 Found 199 hits of ic50 for UniProtKB: P14137
Found 199 hits of ic50 for UniProtKB: P14137 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM83720
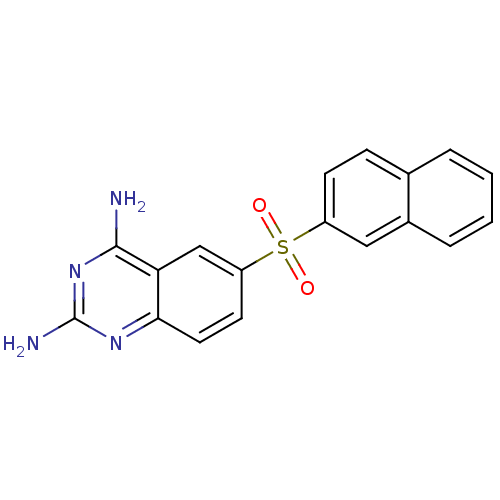
(6-(2-naphthalenylsulfonyl)quinazoline-2,4-diamine ...)Show SMILES Nc1nc(N)c2cc(ccc2n1)S(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C18H14N4O2S/c19-17-15-10-14(7-8-16(15)21-18(20)22-17)25(23,24)13-6-5-11-3-1-2-4-12(11)9-13/h1-10H,(H4,19,20,21,22) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antibacterial activity against Streptococcus faecalis assessed as growth inhibition |
J Med Chem 22: 1247-57 (1979)
Article DOI: 10.1021/jm00196a019
BindingDB Entry DOI: 10.7270/Q28S4SWQ |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50614282
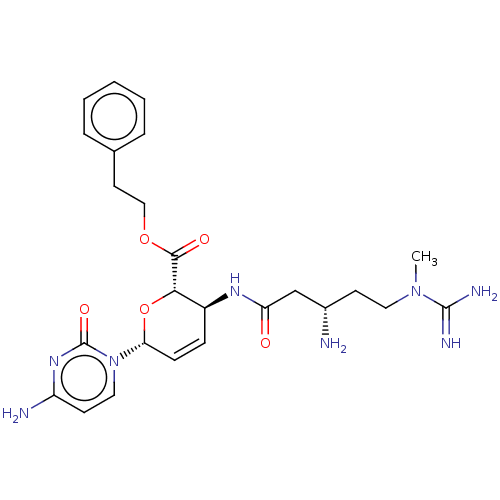
(CHEMBL5279964) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50614275
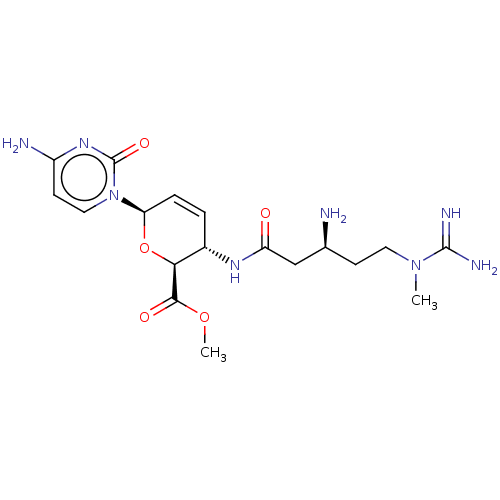
(CHEMBL5277226) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50614276
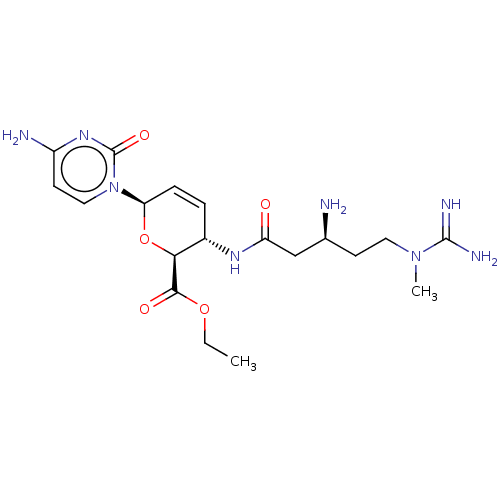
(CHEMBL5286888) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50614277
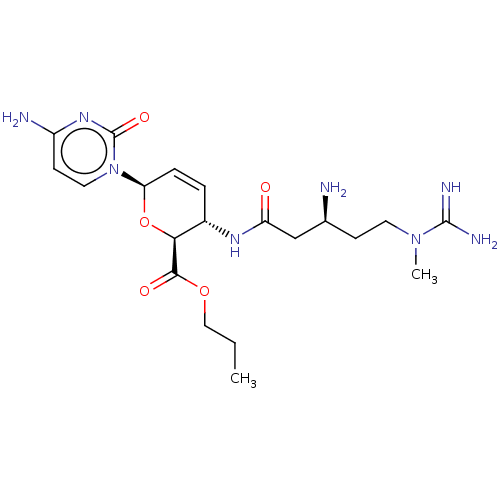
(CHEMBL5267440) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50614279
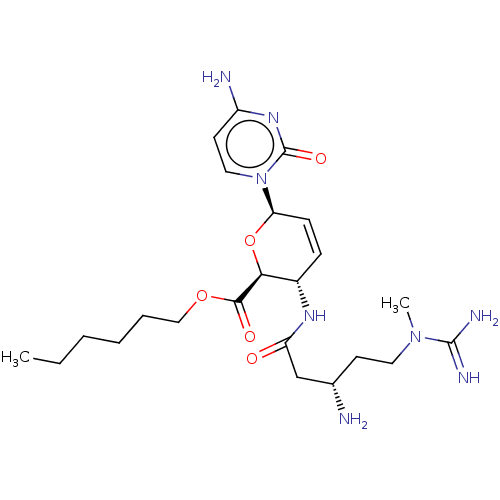
(CHEMBL5279347) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50614280
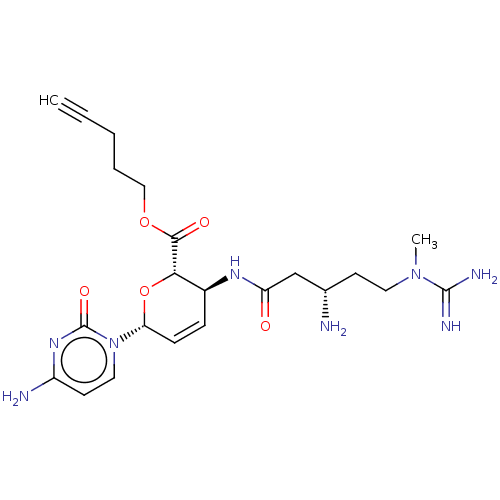
(CHEMBL5276161) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50614431
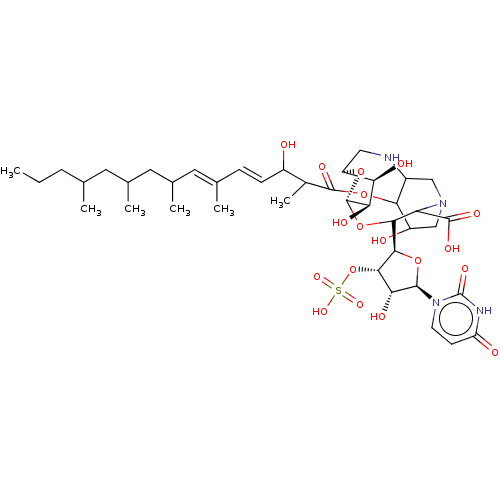
(CHEMBL5289236) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50614274
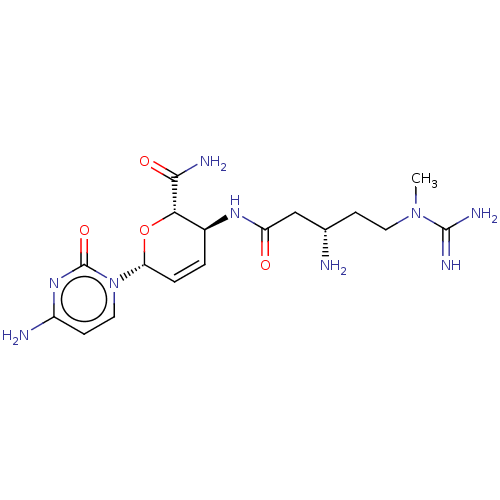
(CHEMBL5272543) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50614281
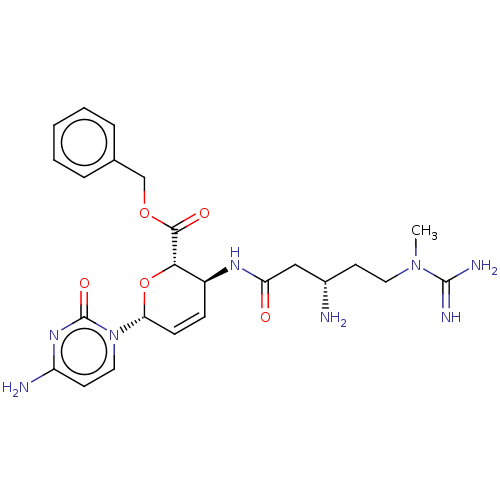
(CHEMBL5288022) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50614278
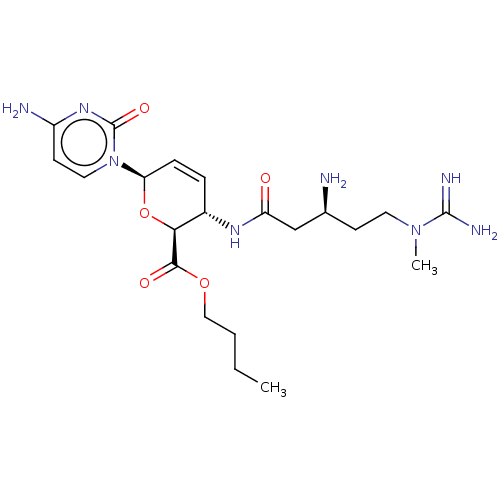
(CHEMBL5285039) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50350465
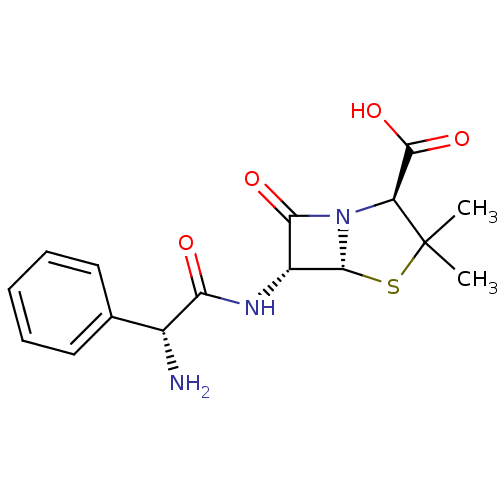
(AMPICILLIN | AY-6108 | Amcill | Aminobenzylpenicil...)Show SMILES CC1(C)S[C@@H]2[C@H](NC(=O)[C@H](N)c3ccccc3)C(=O)N2[C@H]1C(O)=O |r| Show InChI InChI=1S/C16H19N3O4S/c1-16(2)11(15(22)23)19-13(21)10(14(19)24-16)18-12(20)9(17)8-6-4-3-5-7-8/h3-7,9-11,14H,17H2,1-2H3,(H,18,20)(H,22,23)/t9-,10-,11+,14-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lucknow University
Curated by ChEMBL
| Assay Description
Antibacterial activity against Streptococcus faecalis by broth microdilution technique |
Bioorg Med Chem Lett 16: 5883-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.060
BindingDB Entry DOI: 10.7270/Q2MK6GNN |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM21690

(1-cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,...)Show InChI InChI=1S/C17H18FN3O3/c18-13-7-11-14(8-15(13)20-5-3-19-4-6-20)21(10-1-2-10)9-12(16(11)22)17(23)24/h7-10,19H,1-6H2,(H,23,24) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 36.2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antibacterial activity against Enterococcus faecalis MTCC 439 incubated overnight by broth dilution technique |
Citation and Details
Article DOI: 10.1016/j.ejmech.2019.02.055
BindingDB Entry DOI: 10.7270/Q27S7SD8 |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50335519
((S)-3,6-Diamino-hexanoic acid {(3S,9S,12S,15S)-3-(...)Show SMILES CN[C@H](CC(C)C)C(=O)N[C@@H]1[C@H](O)c2ccc(Oc3cc4cc(Oc5ccc(cc5Cl)[C@@H](O)[C@@H]5NC(=O)[C@H](NC(=O)[C@@H]4NC(=O)[C@H](CC(N)=O)NC1=O)c1ccc(O)c(c1)-c1c(O)cc(O)cc1[C@H](NC5=O)C(O)=O)c3O[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O[C@H]1C[C@](C)(N)[C@H](O)[C@H](C)O1)c(Cl)c2 |r| Show InChI InChI=1S/C66H75Cl2N9O24/c1-23(2)12-34(71-5)58(88)76-49-51(83)26-7-10-38(32(67)14-26)97-40-16-28-17-41(55(40)101-65-56(54(86)53(85)42(22-78)99-65)100-44-21-66(4,70)57(87)24(3)96-44)98-39-11-8-27(15-33(39)68)52(84)50-63(93)75-48(64(94)95)31-18-29(79)19-37(81)45(31)30-13-25(6-9-36(30)80)46(60(90)77-50)74-61(91)47(28)73-59(89)35(20-43(69)82)72-62(49)92/h6-11,13-19,23-24,34-35,42,44,46-54,56-57,65,71,78-81,83-87H,12,20-22,70H2,1-5H3,(H2,69,82)(H,72,92)(H,73,89)(H,74,91)(H,75,93)(H,76,88)(H,77,90)(H,94,95)/t24-,34+,35-,42+,44-,46+,47+,48-,49+,50-,51+,52+,53+,54-,56+,57+,65-,66-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 41.4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Chemical Laboratory
