23H36N2O2/c1-21(2,3)25-20(27)17-8-7-15-14-6-9-18-23(5,13-11-19(26)24-18)16(14)10-12-22(15,17)4/h11,13-18H,6-10,12H2,1-5H3,(H,24,26)(H,25,27)/t14-,15-,16-,17+,18+,22-,23+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Aut£noma de M£xico
Curated by ChEMBL
| Assay Description
Inhibition of rat liver type 1 5alpha-reductase assessed as transformation of testosterone to dihydrotestosterone |
Bioorg Med Chem 23: 7535-42 (2015)
Article DOI: 10.1016/j.bmc.2015.10.047
BindingDB Entry DOI: 10.7270/Q2G44S45 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data