 Found 158 hits of ic50 for UniProtKB: P56192
Found 158 hits of ic50 for UniProtKB: P56192 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50112575
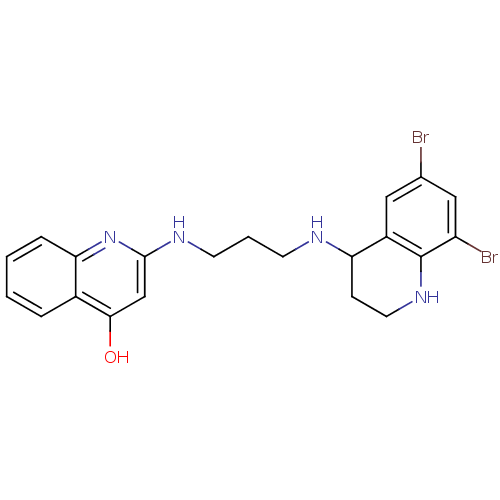
(2-[3-(6,8-Dibromo-1,2,3,4-tetrahydro-quinolin-4-yl...)Show SMILES Oc1cc(NCCCNC2CCNc3c(Br)cc(Br)cc23)nc2ccccc12 Show InChI InChI=1S/C21H22Br2N4O/c22-13-10-15-17(6-9-26-21(15)16(23)11-13)24-7-3-8-25-20-12-19(28)14-4-1-2-5-18(14)27-20/h1-2,4-5,10-12,17,24,26H,3,6-9H2,(H2,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against Methionyl-tRNA synthetase in a pyrophosphate exchange assay |
J Med Chem 45: 1959-62 (2002)
BindingDB Entry DOI: 10.7270/Q2HQ3Z6X |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50222901

(CHEMBL1163059)Show SMILES CSCC[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C15H23N7O7S2/c1-30-3-2-7(16)14(25)21-31(26,27)28-4-8-10(23)11(24)15(29-8)22-6-20-9-12(17)18-5-19-13(9)22/h5-8,10-11,15,23-24H,2-4,16H2,1H3,(H,21,25)(H2,17,18,19)/t7-,8+,10+,11+,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Escherichia coli Methionyl-tRNA synthetase was determined |
Bioorg Med Chem Lett 13: 1087-92 (2003)
BindingDB Entry DOI: 10.7270/Q29K4BRS |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50112577
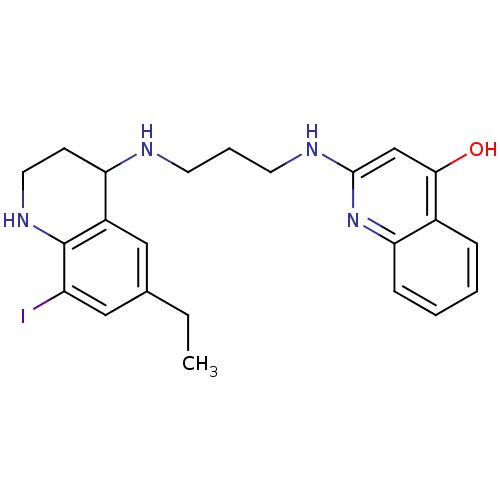
(2-[3-(6-Ethyl-8-iodo-1,2,3,4-tetrahydro-quinolin-4...)Show SMILES CCc1cc(I)c2NCCC(NCCCNc3cc(O)c4ccccc4n3)c2c1 Show InChI InChI=1S/C23H27IN4O/c1-2-15-12-17-19(8-11-27-23(17)18(24)13-15)25-9-5-10-26-22-14-21(29)16-6-3-4-7-20(16)28-22/h3-4,6-7,12-14,19,25,27H,2,5,8-11H2,1H3,(H2,26,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against Staphylococcus aureus Methionyl-tRNA synthetase |
J Med Chem 45: 1959-62 (2002)
BindingDB Entry DOI: 10.7270/Q2HQ3Z6X |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50143330
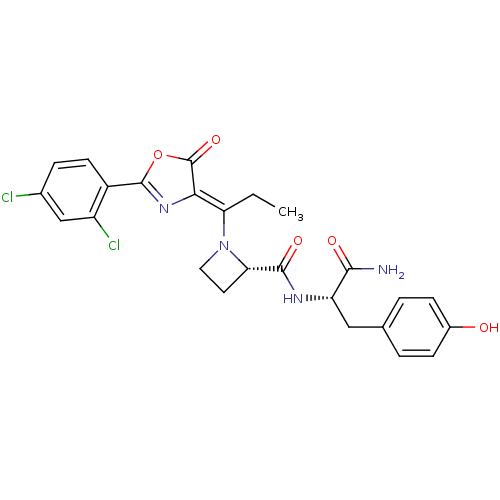
((S)-1-{1-[2-(2,4-Dichloro-phenyl)-5-oxo-oxazol-(4Z...)Show SMILES CC\C(N1CC[C@H]1C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)=C1\N=C(OC1=O)c1ccc(Cl)cc1Cl |c:25| Show InChI InChI=1S/C25H24Cl2N4O5/c1-2-19(21-25(35)36-24(30-21)16-8-5-14(26)12-17(16)27)31-10-9-20(31)23(34)29-18(22(28)33)11-13-3-6-15(32)7-4-13/h3-8,12,18,20,32H,2,9-11H2,1H3,(H2,28,33)(H,29,34)/b21-19-/t18-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 14: 1909-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.094
BindingDB Entry DOI: 10.7270/Q2NG4Q2F |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50143333
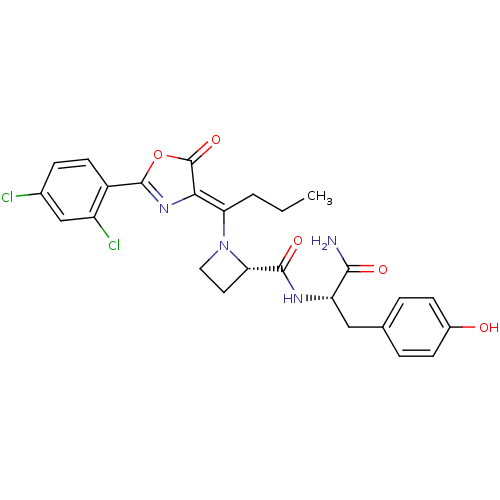
((S)-1-{1-[2-(2,4-Dichloro-phenyl)-5-oxo-oxazol-(4Z...)Show SMILES CCC\C(N1CC[C@H]1C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)=C1\N=C(OC1=O)c1ccc(Cl)cc1Cl |c:26| Show InChI InChI=1S/C26H26Cl2N4O5/c1-2-3-20(22-26(36)37-25(31-22)17-9-6-15(27)13-18(17)28)32-11-10-21(32)24(35)30-19(23(29)34)12-14-4-7-16(33)8-5-14/h4-9,13,19,21,33H,2-3,10-12H2,1H3,(H2,29,34)(H,30,35)/b22-20-/t19-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 14: 1909-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.094
BindingDB Entry DOI: 10.7270/Q2NG4Q2F |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50143329
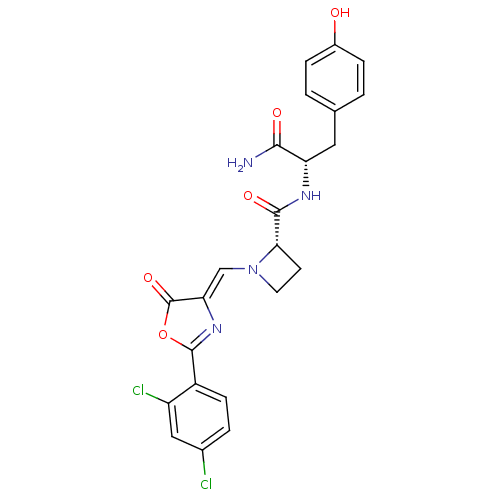