Curated by ChEMBL
| Assay Description
Antibacterial activity against gram positive bacteria Enterococcus faecalis was determined by twofold Micro-broth dilution assay |
Bioorg Med Chem Lett 14: 773-7 (2004)
BindingDB Entry DOI: 10.7270/Q2FJ2JZ3 |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50614273

(CHEMBL5279536) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50498223
(CHEMBL3577359)Show SMILES COc1cc(O)cc2\C=C\C=C\C=C\C=C\C=C(C)\CC(O)CC(O)CC(O)\C=C\CC(O)CC(CC(C)O)OC(=O)c3c(OC)cc(O)cc3\C=C\C=C\C=C\C=C\C=C(C)\CC(O)CC(O)CC(O)\C=C\CC(O)CC(CC(C)O)OC(=O)c12 |c:16,60,t:8,10,12,14,28,52,54,56,58,72| Show InChI InChI=1S/C68H92O18/c1-45-23-17-13-9-7-11-15-19-25-49-35-57(77)43-63(83-5)65(49)67(81)86-62(34-48(4)70)42-54(74)30-22-28-52(72)38-60(80)40-56(76)32-46(2)24-18-14-10-8-12-16-20-26-50-36-58(78)44-64(84-6)66(50)68(82)85-61(33-47(3)69)41-53(73)29-21-27-51(71)37-59(79)39-55(75)31-45/h7-28,35-36,43-44,47-48,51-56,59-62,69-80H,29-34,37-42H2,1-6H3/b9-7+,10-8+,15-11+,16-12+,17-13+,18-14+,25-19+,26-20+,27-21+,28-22+,45-23+,46-24+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 167 | n/a | n/a | n/a | n/a | n/a | n/a |
Lucigen Corporation
Curated by ChEMBL
| Assay Description
Antibacterial activity against Enterococcus faecalis ATCC 51299 assessed as growth inhibition using fresh sample in DMSO by CLSI method |
J Nat Prod 78: 924-8 (2015)
Article DOI: 10.1021/np500911k
BindingDB Entry DOI: 10.7270/Q2GT5R6X |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50225960
(CHEMBL310176)Show InChI InChI=1S/C10H21N2O6P/c1-6(2)4-8(11)10(14)12-9(19(15,16)17)5-18-7(3)13/h6,8-9H,4-5,11H2,1-3H3,(H,12,14)(H2,15,16,17) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 219 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration (IC50) to inhibit the growth of Streptococcus faecalis. |
J Med Chem 29: 2212-7 (1986)
BindingDB Entry DOI: 10.7270/Q27D2X9W |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50225959
(CHEMBL307316)Show InChI InChI=1S/C7H17N2O4P/c1-4(2)7(14(11,12)13)9-6(10)5(3)8/h4-5,7H,8H2,1-3H3,(H,9,10)(H2,11,12,13) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 401 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration (IC50) to inhibit the growth of Streptococcus faecalis. |
J Med Chem 29: 2212-7 (1986)
BindingDB Entry DOI: 10.7270/Q27D2X9W |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50476074
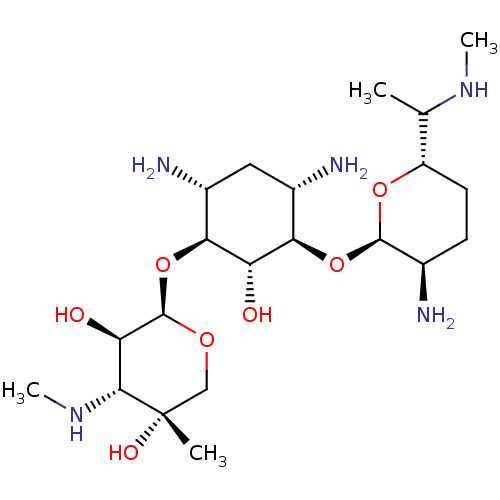
(Gentamicin | SCH-9724 | Uromycine)Show SMILES [H][C@@]1(CN)CC[C@@H](N)[C@@H](O[C@]2([H])[C@@H](N)C[C@@H](N)[C@H](O[C@@]3([H])OC[C@](C)(O)[C@H](NC)[C@H]3O)[C@H]2O)O1.[H][C@]1(CC[C@@H](N)[C@@H](O[C@]2([H])[C@@H](N)C[C@@H](N)[C@H](O[C@@]3([H])OC[C@](C)(O)[C@H](NC)[C@H]3O)[C@H]2O)O1)C(C)N.[H][C@]1(CC[C@@H](N)[C@@H](O[C@]2([H])[C@@H](N)C[C@@H](N)[C@H](O[C@@]3([H])OC[C@](C)(O)[C@H](NC)[C@H]3O)[C@H]2O)O1)C(C)NC |r| Show InChI InChI=1S/C21H43N5O7.C20H41N5O7.C19H39N5O7/c1-9(25-3)13-6-5-10(22)19(31-13)32-16-11(23)7-12(24)17(14(16)27)33-20-15(28)18(26-4)21(2,29)8-30-20;1-8(21)12-5-4-9(22)18(30-12)31-15-10(23)6-11(24)16(13(15)26)32-19-14(27)17(25-3)20(2,28)7-29-19;1-19(27)7-28-18(13(26)16(19)24-2)31-15-11(23)5-10(22)14(12(15)25)30-17-9(21)4-3-8(6-20)29-17/h9-20,25-29H,5-8,22-24H2,1-4H3;8-19,25-28H,4-7,21-24H2,1-3H3;8-18,24-27H,3-7,20-23H2,1-2H3/t9?,10-,11+,12-,13+,14+,15-,16-,17+,18-,19-,20-,21+;8?,9-,10+,11-,12+,13+,14-,15-,16+,17-,18-,19-,20+;8-,9+,10-,11+,12-,13+,14+,15-,16+,17+,18+,19-/m110/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 445 | n/a | n/a | n/a | n/a | n/a | n/a |
