 Found 28 hits of ki for UniProtKB: P0A725
Found 28 hits of ki for UniProtKB: P0A725 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50493081
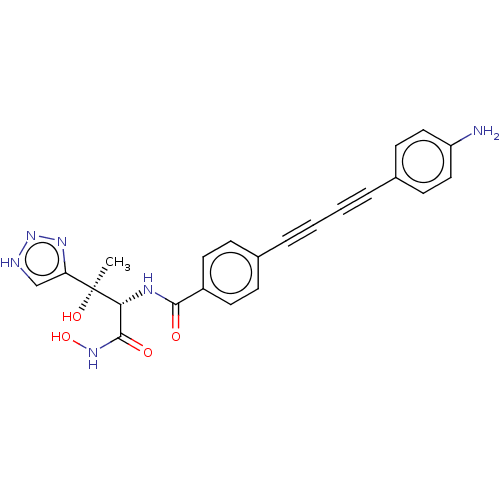
(CHEMBL2420205)Show SMILES C[C@@](O)([C@H](NC(=O)c1ccc(cc1)C#CC#Cc1ccc(N)cc1)C(=O)NO)c1c[nH]nn1 |r| Show InChI InChI=1S/C23H20N6O4/c1-23(32,19-14-25-29-27-19)20(22(31)28-33)26-21(30)17-10-6-15(7-11-17)4-2-3-5-16-8-12-18(24)13-9-16/h6-14,20,32-33H,24H2,1H3,(H,26,30)(H,28,31)(H,25,27,29)/t20-,23+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC enzyme using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetyl glucosamine and [gamma-32P] UDP-3-O-[(R)-3-hydroxymyristoy... |
J Med Chem 56: 6954-6966 (2013)
Article DOI: 10.1021/jm4007774
BindingDB Entry DOI: 10.7270/Q28K7D11 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50493080
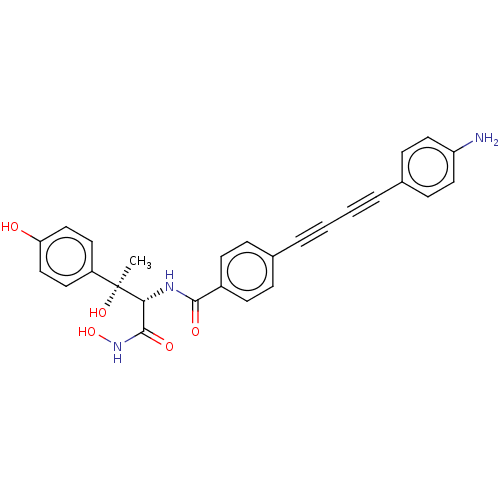
(CHEMBL2420203)Show SMILES C[C@@](O)([C@H](NC(=O)c1ccc(cc1)C#CC#Cc1ccc(N)cc1)C(=O)NO)c1ccc(O)cc1 |r| Show InChI InChI=1S/C27H23N3O5/c1-27(34,21-12-16-23(31)17-13-21)24(26(33)30-35)29-25(32)20-10-6-18(7-11-20)4-2-3-5-19-8-14-22(28)15-9-19/h6-17,24,31,34-35H,28H2,1H3,(H,29,32)(H,30,33)/t24-,27+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC enzyme using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetyl glucosamine and [gamma-32P] UDP-3-O-[(R)-3-hydroxymyristoy... |
J Med Chem 56: 6954-6966 (2013)
Article DOI: 10.1021/jm4007774
BindingDB Entry DOI: 10.7270/Q28K7D11 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50074960

((R)-2-(3,4-Dimethoxy-5-propyl-phenyl)-4,5-dihydro-...)Show SMILES CCCc1cc(cc(OC)c1OC)C1=N[C@H](CO1)C(=O)NO |t:14| Show InChI InChI=1S/C15H20N2O5/c1-4-5-9-6-10(7-12(20-2)13(9)21-3)15-16-11(8-22-15)14(18)17-19/h6-7,11,19H,4-5,8H2,1-3H3,(H,17,18)/t11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Proc Natl Acad Sci U S A 104: 18433-8 (2007)
Article DOI: 10.1073/pnas.0709412104
BindingDB Entry DOI: 10.7270/Q2SB48HR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50478376

(BB-78485 | CHEMBL261713)Show SMILES ONC(=O)[C@@H](Cc1ccc2ccccc2c1)NS(=O)(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C23H20N2O4S/c26-23(24-27)22(14-16-9-10-17-5-1-3-7-19(17)13-16)25-30(28,29)21-12-11-18-6-2-4-8-20(18)15-21/h1-13,15,22,25,27H,14H2,(H,24,26)/t22-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Proc Natl Acad Sci U S A 104: 18433-8 (2007)
Article DOI: 10.1073/pnas.0709412104
BindingDB Entry DOI: 10.7270/Q2SB48HR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50478376