((S)-1-[2-(2,4-Dichloro-phenyl)-5-oxo-oxazol-(4Z)-y...)Show SMILES NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCN1\C=C1/N=C(OC1=O)c1ccc(Cl)cc1Cl |r,c:23| Show InChI InChI=1S/C23H20Cl2N4O5/c24-13-3-6-15(16(25)10-13)22-28-18(23(33)34-22)11-29-8-7-19(29)21(32)27-17(20(26)31)9-12-1-4-14(30)5-2-12/h1-6,10-11,17,19,30H,7-9H2,(H2,26,31)(H,27,32)/b18-11-/t17-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human methionyl-tRNA synthetase (fMetRS) |
Bioorg Med Chem Lett 14: 1909-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.094
BindingDB Entry DOI: 10.7270/Q2NG4Q2F |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50143334
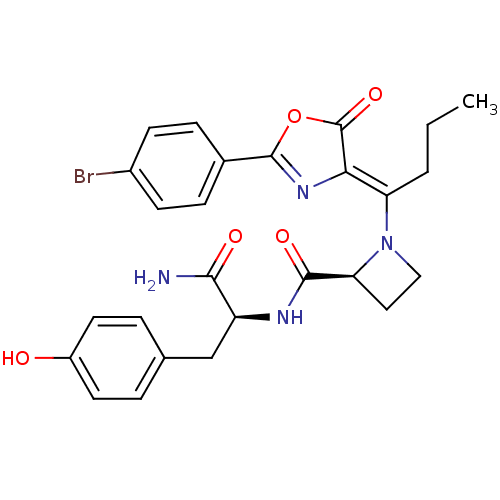
((S)-1-{1-[2-(4-Bromo-phenyl)-5-oxo-oxazol-(4Z)-yli...)Show SMILES CCC\C(N1CC[C@H]1C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)=C1\N=C(OC1=O)c1ccc(Br)cc1 |c:26| Show InChI InChI=1S/C26H27BrN4O5/c1-2-3-20(22-26(35)36-25(30-22)16-6-8-17(27)9-7-16)31-13-12-21(31)24(34)29-19(23(28)33)14-15-4-10-18(32)11-5-15/h4-11,19,21,32H,2-3,12-14H2,1H3,(H2,28,33)(H,29,34)/b22-20-/t19-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 14: 1909-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.094
BindingDB Entry DOI: 10.7270/Q2NG4Q2F |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129541
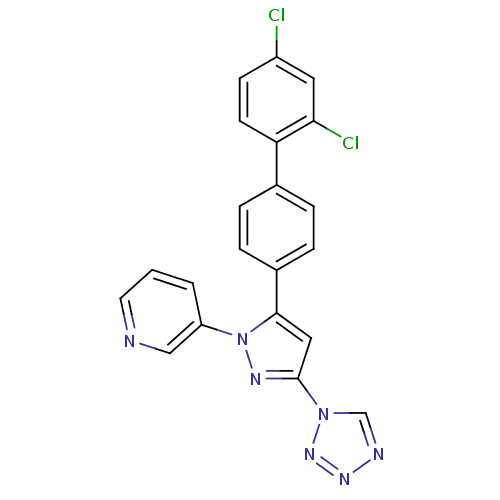
(3-[5-(2',4'-Dichloro-biphenyl-4-yl)-3-tetrazol-1-y...)Show SMILES Clc1ccc(c(Cl)c1)-c1ccc(cc1)-c1cc(nn1-c1cccnc1)-n1cnnn1 Show InChI InChI=1S/C21H13Cl2N7/c22-16-7-8-18(19(23)10-16)14-3-5-15(6-4-14)20-11-21(29-13-25-27-28-29)26-30(20)17-2-1-9-24-12-17/h1-13H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129527
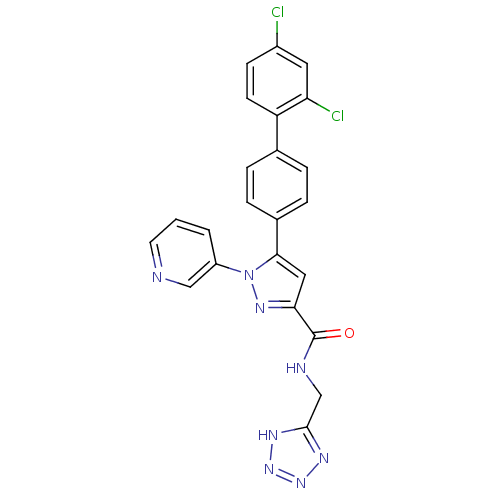
(5-(2',4'-Dichloro-biphenyl-4-yl)-1-pyridin-3-yl-1H...)Show SMILES Clc1ccc(c(Cl)c1)-c1ccc(cc1)-c1cc(nn1-c1cccnc1)C(=O)NCc1nnn[nH]1 Show InChI InChI=1S/C23H16Cl2N8O/c24-16-7-8-18(19(25)10-16)14-3-5-15(6-4-14)21-11-20(23(34)27-13-22-28-31-32-29-22)30-33(21)17-2-1-9-26-12-17/h1-12H,13H2,(H,27,34)(H,28,29,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human methionyl-tRNA synthetase (hMetRS). |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129528

(2-[5-(2',4'-Dichloro-biphenyl-4-yl)-3-tetrazol-1-y...)Show SMILES Clc1ccc(c(Cl)c1)-c1ccc(cc1)-c1cc(nn1-c1ccccn1)-n1cnnn1 Show InChI InChI=1S/C21H13Cl2N7/c22-16-8-9-17(18(23)11-16)14-4-6-15(7-5-14)19-12-21(29-13-25-27-28-29)26-30(19)20-3-1-2-10-24-20/h1-13H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50143331
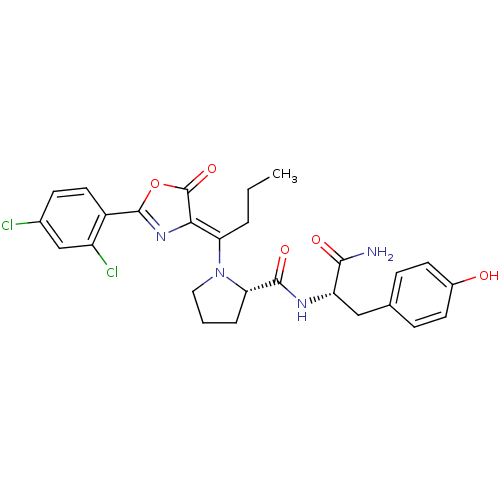
((S)-1-{1-[2-(2,4-Dichloro-phenyl)-5-oxo-oxazol-(4Z...)Show SMILES CCC\C(N1CCC[C@H]1C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)=C1\N=C(OC1=O)c1ccc(Cl)cc1Cl |c:27| Show InChI InChI=1S/C27H28Cl2N4O5/c1-2-4-21(23-27(37)38-26(32-23)18-11-8-16(28)14-19(18)29)33-12-3-5-22(33)25(36)31-20(24(30)35)13-15-6-9-17(34)10-7-15/h6-11,14,20,22,34H,2-5,12-13H2,1H3,(H2,30,35)(H,31,36)/b23-21-/t20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 14: 1909-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.094
BindingDB Entry DOI: 10.7270/Q2NG4Q2F |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50143337
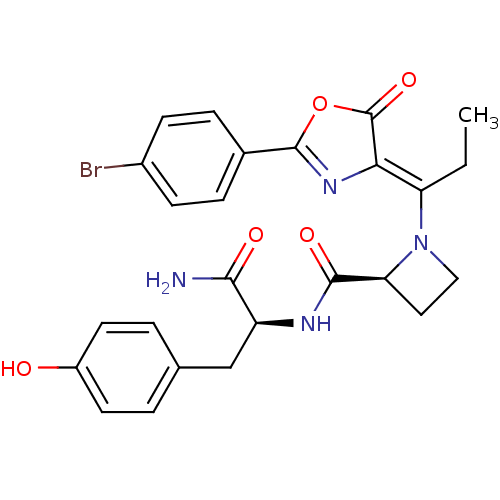
((S)-1-{1-[2-(4-Bromo-phenyl)-5-oxo-oxazol-(4Z)-yli...)Show SMILES CC\C(N1CC[C@H]1C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)=C1\N=C(OC1=O)c1ccc(Br)cc1 |c:25| Show InChI InChI=1S/C25H25BrN4O5/c1-2-19(21-25(34)35-24(29-21)15-5-7-16(26)8-6-15)30-12-11-20(30)23(33)28-18(22(27)32)13-14-3-9-17(31)10-4-14/h3-10,18,20,31H,2,11-13H2,1H3,(H2,27,32)(H,28,33)/b21-19-/t18-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human methionyl-tRNA synthetase (fMetRS) |