Lucknow University
Curated by ChEMBL
| Assay Description
Antibacterial activity against Streptococcus faecalis by broth microdilution technique |
Bioorg Med Chem Lett 16: 5883-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.060
BindingDB Entry DOI: 10.7270/Q2MK6GNN |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50600678
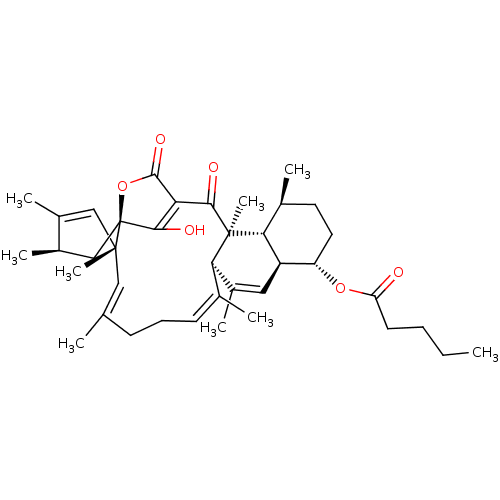
(CHEMBL5176174)Show SMILES [H][C@]12C=C(C)[C@@]3([H])\C(C)=C\CC\C(C)=C\[C@]4(C)C=C(C)[C@H](C)C[C@]44OC(=O)C(=C4O)C(=O)[C@@]3(C)[C@]1([H])[C@@H](C)CC[C@@H]2OC(=O)CCCC |r,c:28,t:2,8,13,17| | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.2c00444
BindingDB Entry DOI: 10.7270/Q2H9997N |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM21690

(1-cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,...)Show InChI InChI=1S/C17H18FN3O3/c18-13-7-11-14(8-15(13)20-5-3-19-4-6-20)21(10-1-2-10)9-12(16(11)22)17(23)24/h7-10,19H,1-6H2,(H,23,24) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 603 | n/a | n/a | n/a | n/a | n/a | n/a |
Lucigen Corporation
Curated by ChEMBL
| Assay Description
Antibacterial activity against Enterococcus faecalis ATCC 51299 assessed as growth inhibition using fresh sample in DMSO by CLSI method |
J Nat Prod 78: 924-8 (2015)
Article DOI: 10.1021/np500911k
BindingDB Entry DOI: 10.7270/Q2GT5R6X |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50366826
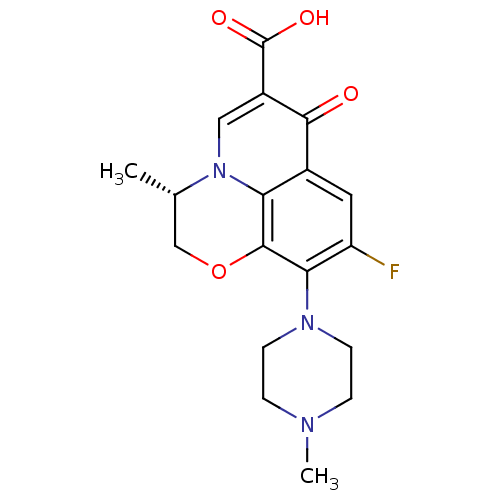
(DR-3355 | Floxacin | Iquix | LEVOFLOXACIN | Levaqu...)Show SMILES C[C@H]1COc2c(N3CCN(C)CC3)c(F)cc3c2n1cc(C(O)=O)c3=O |r| Show InChI InChI=1S/C18H20FN3O4/c1-10-9-26-17-14-11(16(23)12(18(24)25)8-22(10)14)7-13(19)15(17)21-5-3-20(2)4-6-21/h7-8,10H,3-6,9H2,1-2H3,(H,24,25)/t10-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Antibacterial activity against vancomycin-sensitive Enterococcus faecalis 12-5 after 18 hrs by broth microdilution method |
J Nat Prod 77: 1021-30 (2014)
Article DOI: 10.1021/np5000457
BindingDB Entry DOI: 10.7270/Q2280BKR |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50366826

(DR-3355 | Floxacin | Iquix | LEVOFLOXACIN | Levaqu...)Show SMILES C[C@H]1COc2c(N3CCN(C)CC3)c(F)cc3c2n1cc(C(O)=O)c3=O |r| Show InChI InChI=1S/C18H20FN3O4/c1-10-9-26-17-14-11(16(23)12(18(24)25)8-22(10)14)7-13(19)15(17)21-5-3-20(2)4-6-21/h7-8,10H,3-6,9H2,1-2H3,(H,24,25)/t10-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Antibacterial activity against vancomycin-sensitive Enterococcus faecalis ATCC 29212 after 18 hrs by broth microdilution method |
J Nat Prod 77: 1021-30 (2014)
Article DOI: 10.1021/np5000457
BindingDB Entry DOI: 10.7270/Q2280BKR |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50509323
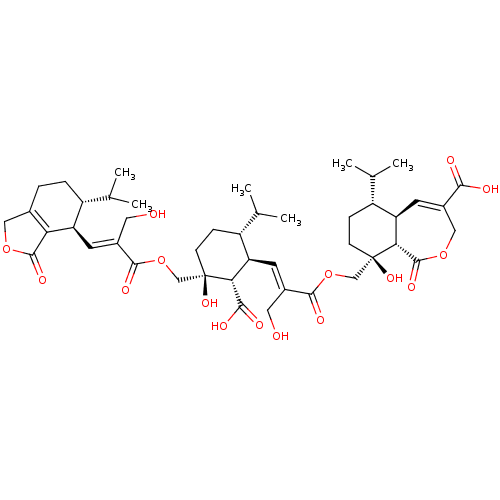
(CHEMBL4436622)Show SMILES [H][C@]12C=C(COC(=O)[C@]1([H])[C@](O)(COC(=O)C(\CO)=C\[C@@H]1[C@H](CC[C@@](O)(COC(=O)C(\CO)=C\[C@@H]3[C@H](CCC4=C3C(=O)OC4)C(C)C)[C@H]1C(O)=O)C(C)C)CC[C@@H]2C(C)C)C(O)=O |r,c:2,39| Show InChI InChI=1S/C45H62O16/c1-22(2)29-8-7-25-18-58-42(54)35(25)32(29)13-26(16-46)40(52)60-20-44(56)11-9-30(23(3)4)33(36(44)39(50)51)14-27(17-47)41(53)61-21-45(57)12-10-31(24(5)6)34-15-28(38(48)49)19-59-43(55)37(34)45/h13-15,22-24,29-34,36-37,46-47,56-57H,7-12,16-21H2,1-6H3,(H,48,49)(H,50,51)/b26-13+,27-14+/t29-,30-,31-,32-,33-,34-,36-,37-,44-,45-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Antibacterial activity against vancomycin-resistant Enterococcus faecalis AUS-RBWH-VRE-01 assessed as reduction in fungal growth incubated for 24 hrs... |
J Nat Prod 82: 3165-3175 (2019)
Article DOI: 10.1021/acs.jnatprod.9b00760
BindingDB Entry DOI: 10.7270/Q2KW5KB8 |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50366826