(BB-78485 | CHEMBL261713)Show SMILES ONC(=O)[C@@H](Cc1ccc2ccccc2c1)NS(=O)(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C23H20N2O4S/c26-23(24-27)22(14-16-9-10-17-5-1-3-7-19(17)13-16)25-30(28,29)21-12-11-18-6-2-4-8-20(18)15-21/h1-13,15,22,25,27H,14H2,(H,24,26)/t22-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC Q202W/G210S mutant |
Proc Natl Acad Sci U S A 104: 18433-8 (2007)
Article DOI: 10.1073/pnas.0709412104
BindingDB Entry DOI: 10.7270/Q2SB48HR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50483252

(CHEMBL1643369)Show SMILES C[C@@H](O)[C@H](NC(=O)c1ccc(cc1)C#CC#Cc1ccc(N)cc1)C(=O)NO |r| Show InChI InChI=1S/C21H19N3O4/c1-14(25)19(21(27)24-28)23-20(26)17-10-6-15(7-11-17)4-2-3-5-16-8-12-18(22)13-9-16/h6-14,19,25,28H,22H2,1H3,(H,23,26)(H,24,27)/t14-,19+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Bioorg Med Chem 19: 852-60 (2011)
Article DOI: 10.1016/j.bmc.2010.12.017
BindingDB Entry DOI: 10.7270/Q2DV1NQM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50483252

(CHEMBL1643369)Show SMILES C[C@@H](O)[C@H](NC(=O)c1ccc(cc1)C#CC#Cc1ccc(N)cc1)C(=O)NO |r| Show InChI InChI=1S/C21H19N3O4/c1-14(25)19(21(27)24-28)23-20(26)17-10-6-15(7-11-17)4-2-3-5-16-8-12-18(22)13-9-16/h6-14,19,25,28H,22H2,1H3,(H,23,26)(H,24,27)/t14-,19+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Bioorg Med Chem 19: 852-60 (2011)
Article DOI: 10.1016/j.bmc.2010.12.017
BindingDB Entry DOI: 10.7270/Q2DV1NQM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50074960

((R)-2-(3,4-Dimethoxy-5-propyl-phenyl)-4,5-dihydro-...)Show SMILES CCCc1cc(cc(OC)c1OC)C1=N[C@H](CO1)C(=O)NO |t:14| Show InChI InChI=1S/C15H20N2O5/c1-4-5-9-6-10(7-12(20-2)13(9)21-3)15-16-11(8-22-15)14(18)17-19/h6-7,11,19H,4-5,8H2,1-3H3,(H,17,18)/t11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC Q202W/G210S mutant |
Proc Natl Acad Sci U S A 104: 18433-8 (2007)
Article DOI: 10.1073/pnas.0709412104
BindingDB Entry DOI: 10.7270/Q2SB48HR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50200120

(CHEMBL260091 | CHIR-090 | US10875832, Compound ChI...)Show SMILES C[C@@H](O)[C@H](NC(=O)c1ccc(cc1)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO |r| Show InChI InChI=1S/C24H27N3O5/c1-17(28)22(24(30)26-31)25-23(29)21-10-8-19(9-11-21)3-2-18-4-6-20(7-5-18)16-27-12-14-32-15-13-27/h4-11,17,22,28,31H,12-16H2,1H3,(H,25,29)(H,26,30)/t17-,22+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Proc Natl Acad Sci U S A 104: 18433-8 (2007)
Article DOI: 10.1073/pnas.0709412104
BindingDB Entry DOI: 10.7270/Q2SB48HR |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50200120

(CHEMBL260091 | CHIR-090 | US10875832, Compound ChI...)Show SMILES C[C@@H](O)[C@H](NC(=O)c1ccc(cc1)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO |r| Show InChI InChI=1S/C24H27N3O5/c1-17(28)22(24(30)26-31)25-23(29)21-10-8-19(9-11-21)3-2-18-4-6-20(7-5-18)16-27-12-14-32-15-13-27/h4-11,17,22,28,31H,12-16H2,1H3,(H,25,29)(H,26,30)/t17-,22+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC enzyme |
J Med Chem 56: 6954-6966 (2013)
Article DOI: 10.1021/jm4007774
BindingDB Entry DOI: 10.7270/Q28K7D11 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM92267

(CS257)Show SMILES C[C@@H](O)[C@H](NC(=O)c1ccc(cc1)C#CC#Cc1ccccc1)C(=O)NO Show InChI InChI=1S/C21H18N2O4/c1-15(24)19(21(26)23-27)22-20(25)18-13-11-17(12-14-18)10-6-5-9-16-7-3-2-4-8-16/h2-4,7-8,11-15,19,24,27H,1H3,(H,22,25)(H,23,26)/t15-,19+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Bioorg Med Chem 19: 852-60 (2011)
Article DOI: 10.1016/j.bmc.2010.12.017
BindingDB Entry DOI: 10.7270/Q2DV1NQM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM92267