Bioorg Med Chem Lett 14: 1909-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.094
BindingDB Entry DOI: 10.7270/Q2NG4Q2F |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50143332
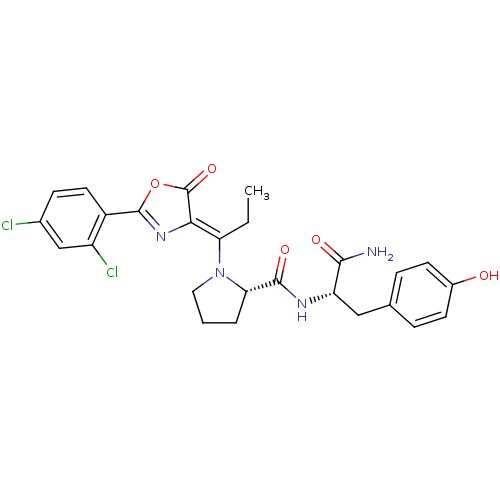
((S)-1-{1-[2-(2,4-Dichloro-phenyl)-5-oxo-oxazol-(4Z...)Show SMILES CC\C(N1CCC[C@H]1C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)=C1\N=C(OC1=O)c1ccc(Cl)cc1Cl |c:26| Show InChI InChI=1S/C26H26Cl2N4O5/c1-2-20(22-26(36)37-25(31-22)17-10-7-15(27)13-18(17)28)32-11-3-4-21(32)24(35)30-19(23(29)34)12-14-5-8-16(33)9-6-14/h5-10,13,19,21,33H,2-4,11-12H2,1H3,(H2,29,34)(H,30,35)/b22-20-/t19-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 14: 1909-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.094
BindingDB Entry DOI: 10.7270/Q2NG4Q2F |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129534

(5-(2',4'-Dichloro-biphenyl-4-yl)-1-pyridin-3-yl-1H...)Show SMILES OC(=O)c1cc(-c2ccc(cc2)-c2ccc(Cl)cc2Cl)n(n1)-c1cccnc1 Show InChI InChI=1S/C21H13Cl2N3O2/c22-15-7-8-17(18(23)10-15)13-3-5-14(6-4-13)20-11-19(21(27)28)25-26(20)16-2-1-9-24-12-16/h1-12H,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human methionyl-tRNA synthetase (hMetRS). |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129551
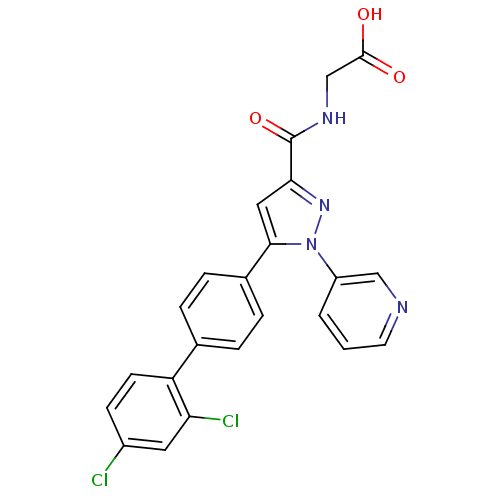
(CHEMBL74224 | {[5-(2',4'-Dichloro-biphenyl-4-yl)-1...)Show SMILES OC(=O)CNC(=O)c1cc(-c2ccc(cc2)-c2ccc(Cl)cc2Cl)n(n1)-c1cccnc1 Show InChI InChI=1S/C23H16Cl2N4O3/c24-16-7-8-18(19(25)10-16)14-3-5-15(6-4-14)21-11-20(23(32)27-13-22(30)31)28-29(21)17-2-1-9-26-12-17/h1-12H,13H2,(H,27,32)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50143343
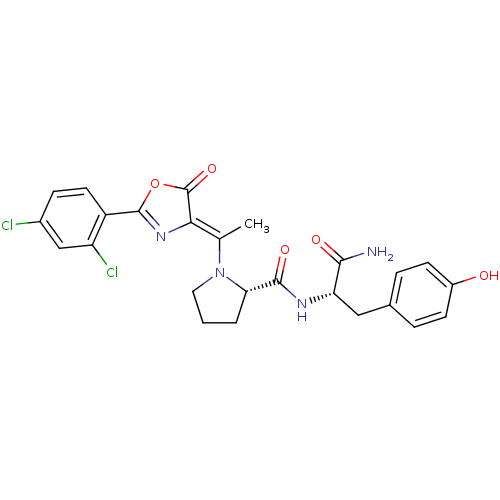
((S)-1-{1-[2-(2,4-Dichloro-phenyl)-5-oxo-oxazol-(4Z...)Show SMILES C\C(N1CCC[C@H]1C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)=C1\N=C(OC1=O)c1ccc(Cl)cc1Cl |c:25| Show InChI InChI=1S/C25H24Cl2N4O5/c1-13(21-25(35)36-24(30-21)17-9-6-15(26)12-18(17)27)31-10-2-3-20(31)23(34)29-19(22(28)33)11-14-4-7-16(32)8-5-14/h4-9,12,19-20,32H,2-3,10-11H2,1H3,(H2,28,33)(H,29,34)/b21-13-/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human methionyl-tRNA synthetase (fMetRS) |
Bioorg Med Chem Lett 14: 1909-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.094
BindingDB Entry DOI: 10.7270/Q2NG4Q2F |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50143341
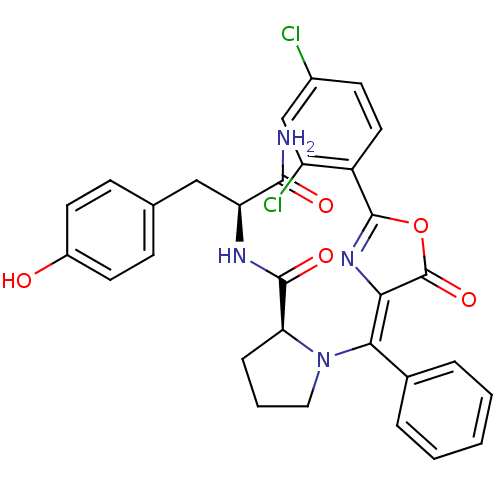
((S)-1-{[2-(2,4-Dichloro-phenyl)-5-oxo-oxazol-(4Z)-...)Show SMILES NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1\C(=C1/N=C(OC1=O)c1ccc(Cl)cc1Cl)c1ccccc1 |c:24| Show InChI InChI=1S/C30H26Cl2N4O5/c31-19-10-13-21(22(32)16-19)29-35-25(30(40)41-29)26(18-5-2-1-3-6-18)36-14-4-7-24(36)28(39)34-23(27(33)38)15-17-8-11-20(37)12-9-17/h1-3,5-6,8-13,16,23-24,37H,4,7,14-15H2,(H2,33,38)(H,34,39)/b26-25-/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 14: 1909-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.094
BindingDB Entry DOI: 10.7270/Q2NG4Q2F |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50112576
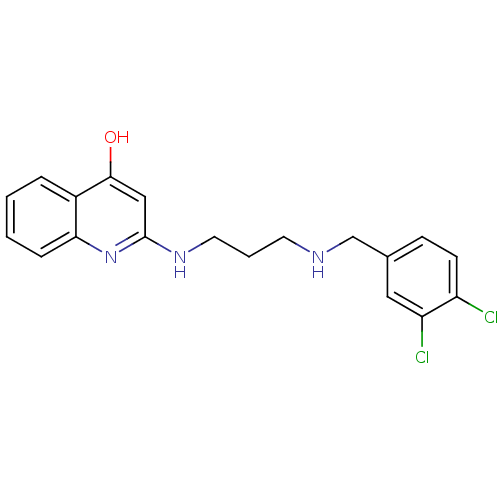
(2-(3-(3,4-dichlorobenzylamino)propylamino)quinolin...)Show InChI InChI=1S/C19H19Cl2N3O/c20-15-7-6-13(10-16(15)21)12-22-8-3-9-23-19-11-18(25)14-4-1-2-5-17(14)24-19/h1-2,4-7,10-11,22H,3,8-9,12H2,(H2,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against Methionyl-tRNA synthetase in a pyrophosphate exchange assay |