(DR-3355 | Floxacin | Iquix | LEVOFLOXACIN | Levaqu...)Show SMILES C[C@H]1COc2c(N3CCN(C)CC3)c(F)cc3c2n1cc(C(O)=O)c3=O |r| Show InChI InChI=1S/C18H20FN3O4/c1-10-9-26-17-14-11(16(23)12(18(24)25)8-22(10)14)7-13(19)15(17)21-5-3-20(2)4-6-21/h7-8,10H,3-6,9H2,1-2H3,(H,24,25)/t10-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Antibacterial activity against vancomycin-resistant Enterococcus faecalis ATCC 51299 after 18 hrs by broth microdilution method |
J Nat Prod 77: 1021-30 (2014)
Article DOI: 10.1021/np5000457
BindingDB Entry DOI: 10.7270/Q2280BKR |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM31768

(CHEMBL295698 | Ketoconazole | Nizoral | Panfungol)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OC[C@@H]2CO[C@](Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research
Curated by ChEMBL
| Assay Description
Inhibition of cholesterol side chain cleavage cytochrome P450 |
J Med Chem 35: 2818-25 (1992)
BindingDB Entry DOI: 10.7270/Q24Q7SZT |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50497194
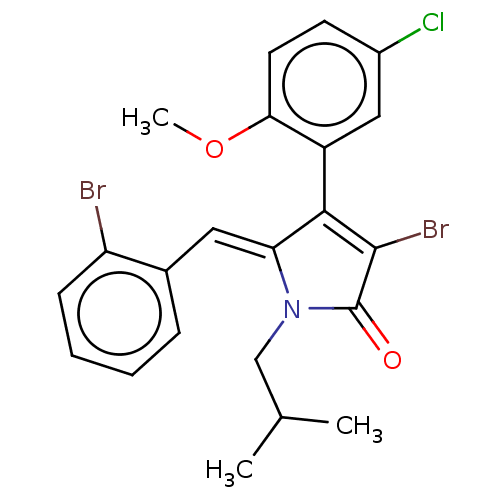
(CHEMBL3116168)Show SMILES COc1ccc(Cl)cc1C1=C(Br)C(=O)N(CC(C)C)\C1=C\c1ccccc1Br |c:10| Show InChI InChI=1S/C22H20Br2ClNO2/c1-13(2)12-26-18(10-14-6-4-5-7-17(14)23)20(21(24)22(26)27)16-11-15(25)8-9-19(16)28-3/h4-11,13H,12H2,1-3H3/b18-10+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Vi£osa
Curated by ChEMBL
| Assay Description
Antimicrobial activity against Enterococcus faecalis assessed as inhibition of biofilm formation after 20 hrs by crystal violet staining analysis |
Eur J Med Chem 82: 127-38 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.035
BindingDB Entry DOI: 10.7270/Q29K4F6V |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50237605
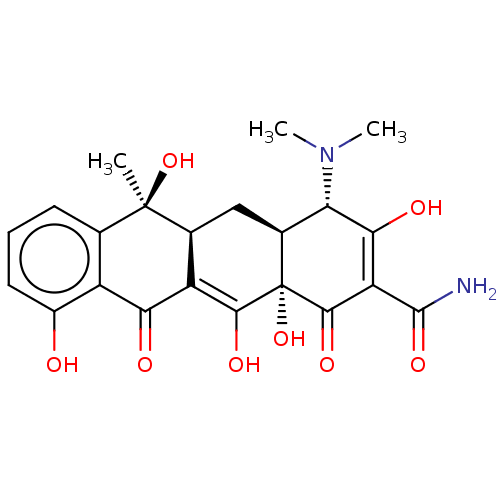
(Achromycin | Achromycin V | CHEBI:27902 | Cyclopar...)Show SMILES [H][C@@]12C[C@@]3([H])C(=C(O)[C@]1(O)C(=O)C(C(N)=O)=C(O)[C@H]2N(C)C)C(=O)c1c(O)cccc1[C@@]3(C)O |r,t:5,16| Show InChI InChI=1S/C22H24N2O8/c1-21(31)8-5-4-6-11(25)12(8)16(26)13-9(21)7-10-15(24(2)3)17(27)14(20(23)30)19(29)22(10,32)18(13)28/h4-6,9-10,15,25,27-28,31-32H,7H2,1-3H3,(H2,23,30)/t9-,10-,15-,21+,22-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island
Curated by ChEMBL
| Assay Description
Antibacterial activity against vancomycin-resistant Enterococcus faecalis ATCC 51299 after 18 hrs by broth dilution assay |
J Nat Prod 69: 1070-3 (2006)
Article DOI: 10.1021/np050449b
BindingDB Entry DOI: 10.7270/Q22J6FNX |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50509324
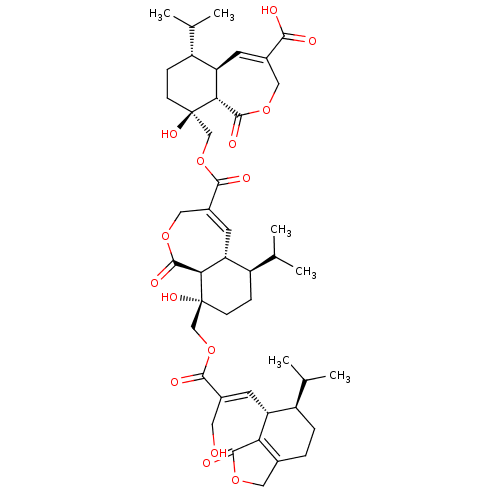
(CHEMBL4463460)Show SMILES [H][C@]12C=C(COC(=O)[C@]1([H])[C@](O)(COC(=O)C1=C[C@]3([H])[C@H](CC[C@@](O)(COC(=O)C(\CO)=C\[C@@H]4[C@H](CCC5=C4C(=O)OC5)C(C)C)[C@@]3([H])C(=O)OC1)C(C)C)CC[C@@H]2C(C)C)C(O)=O |r,c:2,38,t:17| Show InChI InChI=1S/C45H60O15/c1-22(2)29-8-7-25-17-56-41(51)35(25)32(29)13-26(16-46)39(49)59-20-44(54)12-10-31(24(5)6)34-15-28(19-58-43(53)37(34)44)40(50)60-21-45(55)11-9-30(23(3)4)33-14-27(38(47)48)18-57-42(52)36(33)45/h13-15,22-24,29-34,36-37,46,54-55H,7-12,16-21H2,1-6H3,(H,47,48)/b26-13+/t29-,30-,31-,32-,33-,34-,36-,37-,44-,45-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Antibacterial activity against vancomycin-resistant Enterococcus faecalis AUS-RBWH-VRE-01 assessed as reduction in fungal growth incubated for 24 hrs... |
J Nat Prod 82: 3165-3175 (2019)
Article DOI: 10.1021/acs.jnatprod.9b00760
BindingDB Entry DOI: 10.7270/Q2KW5KB8 |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50478886
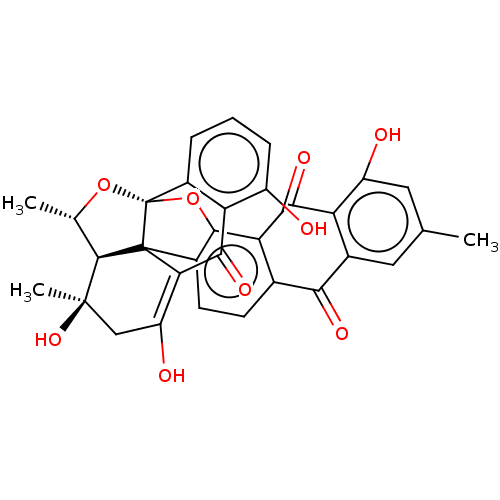
(CHEMBL501116)Show SMILES [H][C@]12[C@H](C)O[C@]34Oc5c(ccc6C(=O)c7cc(C)cc(O)c7C(=O)c56)C13C(=C(O)C[C@@]2(C)O)C(=O)c1c(O)cccc41 |r,t:31| Show InChI InChI=1S/C32H24O9/c1-12-9-15-21(19(34)10-12)26(37)22-14(25(15)36)7-8-17-28(22)41-32-16-5-4-6-18(33)23(16)27(38)24-20(35)11-30(3,39)29(13(2)40-32)31(17,24)32/h4-10,13,29,33-35,39H,11H2,1-3H3/t13-,29+,30+,31?,32+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island
Curated by ChEMBL
| Assay Description