J Med Chem 45: 1959-62 (2002)
BindingDB Entry DOI: 10.7270/Q2HQ3Z6X |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129553
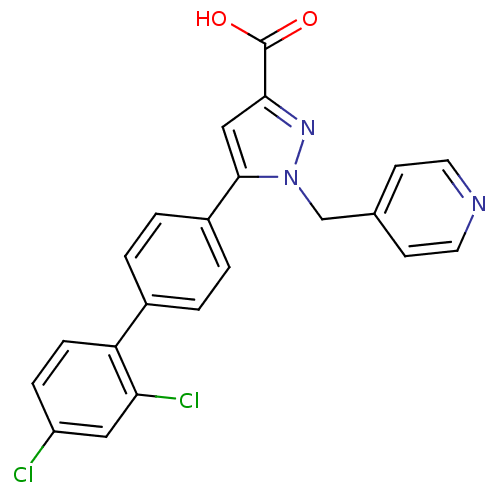
(5-(2',4'-Dichloro-biphenyl-4-yl)-1-pyridin-4-ylmet...)Show SMILES OC(=O)c1cc(-c2ccc(cc2)-c2ccc(Cl)cc2Cl)n(Cc2ccncc2)n1 Show InChI InChI=1S/C22H15Cl2N3O2/c23-17-5-6-18(19(24)11-17)15-1-3-16(4-2-15)21-12-20(22(28)29)26-27(21)13-14-7-9-25-10-8-14/h1-12H,13H2,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50143342
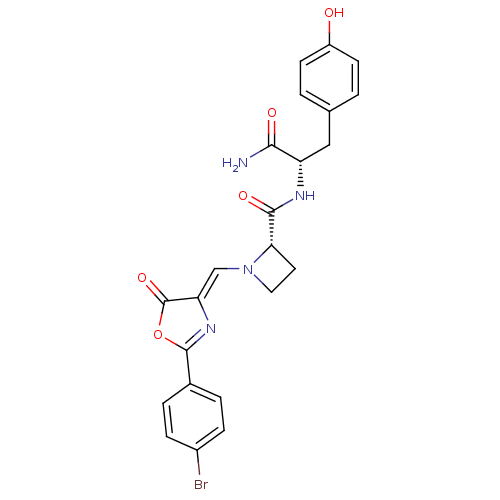
((S)-1-[2-(4-Bromo-phenyl)-5-oxo-oxazol-(4Z)-yliden...)Show SMILES NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCN1\C=C1/N=C(OC1=O)c1ccc(Br)cc1 |c:23| Show InChI InChI=1S/C23H21BrN4O5/c24-15-5-3-14(4-6-15)22-27-18(23(32)33-22)12-28-10-9-19(28)21(31)26-17(20(25)30)11-13-1-7-16(29)8-2-13/h1-8,12,17,19,29H,9-11H2,(H2,25,30)(H,26,31)/b18-12-/t17-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 14: 1909-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.094
BindingDB Entry DOI: 10.7270/Q2NG4Q2F |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129539
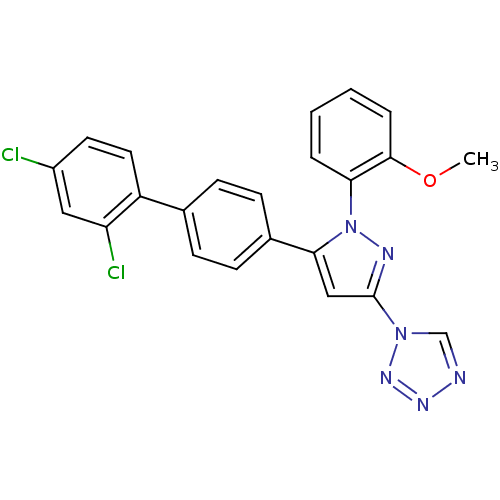
(1-[5-(2',4'-Dichloro-biphenyl-4-yl)-1-(2-methoxy-p...)Show SMILES COc1ccccc1-n1nc(cc1-c1ccc(cc1)-c1ccc(Cl)cc1Cl)-n1cnnn1 Show InChI InChI=1S/C23H16Cl2N6O/c1-32-22-5-3-2-4-20(22)31-21(13-23(27-31)30-14-26-28-29-30)16-8-6-15(7-9-16)18-11-10-17(24)12-19(18)25/h2-14H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Enterococci faecalis methionyl-tRNA synthetase (EfMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129546
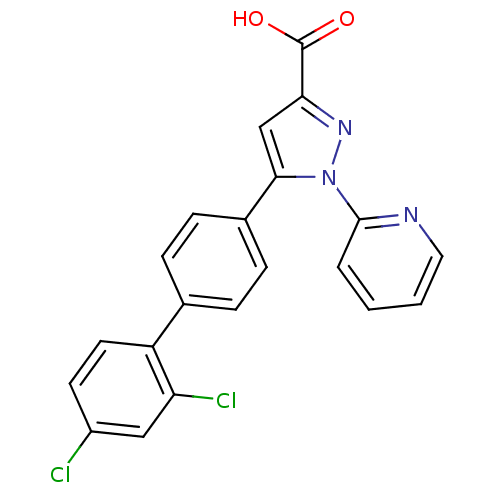
(5-(2',4'-Dichloro-biphenyl-4-yl)-1-pyridin-2-yl-1H...)Show SMILES OC(=O)c1cc(-c2ccc(cc2)-c2ccc(Cl)cc2Cl)n(n1)-c1ccccn1 Show InChI InChI=1S/C21H13Cl2N3O2/c22-15-8-9-16(17(23)11-15)13-4-6-14(7-5-13)19-12-18(21(27)28)25-26(19)20-3-1-2-10-24-20/h1-12H,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human methionyl-tRNA synthetase (hMetRS). |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129533
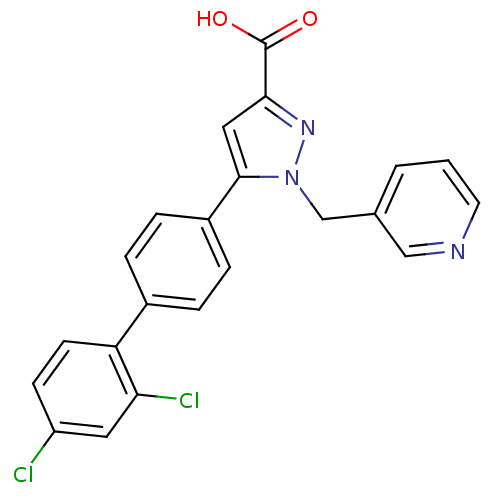
(5-(2',4'-Dichloro-biphenyl-4-yl)-1-pyridin-3-ylmet...)Show SMILES OC(=O)c1cc(-c2ccc(cc2)-c2ccc(Cl)cc2Cl)n(Cc2cccnc2)n1 Show InChI InChI=1S/C22H15Cl2N3O2/c23-17-7-8-18(19(24)10-17)15-3-5-16(6-4-15)21-11-20(22(28)29)26-27(21)13-14-2-1-9-25-12-14/h1-12H,13H2,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50143328
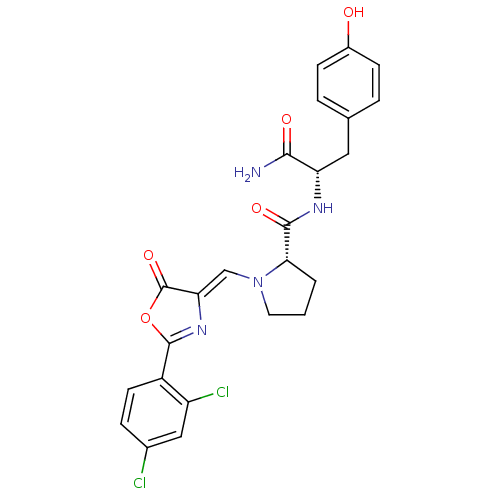
((S)-1-[2-(2,4-Dichloro-phenyl)-5-oxo-oxazol-(4Z)-y...)Show SMILES NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1\C=C1/N=C(OC1=O)c1ccc(Cl)cc1Cl |c:24| Show InChI InChI=1S/C24H22Cl2N4O5/c25-14-5-8-16(17(26)11-14)23-29-19(24(34)35-23)12-30-9-1-2-20(30)22(33)28-18(21(27)32)10-13-3-6-15(31)7-4-13/h3-8,11-12,18,20,31H,1-2,9-10H2,(H2,27,32)(H,28,33)/b19-12-/t18-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human methionyl-tRNA synthetase (fMetRS) |
Bioorg Med Chem Lett 14: 1909-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.094
BindingDB Entry DOI: 10.7270/Q2NG4Q2F |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50143329

((S)-1-[2-(2,4-Dichloro-phenyl)-5-oxo-oxazol-(4Z)-y...)Show SMILES NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCN1\C=C1/N=C(OC1=O)c1ccc(Cl)cc1Cl |r,c:23| Show InChI InChI=1S/C23H20Cl2N4O5/c24-13-3-6-15(16(25)10-13)22-28-18(23(33)34-22)11-29-8-7-19(29)21(32)27-17(20(26)31)9-12-1-4-14(30)5-2-12/h1-6,10-11,17,19,30H,7-9H2,(H2,26,31)(H,27,32)/b18-11-/t17-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Enterococci faecalis methionyl-tRNA synthetase (EfMetRS) |
Bioorg Med Chem Lett 14: 1909-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.094
BindingDB Entry DOI: 10.7270/Q2NG4Q2F |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50143339

((S)-1-{1-[2-(4-Bromo-phenyl)-5-oxo-oxazol-(4Z)-yli...)Show SMILES CCC\C(N1CCC[C@H]1C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)=C1\N=C(OC1=O)c1ccc(Br)cc1 |c:27| Show InChI InChI=1S/C27H29BrN4O5/c1-2-4-21(23-27(36)37-26(31-23)17-8-10-18(28)11-9-17)32-14-3-5-22(32)25(35)30-20(24(29)34)15-16-6-12-19(33)13-7-16/h6-13,20,22,33H,2-5,14-15H2,1H3,(H2,29,34)(H,30,35)/b23-21-/t20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 14: 1909-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.094
BindingDB Entry DOI: 10.7270/Q2NG4Q2F |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50143338
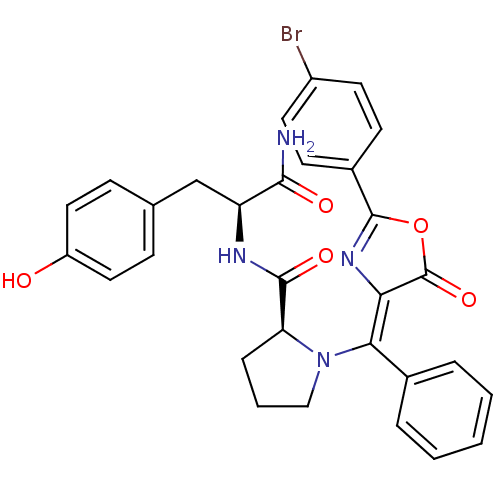
((S)-1-{[2-(4-Bromo-phenyl)-5-oxo-oxazol-(4Z)-ylide...)Show SMILES NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1\C(=C1/N=C(OC1=O)c1ccc(Br)cc1)c1ccccc1 |c:24| Show InChI InChI=1S/C30H27BrN4O5/c31-21-12-10-20(11-13-21)29-34-25(30(39)40-29)26(19-5-2-1-3-6-19)35-16-4-7-24(35)28(38)33-23(27(32)37)17-18-8-14-22(36)15-9-18/h1-3,5-6,8-15,23-24,36H,4,7,16-17H2,(H2,32,37)(H,33,38)/b26-25-/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 14: 1909-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.094
BindingDB Entry DOI: 10.7270/Q2NG4Q2F |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50143340
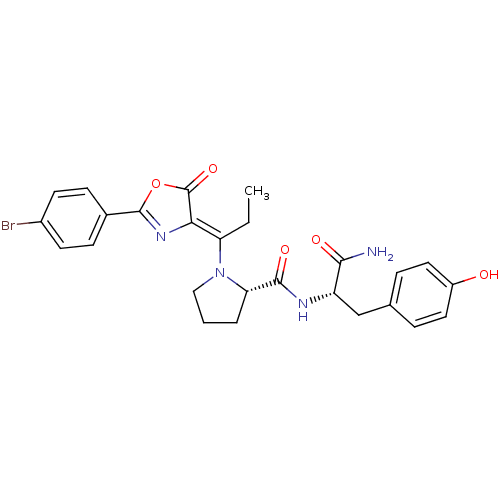
((S)-1-{1-[2-(4-Bromo-phenyl)-5-oxo-oxazol-(4Z)-yli...)Show SMILES CC\C(N1CCC[C@H]1C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)=C1\N=C(OC1=O)c1ccc(Br)cc1 |c:26| Show InChI InChI=1S/C26H27BrN4O5/c1-2-20(22-26(35)36-25(30-22)16-7-9-17(27)10-8-16)31-13-3-4-21(31)24(34)29-19(23(28)33)14-15-5-11-18(32)12-6-15/h5-12,19,21,32H,2-4,13-14H2,1H3,(H2,28,33)(H,29,34)/b22-20-/t19-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human methionyl-tRNA synthetase (fMetRS) |
Bioorg Med Chem Lett 14: 1909-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.094
BindingDB Entry DOI: 10.7270/Q2NG4Q2F |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129550
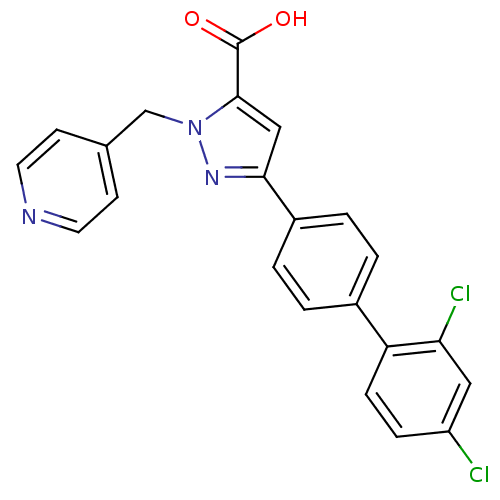
(5-(2',4'-Dichloro-biphenyl-4-yl)-2-pyridin-4-ylmet...)Show SMILES OC(=O)c1cc(nn1Cc1ccncc1)-c1ccc(cc1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C22H15Cl2N3O2/c23-17-5-6-18(19(24)11-17)15-1-3-16(4-2-15)20-12-21(22(28)29)27(26-20)13-14-7-9-25-10-8-14/h1-12H,13H2,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129532
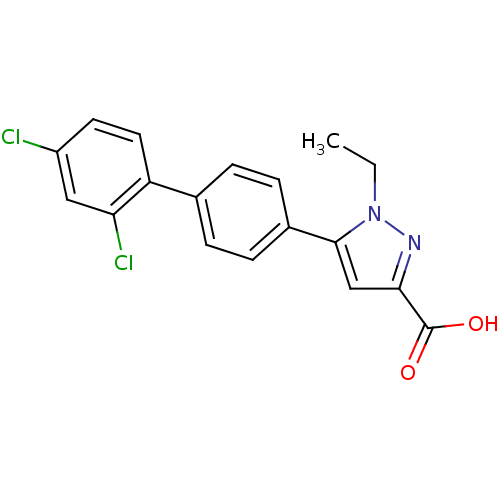
(5-(2',4'-Dichloro-biphenyl-4-yl)-1-ethyl-1H-pyrazo...)Show SMILES CCn1nc(cc1-c1ccc(cc1)-c1ccc(Cl)cc1Cl)C(O)=O Show InChI InChI=1S/C18H14Cl2N2O2/c1-2-22-17(10-16(21-22)18(23)24)12-5-3-11(4-6-12)14-8-7-13(19)9-15(14)20/h3-10H,2H2,1H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129543
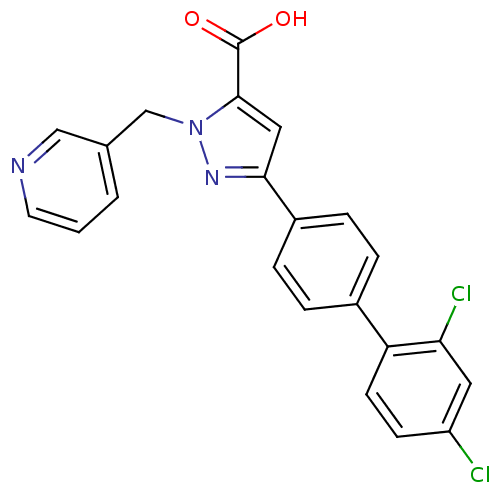