Antibacterial activity against vancomycin-resistant Enterococcus faecalis ATCC 51299 after 18 hrs by broth dilution assay |
J Nat Prod 69: 1070-3 (2006)
Article DOI: 10.1021/np050449b
BindingDB Entry DOI: 10.7270/Q22J6FNX |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50497201
(CHEMBL3116167)Show SMILES COc1ccc(C)cc1C1=C(Br)C(=O)N(CC(C)C)\C1=C\c1ccccc1Cl |c:10| Show InChI InChI=1S/C23H23BrClNO2/c1-14(2)13-26-19(12-16-7-5-6-8-18(16)25)21(22(24)23(26)27)17-11-15(3)9-10-20(17)28-4/h5-12,14H,13H2,1-4H3/b19-12+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Vi£osa
Curated by ChEMBL
| Assay Description
Antimicrobial activity against Enterococcus faecalis assessed as inhibition of biofilm formation after 20 hrs by crystal violet staining analysis |
Eur J Med Chem 82: 127-38 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.035
BindingDB Entry DOI: 10.7270/Q29K4F6V |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50103513
(CHEBI:17076 | Chemform | Gerox | NSC-14083 | Strep...)Show SMILES CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@H]1[C@H](O[C@H]2[C@H](O)[C@@H](O)[C@H](NC(N)=N)[C@@H](O)[C@@H]2NC(N)=N)O[C@@H](C)[C@]1(O)C=O Show InChI InChI=1S/C21H39N7O12/c1-5-21(36,4-30)16(40-17-9(26-2)13(34)10(31)6(3-29)38-17)18(37-5)39-15-8(28-20(24)25)11(32)7(27-19(22)23)12(33)14(15)35/h4-18,26,29,31-36H,3H2,1-2H3,(H4,22,23,27)(H4,24,25,28)/t5-,6-,7+,8-,9-,10-,11+,12-,13-,14+,15+,16-,17-,18-,21+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 2.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Chemical Laboratory
Curated by ChEMBL
| Assay Description
Antibacterial activity against gram positive bacteria Enterococcus faecalis was determined by twofold Micro-broth dilution assay |
Bioorg Med Chem Lett 14: 773-7 (2004)
BindingDB Entry DOI: 10.7270/Q2FJ2JZ3 |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50499513
(CHEMBL4299436)Show SMILES [H][C@]1(C[C@@](C)(N)[C@@H](O)[C@@H](C)O1)O[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@@H]1Oc1c2Oc3ccc(cc3Cl)[C@@H](O)[C@@H](NC(=O)[C@H](CC(C)C)NC)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@]3([H])c(c2)cc1Oc1ccc(cc1Cl)[C@@H](O)[C@]1([H])NC(=O)[C@]([H])(NC3=O)c2ccc(O)c(c2)-c2c(O)cc(O)cc2[C@@H](NC1=O)C(O)=O |r| Show InChI InChI=1S/C66H75Cl2N9O24/c1-23(2)12-34(71-5)58(88)76-49-51(83)26-7-10-38(32(67)14-26)97-40-16-28-17-41(55(40)101-65-56(54(86)53(85)42(22-78)99-65)100-44-21-66(4,70)57(87)24(3)96-44)98-39-11-8-27(15-33(39)68)52(84)50-63(93)75-48(64(94)95)31-18-29(79)19-37(81)45(31)30-13-25(6-9-36(30)80)46(60(90)77-50)74-61(91)47(28)73-59(89)35(20-43(69)82)72-62(49)92/h6-11,13-19,23-24,34-35,42,44,46-54,56-57,65,71,78-81,83-87H,12,20-22,70H2,1-5H3,(H2,69,82)(H,72,92)(H,73,89)(H,74,91)(H,75,93)(H,76,88)(H,77,90)(H,94,95)/t24-,34+,35+,42+,44-,46-,47-,48-,49-,50+,51-,52-,53+,54-,56+,57+,65-,66-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antibacterial activity against low level vancomycin-resistant Enterococcus faecalis ATCC 51299 by plate reader analysis |
J Nat Prod 78: 2748-53 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00721
BindingDB Entry DOI: 10.7270/Q2M61P8H |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50497203
(CHEMBL3115985)Show SMILES COc1ccc(C)cc1C1=C(Br)C(=O)N(CC(C)C)C1(O)Cc1ccccc1Cl |c:10| Show InChI InChI=1S/C23H25BrClNO3/c1-14(2)13-26-22(27)21(24)20(17-11-15(3)9-10-19(17)29-4)23(26,28)12-16-7-5-6-8-18(16)25/h5-11,14,28H,12-13H2,1-4H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Vi£osa
Curated by ChEMBL
| Assay Description
Antimicrobial activity against Enterococcus faecalis assessed as inhibition of biofilm formation after 20 hrs by crystal violet staining analysis |
Eur J Med Chem 82: 127-38 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.035
BindingDB Entry DOI: 10.7270/Q29K4F6V |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50335519