(5-(2',4'-Dichloro-biphenyl-4-yl)-2-pyridin-3-ylmet...)Show SMILES OC(=O)c1cc(nn1Cc1cccnc1)-c1ccc(cc1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C22H15Cl2N3O2/c23-17-7-8-18(19(24)10-17)15-3-5-16(6-4-15)20-11-21(22(28)29)27(26-20)13-14-2-1-9-25-12-14/h1-12H,13H2,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human methionyl-tRNA synthetase (hMetRS). |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50143336
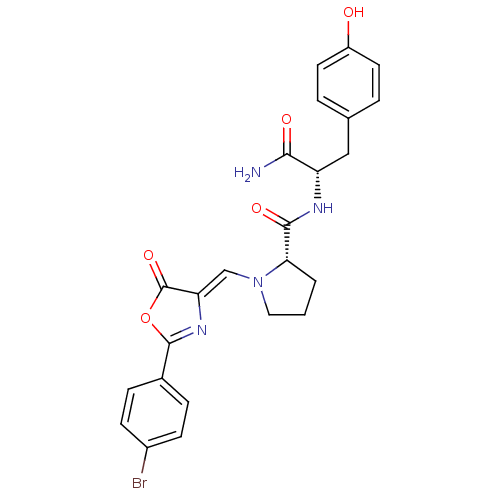
((S)-1-[2-(4-Bromo-phenyl)-5-oxo-oxazol-(4Z)-yliden...)Show SMILES NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1\C=C1/N=C(OC1=O)c1ccc(Br)cc1 |c:24| Show InChI InChI=1S/C24H23BrN4O5/c25-16-7-5-15(6-8-16)23-28-19(24(33)34-23)13-29-11-1-2-20(29)22(32)27-18(21(26)31)12-14-3-9-17(30)10-4-14/h3-10,13,18,20,30H,1-2,11-12H2,(H2,26,31)(H,27,32)/b19-13-/t18-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 14: 1909-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.094
BindingDB Entry DOI: 10.7270/Q2NG4Q2F |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50366861
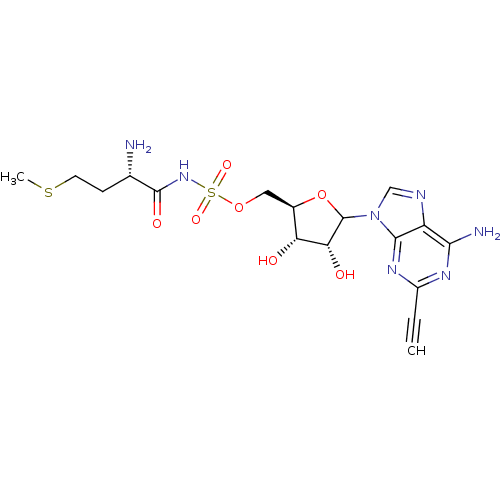
(CHEMBL605595)Show SMILES CSCC[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(N)nc(nc12)C#C |r| Show InChI InChI=1S/C17H23N7O7S2/c1-3-10-21-14(19)11-15(22-10)24(7-20-11)17-13(26)12(25)9(31-17)6-30-33(28,29)23-16(27)8(18)4-5-32-2/h1,7-9,12-13,17,25-26H,4-6,18H2,2H3,(H,23,27)(H2,19,21,22)/t8-,9+,12+,13+,17?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Escherichia coli Methionyl-tRNA synthetase was determined |
Bioorg Med Chem Lett 13: 1087-92 (2003)
BindingDB Entry DOI: 10.7270/Q29K4BRS |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129526
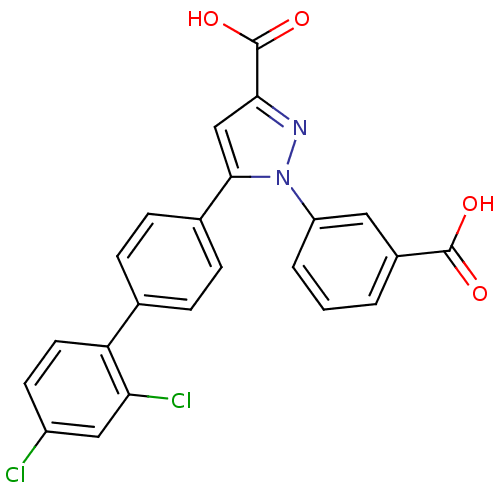
(1-(3-Carboxy-phenyl)-5-(2',4'-dichloro-biphenyl-4-...)Show SMILES OC(=O)c1cc(-c2ccc(cc2)-c2ccc(Cl)cc2Cl)n(n1)-c1cccc(c1)C(O)=O Show InChI InChI=1S/C23H14Cl2N2O4/c24-16-8-9-18(19(25)11-16)13-4-6-14(7-5-13)21-12-20(23(30)31)26-27(21)17-3-1-2-15(10-17)22(28)29/h1-12H,(H,28,29)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50366868

(CHEMBL605589)Show SMILES CSCC[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(N)nc(I)nc12 |r| Show InChI InChI=1S/C15H22IN7O7S2/c1-31-3-2-6(17)13(26)22-32(27,28)29-4-7-9(24)10(25)14(30-7)23-5-19-8-11(18)20-15(16)21-12(8)23/h5-7,9-10,14,24-25H,2-4,17H2,1H3,(H,22,26)(H2,18,20,21)/t6-,7+,9+,10+,14?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Escherichia coli Methionyl-tRNA synthetase was determined |
Bioorg Med Chem Lett 13: 1087-92 (2003)
BindingDB Entry DOI: 10.7270/Q29K4BRS |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50143332

((S)-1-{1-[2-(2,4-Dichloro-phenyl)-5-oxo-oxazol-(4Z...)Show SMILES CC\C(N1CCC[C@H]1C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)=C1\N=C(OC1=O)c1ccc(Cl)cc1Cl |c:26| Show InChI InChI=1S/C26H26Cl2N4O5/c1-2-20(22-26(36)37-25(31-22)17-10-7-15(27)13-18(17)28)32-11-3-4-21(32)24(35)30-19(23(29)34)12-14-5-8-16(33)9-6-14/h5-10,13,19,21,33H,2-4,11-12H2,1H3,(H2,29,34)(H,30,35)/b22-20-/t19-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Enterococci faecalis methionyl-tRNA synthetase (EfMetRS) |
Bioorg Med Chem Lett 14: 1909-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.094
BindingDB Entry DOI: 10.7270/Q2NG4Q2F |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129548
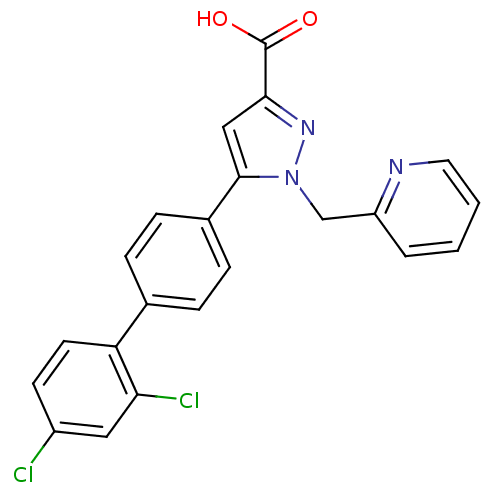
(5-(2',4'-Dichloro-biphenyl-4-yl)-1-pyridin-2-ylmet...)Show SMILES OC(=O)c1cc(-c2ccc(cc2)-c2ccc(Cl)cc2Cl)n(Cc2ccccn2)n1 Show InChI InChI=1S/C22H15Cl2N3O2/c23-16-8-9-18(19(24)11-16)14-4-6-15(7-5-14)21-12-20(22(28)29)26-27(21)13-17-3-1-2-10-25-17/h1-12H,13H2,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50143342

((S)-1-[2-(4-Bromo-phenyl)-5-oxo-oxazol-(4Z)-yliden...)Show SMILES NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCN1\C=C1/N=C(OC1=O)c1ccc(Br)cc1 |c:23| Show InChI InChI=1S/C23H21BrN4O5/c24-15-5-3-14(4-6-15)22-27-18(23(32)33-22)12-28-10-9-19(28)21(31)26-17(20(25)30)11-13-1-7-16(29)8-2-13/h1-8,12,17,19,29H,9-11H2,(H2,25,30)(H,26,31)/b18-12-/t17-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Enterococci faecalis methionyl-tRNA synthetase (EfMetRS) |
Bioorg Med Chem Lett 14: 1909-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.094
BindingDB Entry DOI: 10.7270/Q2NG4Q2F |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129544
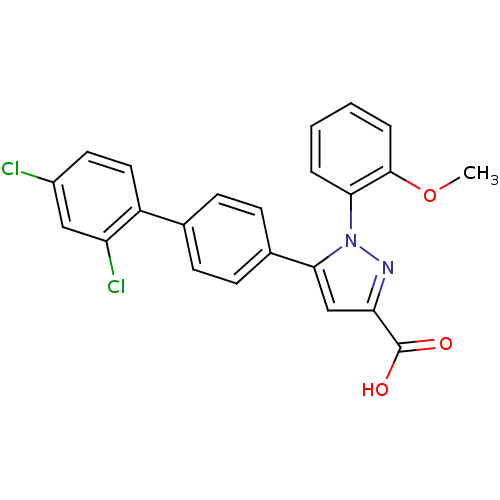
(5-(2',4'-Dichloro-biphenyl-4-yl)-1-(2-methoxy-phen...)Show SMILES COc1ccccc1-n1nc(cc1-c1ccc(cc1)-c1ccc(Cl)cc1Cl)C(O)=O Show InChI InChI=1S/C23H16Cl2N2O3/c1-30-22-5-3-2-4-20(22)27-21(13-19(26-27)23(28)29)15-8-6-14(7-9-15)17-11-10-16(24)12-18(17)25/h2-13H,1H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50143335
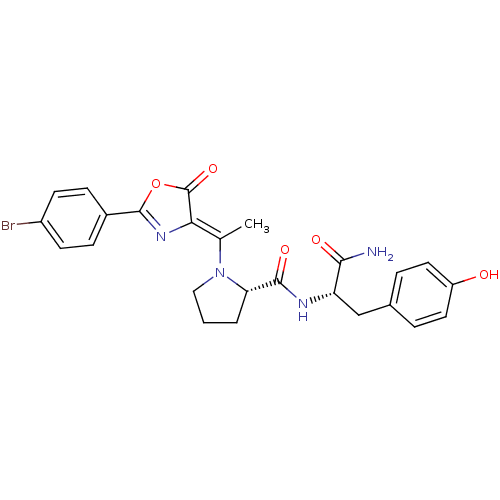
((S)-1-{1-[2-(4-Bromo-phenyl)-5-oxo-oxazol-(4Z)-yli...)Show SMILES C\C(N1CCC[C@H]1C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)=C1\N=C(OC1=O)c1ccc(Br)cc1 |c:25| Show InChI InChI=1S/C25H25BrN4O5/c1-14(21-25(34)35-24(29-21)16-6-8-17(26)9-7-16)30-12-2-3-20(30)23(33)28-19(22(27)32)13-15-4-10-18(31)11-5-15/h4-11,19-20,31H,2-3,12-13H2,1H3,(H2,27,32)(H,28,33)/b21-14-/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 14: 1909-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.094
BindingDB Entry DOI: 10.7270/Q2NG4Q2F |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129528

(2-[5-(2',4'-Dichloro-biphenyl-4-yl)-3-tetrazol-1-y...)Show SMILES Clc1ccc(c(Cl)c1)-c1ccc(cc1)-c1cc(nn1-c1ccccn1)-n1cnnn1 Show InChI InChI=1S/C21H13Cl2N7/c22-16-8-9-17(18(23)11-16)14-4-6-15(7-5-14)19-12-21(29-13-25-27-28-29)26-30(19)20-3-1-2-10-24-20/h1-13H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Enterococci faecalis methionyl-tRNA synthetase (EfMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50366863
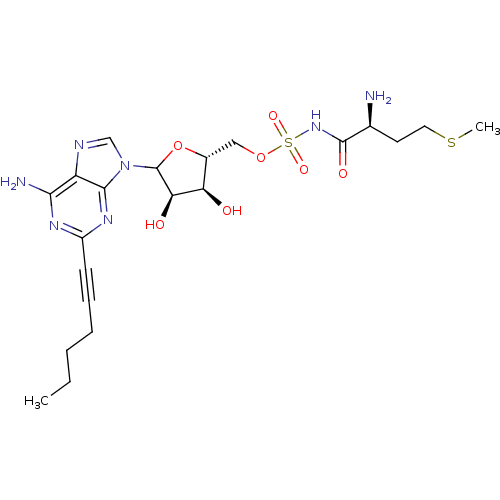
(CHEMBL605806)Show SMILES CCCCC#Cc1nc(N)c2ncn(C3O[C@H](COS(=O)(=O)NC(=O)[C@@H](N)CCSC)[C@@H](O)[C@H]3O)c2n1 |r| Show InChI InChI=1S/C21H31N7O7S2/c1-3-4-5-6-7-14-25-18(23)15-19(26-14)28(11-24-15)21-17(30)16(29)13(35-21)10-34-37(32,33)27-20(31)12(22)8-9-36-2/h11-13,16-17,21,29-30H,3-5,8-10,22H2,1-2H3,(H,27,31)(H2,23,25,26)/t12-,13+,16+,17+,21?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Escherichia coli Methionyl-tRNA synthetase was determined |
Bioorg Med Chem Lett 13: 1087-92 (2003)
BindingDB Entry DOI: 10.7270/Q29K4BRS |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129546

(5-(2',4'-Dichloro-biphenyl-4-yl)-1-pyridin-2-yl-1H...)Show SMILES OC(=O)c1cc(-c2ccc(cc2)-c2ccc(Cl)cc2Cl)n(n1)-c1ccccn1 Show InChI InChI=1S/C21H13Cl2N3O2/c22-15-8-9-16(17(23)11-15)13-4-6-14(7-5-13)19-12-18(21(27)28)25-26(19)20-3-1-2-10-24-20/h1-12H,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Enterococci faecalis methionyl-tRNA synthetase (EfMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