((S)-3,6-Diamino-hexanoic acid {(3S,9S,12S,15S)-3-(...)Show SMILES CN[C@H](CC(C)C)C(=O)N[C@@H]1[C@H](O)c2ccc(Oc3cc4cc(Oc5ccc(cc5Cl)[C@@H](O)[C@@H]5NC(=O)[C@H](NC(=O)[C@@H]4NC(=O)[C@H](CC(N)=O)NC1=O)c1ccc(O)c(c1)-c1c(O)cc(O)cc1[C@H](NC5=O)C(O)=O)c3O[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O[C@H]1C[C@](C)(N)[C@H](O)[C@H](C)O1)c(Cl)c2 |r| Show InChI InChI=1S/C66H75Cl2N9O24/c1-23(2)12-34(71-5)58(88)76-49-51(83)26-7-10-38(32(67)14-26)97-40-16-28-17-41(55(40)101-65-56(54(86)53(85)42(22-78)99-65)100-44-21-66(4,70)57(87)24(3)96-44)98-39-11-8-27(15-33(39)68)52(84)50-63(93)75-48(64(94)95)31-18-29(79)19-37(81)45(31)30-13-25(6-9-36(30)80)46(60(90)77-50)74-61(91)47(28)73-59(89)35(20-43(69)82)72-62(49)92/h6-11,13-19,23-24,34-35,42,44,46-54,56-57,65,71,78-81,83-87H,12,20-22,70H2,1-5H3,(H2,69,82)(H,72,92)(H,73,89)(H,74,91)(H,75,93)(H,76,88)(H,77,90)(H,94,95)/t24-,34+,35-,42+,44-,46+,47+,48-,49+,50-,51+,52+,53+,54-,56+,57+,65-,66-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lucigen Corporation
Curated by ChEMBL
| Assay Description
Antibacterial activity against Enterococcus faecalis ATCC 51299 assessed as growth inhibition using fresh sample in DMSO by CLSI method |
J Nat Prod 78: 924-8 (2015)
Article DOI: 10.1021/np500911k
BindingDB Entry DOI: 10.7270/Q2GT5R6X |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50497199
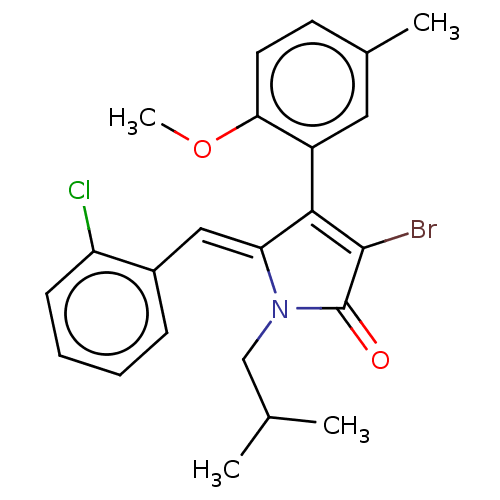
(CHEMBL3115992)Show SMILES COc1ccc(C)cc1C1=C(Br)C(=O)N(CC(C)C)\C1=C/c1ccccc1Cl |c:10| Show InChI InChI=1S/C23H23BrClNO2/c1-14(2)13-26-19(12-16-7-5-6-8-18(16)25)21(22(24)23(26)27)17-11-15(3)9-10-20(17)28-4/h5-12,14H,13H2,1-4H3/b19-12- | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Vi£osa
Curated by ChEMBL
| Assay Description
Antimicrobial activity against Enterococcus faecalis assessed as inhibition of biofilm formation after 20 hrs by crystal violet staining analysis |
Eur J Med Chem 82: 127-38 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.035
BindingDB Entry DOI: 10.7270/Q29K4F6V |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50497204

(CHEMBL3115986)Show SMILES COc1ccc(Cl)cc1C1=C(Br)C(=O)N(CC(C)C)C1(O)Cc1ccccc1Br |c:10| Show InChI InChI=1S/C22H22Br2ClNO3/c1-13(2)12-26-21(27)20(24)19(16-10-15(25)8-9-18(16)29-3)22(26,28)11-14-6-4-5-7-17(14)23/h4-10,13,28H,11-12H2,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Vi£osa
Curated by ChEMBL
| Assay Description
Antimicrobial activity against Enterococcus faecalis assessed as inhibition of biofilm formation after 20 hrs by crystal violet staining analysis |
Eur J Med Chem 82: 127-38 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.035
BindingDB Entry DOI: 10.7270/Q29K4F6V |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50218278

(CHEMBL279571)Show SMILES CCCCCCCCCCCCO[C@H](COP(O)(=O)OC1OC(C(O)C(NC(=O)Nc2ccc(Cl)c(c2)C(F)(F)F)C1OC1OC(CO)C(O)C(O)C1NC(=O)c1cccc(c1)C(F)(F)F)C(N)=O)C(O)=O Show InChI InChI=1S/C43H58ClF6N4O17P/c1-2-3-4-5-6-7-8-9-10-11-17-66-28(38(61)62)21-67-72(64,65)71-40-34(29(33(58)35(70-40)36(51)59)54-41(63)52-24-15-16-26(44)25(19-24)43(48,49)50)69-39-30(32(57)31(56)27(20-55)68-39)53-37(60)22-13-12-14-23(18-22)42(45,46)47/h12-16,18-19,27-35,39-40,55-58H,2-11,17,20-21H2,1H3,(H2,51,59)(H,53,60)(H,61,62)(H,64,65)(H2,52,54,63)/t27?,28-,29?,30?,31?,32?,33?,34?,35?,39?,40?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Advanced Medicine, Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of peptidoglycan synthesis in Enterococcus faecalis using [14C]lysine radioligand |
Bioorg Med Chem Lett 10: 2251-4 (2000)
BindingDB Entry DOI: 10.7270/Q2D79DM6 |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50335519