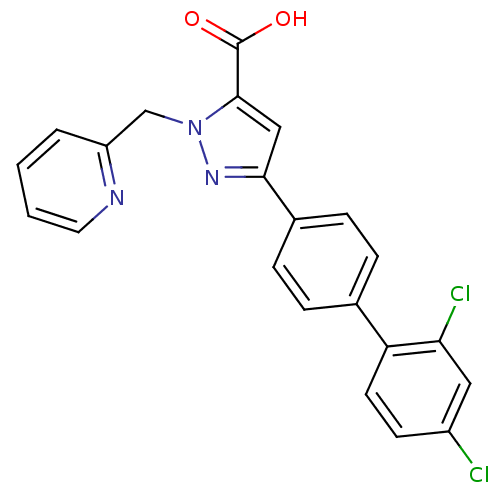
(Homo sapiens (Human)) | BDBM50129542
(5-(2',4'-Dichloro-biphenyl-4-yl)-2-pyridin-2-ylmet...)Show SMILES OC(=O)c1cc(nn1Cc1ccccn1)-c1ccc(cc1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C22H15Cl2N3O2/c23-16-8-9-18(19(24)11-16)14-4-6-15(7-5-14)20-12-21(22(28)29)27(26-20)13-17-3-1-2-10-25-17/h1-12H,13H2,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
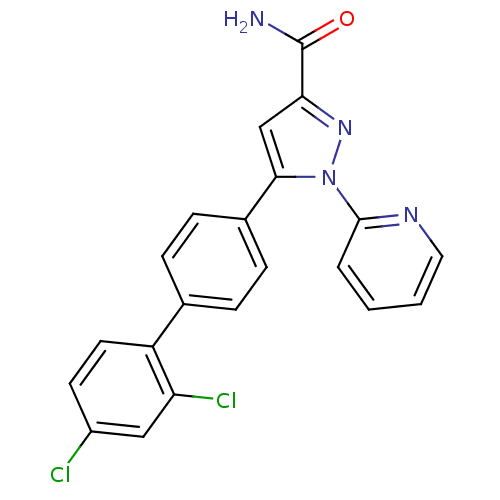
(Homo sapiens (Human)) | BDBM50129529
(5-(2',4'-Dichloro-biphenyl-4-yl)-1-pyridin-2-yl-1H...)Show SMILES NC(=O)c1cc(-c2ccc(cc2)-c2ccc(Cl)cc2Cl)n(n1)-c1ccccn1 Show InChI InChI=1S/C21H14Cl2N4O/c22-15-8-9-16(17(23)11-15)13-4-6-14(7-5-13)19-12-18(21(24)28)26-27(19)20-3-1-2-10-25-20/h1-12H,(H2,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
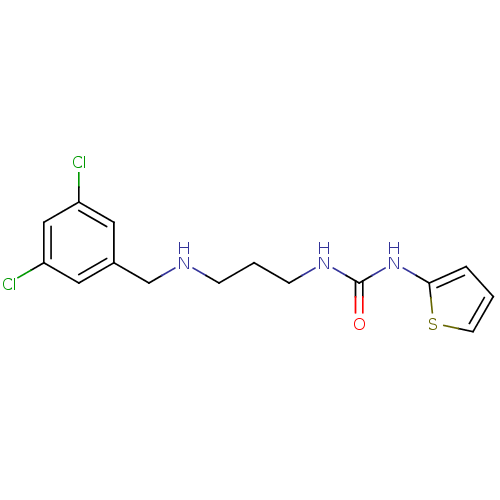
(Homo sapiens (Human)) | BDBM50393794
(CHEMBL2159537)Show InChI InChI=1S/C15H17Cl2N3OS/c16-12-7-11(8-13(17)9-12)10-18-4-2-5-19-15(21)20-14-3-1-6-22-14/h1,3,6-9,18H,2,4-5,10H2,(H2,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human mitochondrial MetRS aminoacylation activity assessed as reduction in [3H]methionine incorporation into Escherichia coli tRNA pre-... |
J Med Chem 55: 6342-51 (2012)
Article DOI: 10.1021/jm300303e
BindingDB Entry DOI: 10.7270/Q2SJ1MQH |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50143339

((S)-1-{1-[2-(4-Bromo-phenyl)-5-oxo-oxazol-(4Z)-yli...)Show SMILES CCC\C(N1CCC[C@H]1C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)=C1\N=C(OC1=O)c1ccc(Br)cc1 |c:27| Show InChI InChI=1S/C27H29BrN4O5/c1-2-4-21(23-27(36)37-26(31-23)17-8-10-18(28)11-9-17)32-14-3-5-22(32)25(35)30-20(24(29)34)15-16-6-12-19(33)13-7-16/h6-13,20,22,33H,2-5,14-15H2,1H3,(H2,29,34)(H,30,35)/b23-21-/t20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 14: 1909-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.094
BindingDB Entry DOI: 10.7270/Q2NG4Q2F |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129535
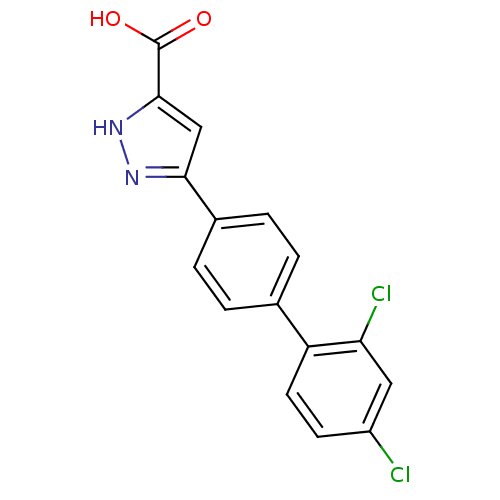
(5-(2',4'-Dichloro-biphenyl-4-yl)-1H-pyrazole-3-car...)Show SMILES OC(=O)c1cc(n[nH]1)-c1ccc(cc1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C16H10Cl2N2O2/c17-11-5-6-12(13(18)7-11)9-1-3-10(4-2-9)14-8-15(16(21)22)20-19-14/h1-8H,(H,19,20)(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129554
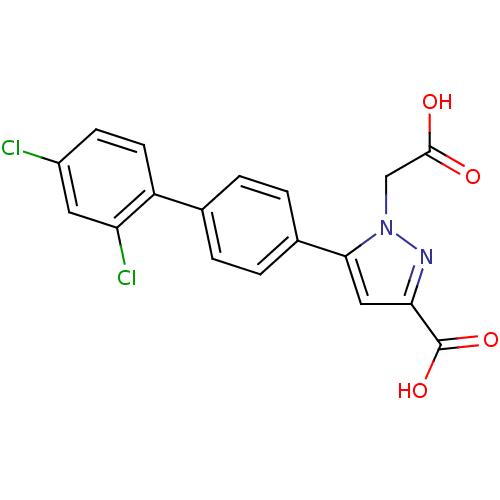
(1-Carboxymethyl-5-(2',4'-dichloro-biphenyl-4-yl)-1...)Show SMILES OC(=O)Cn1nc(cc1-c1ccc(cc1)-c1ccc(Cl)cc1Cl)C(O)=O Show InChI InChI=1S/C18H12Cl2N2O4/c19-12-5-6-13(14(20)7-12)10-1-3-11(4-2-10)16-8-15(18(25)26)21-22(16)9-17(23)24/h1-8H,9H2,(H,23,24)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50143330

((S)-1-{1-[2-(2,4-Dichloro-phenyl)-5-oxo-oxazol-(4Z...)Show SMILES CC\C(N1CC[C@H]1C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)=C1\N=C(OC1=O)c1ccc(Cl)cc1Cl |c:25| Show InChI InChI=1S/C25H24Cl2N4O5/c1-2-19(21-25(35)36-24(30-21)16-8-5-14(26)12-17(16)27)31-10-9-20(31)23(34)29-18(22(28)33)11-13-3-6-15(32)7-4-13/h3-8,12,18,20,32H,2,9-11H2,1H3,(H2,28,33)(H,29,34)/b21-19-/t18-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 14: 1909-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.094
BindingDB Entry DOI: 10.7270/Q2NG4Q2F |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data