((S)-3,6-Diamino-hexanoic acid {(3S,9S,12S,15S)-3-(...)Show SMILES CN[C@H](CC(C)C)C(=O)N[C@@H]1[C@H](O)c2ccc(Oc3cc4cc(Oc5ccc(cc5Cl)[C@@H](O)[C@@H]5NC(=O)[C@H](NC(=O)[C@@H]4NC(=O)[C@H](CC(N)=O)NC1=O)c1ccc(O)c(c1)-c1c(O)cc(O)cc1[C@H](NC5=O)C(O)=O)c3O[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O[C@H]1C[C@](C)(N)[C@H](O)[C@H](C)O1)c(Cl)c2 |r| Show InChI InChI=1S/C66H75Cl2N9O24/c1-23(2)12-34(71-5)58(88)76-49-51(83)26-7-10-38(32(67)14-26)97-40-16-28-17-41(55(40)101-65-56(54(86)53(85)42(22-78)99-65)100-44-21-66(4,70)57(87)24(3)96-44)98-39-11-8-27(15-33(39)68)52(84)50-63(93)75-48(64(94)95)31-18-29(79)19-37(81)45(31)30-13-25(6-9-36(30)80)46(60(90)77-50)74-61(91)47(28)73-59(89)35(20-43(69)82)72-62(49)92/h6-11,13-19,23-24,34-35,42,44,46-54,56-57,65,71,78-81,83-87H,12,20-22,70H2,1-5H3,(H2,69,82)(H,72,92)(H,73,89)(H,74,91)(H,75,93)(H,76,88)(H,77,90)(H,94,95)/t24-,34+,35-,42+,44-,46+,47+,48-,49+,50-,51+,52+,53+,54-,56+,57+,65-,66-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Advanced Medicine, Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of peptidoglycan synthesis in Enterococcus faecalis using [14C]lysine radioligand |
Bioorg Med Chem Lett 10: 2251-4 (2000)
BindingDB Entry DOI: 10.7270/Q2D79DM6 |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50600667
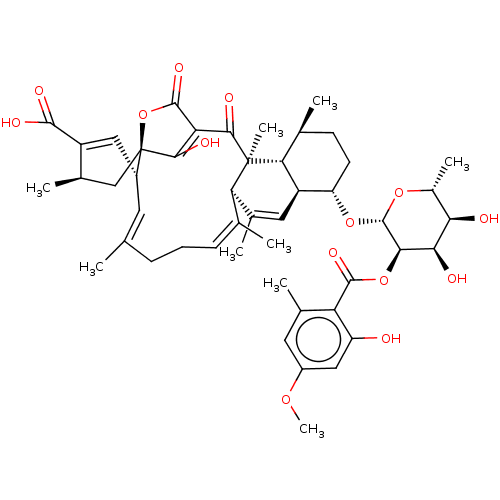
(CHEMBL5180164)Show SMILES [H][C@]12C=C(C)[C@@]3([H])\C(C)=C\CC\C(C)=C\[C@]4([H])C=C([C@H](C)C[C@]44OC(=O)C(=C4O)C(=O)[C@@]3(C)[C@]1([H])[C@@H](C)CC[C@@H]2O[C@@H]1O[C@H](C)[C@@H](O)[C@@H](O)[C@H]1OC(=O)c1c(C)cc(OC)cc1O)C(O)=O |r,c:17,27,t:2,8,13| | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.2c00444
BindingDB Entry DOI: 10.7270/Q2H9997N |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50498339
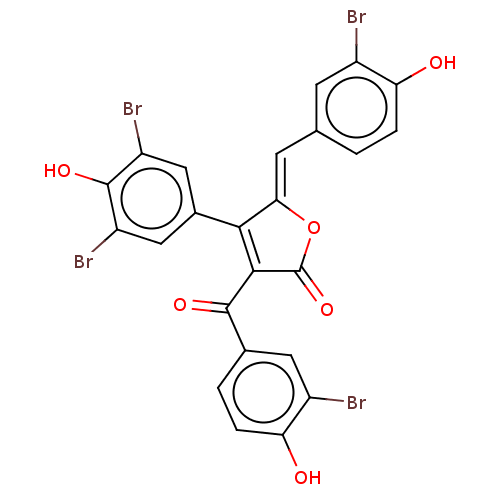
(CHEMBL3585711)Show SMILES Oc1ccc(\C=C2/OC(=O)C(C(=O)c3ccc(O)c(Br)c3)=C2c2cc(Br)c(O)c(Br)c2)cc1Br |c:21| Show InChI InChI=1S/C24H12Br4O6/c25-13-5-10(1-3-17(13)29)6-19-20(12-8-15(27)23(32)16(28)9-12)21(24(33)34-19)22(31)11-2-4-18(30)14(26)7-11/h1-9,29-30,32H/b19-6- | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ
Curated by ChEMBL
| Assay Description
Antibacterial activity against Enterococcus faecalis CECT 481 assessed as inhibition of microbial growth incubated for 18 hrs by 2-fold microtiter br... |
Bioorg Med Chem 23: 3618-28 (2015)
Article DOI: 10.1016/j.bmc.2015.04.010
BindingDB Entry DOI: 10.7270/Q2B56NRF |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50497190
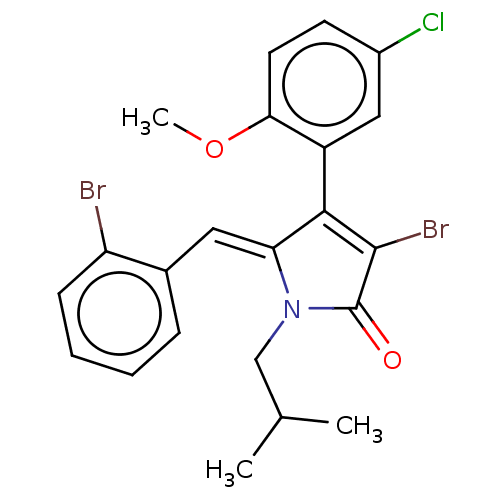
(CHEMBL3115993)Show SMILES COc1ccc(Cl)cc1C1=C(Br)C(=O)N(CC(C)C)\C1=C/c1ccccc1Br |c:10| Show InChI InChI=1S/C22H20Br2ClNO2/c1-13(2)12-26-18(10-14-6-4-5-7-17(14)23)20(21(24)22(26)27)16-11-15(25)8-9-19(16)28-3/h4-11,13H,12H2,1-3H3/b18-10- | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Vi£osa
Curated by ChEMBL
| Assay Description
Antimicrobial activity against Enterococcus faecalis assessed as inhibition of biofilm formation after 20 hrs by crystal violet staining analysis |
Eur J Med Chem 82: 127-38 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.035
BindingDB Entry DOI: 10.7270/Q29K4F6V |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50004442
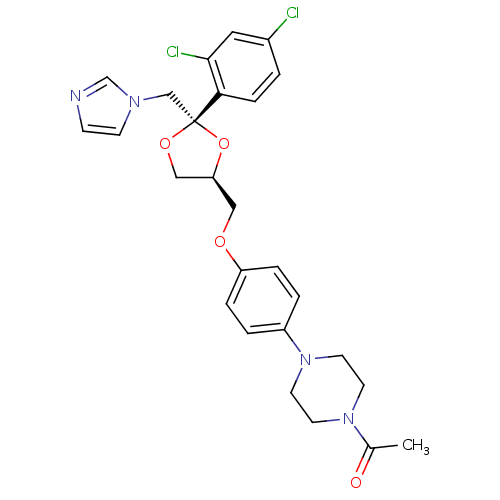
((2S,4S)-ketoconazole | 1-acetyl-4-(4-{[(2S,4S)-2-(...)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OC[C@H]2CO[C@](Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research
Curated by ChEMBL
| Assay Description
Inhibition of cholesterol side chain cleavage cytochrome P450 |
J Med Chem 35: 2818-25 (1992)
BindingDB Entry DOI: 10.7270/Q24Q7SZT |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50600670
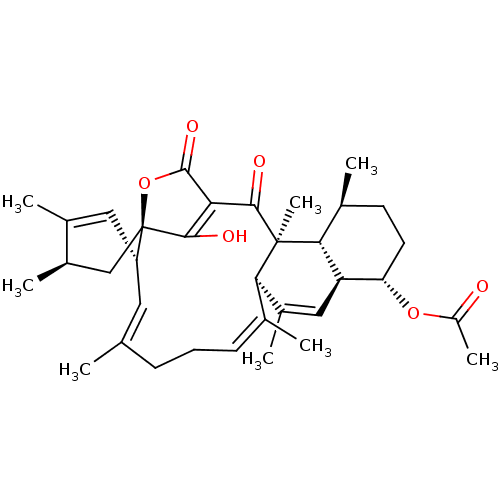
(CHEMBL5204687)Show SMILES [H][C@@]12C=C(C)[C@H](C)C[C@]11OC(=O)C(=C1O)C(=O)[C@]1(C)[C@@]([H])(C(C)=C[C@]3([H])[C@H](CC[C@H](C)[C@@]13[H])OC(C)=O)\C(C)=C\CC\C(C)=C\2 |r,c:13,24,t:2,42,47| | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.2c00444
BindingDB Entry DOI: 10.7270/Q2H9997N |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
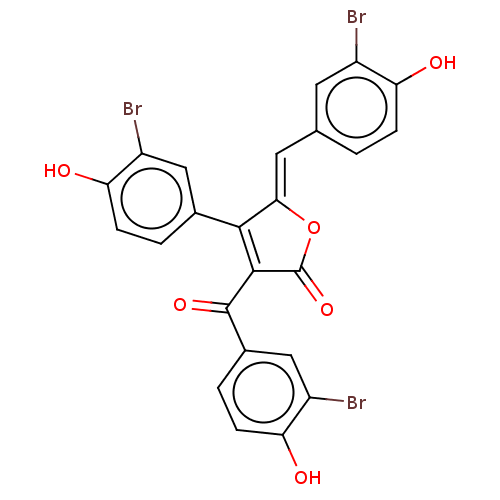
(Rattus norvegicus) | BDBM50498341
(CHEMBL3585709)Show SMILES Oc1ccc(\C=C2/OC(=O)C(C(=O)c3ccc(O)c(Br)c3)=C2c2ccc(O)c(Br)c2)cc1Br |c:21| Show InChI InChI=1S/C24H13Br3O6/c25-14-7-11(1-4-17(14)28)8-20-21(12-2-5-18(29)15(26)9-12)22(24(32)33-20)23(31)13-3-6-19(30)16(27)10-13/h1-10,28-30H/b20-8- | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ
Curated by ChEMBL
| Assay Description
Antibacterial activity against Enterococcus faecalis CECT 481 assessed as inhibition of microbial growth incubated for 18 hrs by 2-fold microtiter br... |
Bioorg Med Chem 23: 3618-28 (2015)
Article DOI: 10.1016/j.bmc.2015.04.010
BindingDB Entry DOI: 10.7270/Q2B56NRF |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
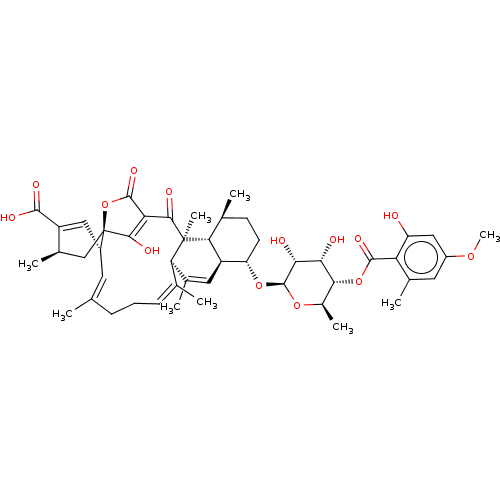
(Rattus norvegicus) | BDBM50600662
(CHEMBL5193721)Show SMILES [H][C@]12C=C(C)[C@@]3([H])\C(C)=C\CC\C(C)=C\[C@]4([H])C=C([C@H](C)C[C@]44OC(=O)C(=C4O)C(=O)[C@@]3(C)[C@]1([H])[C@@H](C)CC[C@@H]2O[C@@H]1O[C@H](C)[C@@H](OC(=O)c2c(C)cc(OC)cc2O)[C@@H](O)[C@H]1O)C(O)=O |r,c:17,27,t:2,8,13| | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.2c00444
BindingDB Entry DOI: 10.7270/Q2H9997N |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50497191

(CHEMBL3116162)Show SMILES COc1cccc(\C=C2/N(CC(C)C)C(=O)C(Br)=C2c2cc(Cl)ccc2OC)c1 |c:17| Show InChI InChI=1S/C23H23BrClNO3/c1-14(2)13-26-19(11-15-6-5-7-17(10-15)28-3)21(22(24)23(26)27)18-12-16(25)8-9-20(18)29-4/h5-12,14H,13H2,1-4H3/b19-11- | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Vi£osa
Curated by ChEMBL
| Assay Description
Antimicrobial activity against Enterococcus faecalis assessed as inhibition of biofilm formation after 20 hrs by crystal violet staining analysis |
Eur J Med Chem 82: 127-38 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.035
BindingDB Entry DOI: 10.7270/Q29K4F6V |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50498340
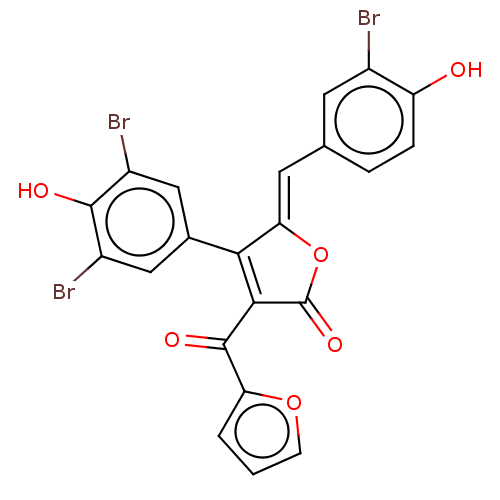
(CHEMBL3585717)Show SMILES Oc1ccc(\C=C2/OC(=O)C(C(=O)c3ccco3)=C2c2cc(Br)c(O)c(Br)c2)cc1Br |c:18| Show InChI InChI=1S/C22H11Br3O6/c23-12-6-10(3-4-15(12)26)7-17-18(11-8-13(24)20(27)14(25)9-11)19(22(29)31-17)21(28)16-2-1-5-30-16/h1-9,26-27H/b17-7- | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ
Curated by ChEMBL
| Assay Description
Antibacterial activity against Enterococcus faecalis CECT 481 assessed as inhibition of microbial growth incubated for 18 hrs by 2-fold microtiter br... |
Bioorg Med Chem 23: 3618-28 (2015)
Article DOI: 10.1016/j.bmc.2015.04.010
BindingDB Entry DOI: 10.7270/Q2B56NRF |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50498338
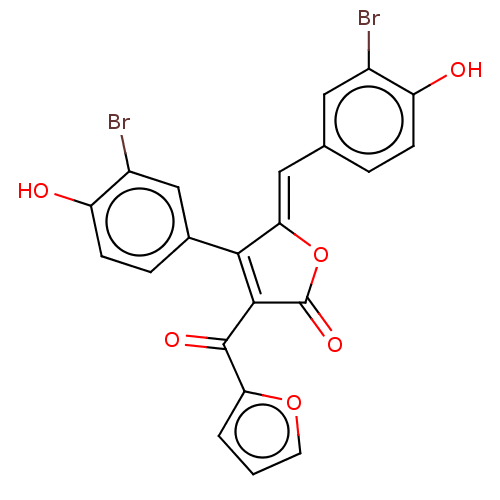
(CHEMBL3585716)Show SMILES Oc1ccc(\C=C2/OC(=O)C(C(=O)c3ccco3)=C2c2ccc(O)c(Br)c2)cc1Br |c:18| Show InChI InChI=1S/C22H12Br2O6/c23-13-8-11(3-5-15(13)25)9-18-19(12-4-6-16(26)14(24)10-12)20(22(28)30-18)21(27)17-2-1-7-29-17/h1-10,25-26H/b18-9- | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ
Curated by ChEMBL
| Assay Description
Antibacterial activity against Enterococcus faecalis CECT 481 assessed as inhibition of microbial growth incubated for 18 hrs by 2-fold microtiter br... |
Bioorg Med Chem 23: 3618-28 (2015)
Article DOI: 10.1016/j.bmc.2015.04.010
BindingDB Entry DOI: 10.7270/Q2B56NRF |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50497202
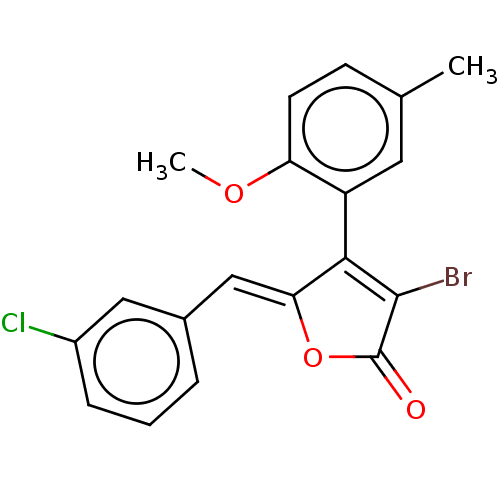
(CHEMBL3115977)Show SMILES COc1ccc(C)cc1C1=C(Br)C(=O)O\C1=C/c1cccc(Cl)c1 |c:10| Show InChI InChI=1S/C19H14BrClO3/c1-11-6-7-15(23-2)14(8-11)17-16(24-19(22)18(17)20)10-12-4-3-5-13(21)9-12/h3-10H,1-2H3/b16-10- | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Vi£osa
Curated by ChEMBL
| Assay Description
Antimicrobial activity against Enterococcus faecalis assessed as inhibition of biofilm formation after 20 hrs by crystal violet staining analysis |
Eur J Med Chem 82: 127-38 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.035
BindingDB Entry DOI: 10.7270/Q29K4F6V |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data