(CS257)Show SMILES C[C@@H](O)[C@H](NC(=O)c1ccc(cc1)C#CC#Cc1ccccc1)C(=O)NO Show InChI InChI=1S/C21H18N2O4/c1-15(24)19(21(26)23-27)22-20(25)18-13-11-17(12-14-18)10-6-5-9-16-7-3-2-4-8-16/h2-4,7-8,11-15,19,24,27H,1H3,(H,22,25)(H,23,26)/t15-,19+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Bioorg Med Chem 19: 852-60 (2011)
Article DOI: 10.1016/j.bmc.2010.12.017
BindingDB Entry DOI: 10.7270/Q2DV1NQM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50200120

(CHEMBL260091 | CHIR-090 | US10875832, Compound ChI...)Show SMILES C[C@@H](O)[C@H](NC(=O)c1ccc(cc1)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO |r| Show InChI InChI=1S/C24H27N3O5/c1-17(28)22(24(30)26-31)25-23(29)21-10-8-19(9-11-21)3-2-18-4-6-20(7-5-18)16-27-12-14-32-15-13-27/h4-11,17,22,28,31H,12-16H2,1H3,(H,25,29)(H,26,30)/t17-,22+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Bioorg Med Chem Lett 21: 1155-61 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.111
BindingDB Entry DOI: 10.7270/Q2Q52SFZ |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50200120

(CHEMBL260091 | CHIR-090 | US10875832, Compound ChI...)Show SMILES C[C@@H](O)[C@H](NC(=O)c1ccc(cc1)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO |r| Show InChI InChI=1S/C24H27N3O5/c1-17(28)22(24(30)26-31)25-23(29)21-10-8-19(9-11-21)3-2-18-4-6-20(7-5-18)16-27-12-14-32-15-13-27/h4-11,17,22,28,31H,12-16H2,1H3,(H,25,29)(H,26,30)/t17-,22+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50200120

(CHEMBL260091 | CHIR-090 | US10875832, Compound ChI...)Show SMILES C[C@@H](O)[C@H](NC(=O)c1ccc(cc1)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO |r| Show InChI InChI=1S/C24H27N3O5/c1-17(28)22(24(30)26-31)25-23(29)21-10-8-19(9-11-21)3-2-18-4-6-20(7-5-18)16-27-12-14-32-15-13-27/h4-11,17,22,28,31H,12-16H2,1H3,(H,25,29)(H,26,30)/t17-,22+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC Q202W/G210S mutant |
Proc Natl Acad Sci U S A 104: 18433-8 (2007)
Article DOI: 10.1073/pnas.0709412104
BindingDB Entry DOI: 10.7270/Q2SB48HR |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50200120

(CHEMBL260091 | CHIR-090 | US10875832, Compound ChI...)Show SMILES C[C@@H](O)[C@H](NC(=O)c1ccc(cc1)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO |r| Show InChI InChI=1S/C24H27N3O5/c1-17(28)22(24(30)26-31)25-23(29)21-10-8-19(9-11-21)3-2-18-4-6-20(7-5-18)16-27-12-14-32-15-13-27/h4-11,17,22,28,31H,12-16H2,1H3,(H,25,29)(H,26,30)/t17-,22+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Westf£lischen Wilhelms-Universit£t M£nster
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetylglucosamine as substrate incubated for 30 mins prior to enzyme add... |
Bioorg Med Chem 22: 1016-28 (2014)
Article DOI: 10.1016/j.bmc.2013.12.057
BindingDB Entry DOI: 10.7270/Q2X92F7C |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50478376

(BB-78485 | CHEMBL261713)Show SMILES ONC(=O)[C@@H](Cc1ccc2ccccc2c1)NS(=O)(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C23H20N2O4S/c26-23(24-27)22(14-16-9-10-17-5-1-3-7-19(17)13-16)25-30(28,29)21-12-11-18-6-2-4-8-20(18)15-21/h1-13,15,22,25,27H,14H2,(H,24,26)/t22-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50074960

((R)-2-(3,4-Dimethoxy-5-propyl-phenyl)-4,5-dihydro-...)Show SMILES CCCc1cc(cc(OC)c1OC)C1=N[C@H](CO1)C(=O)NO |t:14| Show InChI InChI=1S/C15H20N2O5/c1-4-5-9-6-10(7-12(20-2)13(9)21-3)15-16-11(8-22-15)14(18)17-19/h6-7,11,19H,4-5,8H2,1-3H3,(H,17,18)/t11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Bioorg Med Chem Lett 21: 1155-61 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.111
BindingDB Entry DOI: 10.7270/Q2Q52SFZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50074960

((R)-2-(3,4-Dimethoxy-5-propyl-phenyl)-4,5-dihydro-...)Show SMILES CCCc1cc(cc(OC)c1OC)C1=N[C@H](CO1)C(=O)NO |t:14| Show InChI InChI=1S/C15H20N2O5/c1-4-5-9-6-10(7-12(20-2)13(9)21-3)15-16-11(8-22-15)14(18)17-19/h6-7,11,19H,4-5,8H2,1-3H3,(H,17,18)/t11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC enzyme |
J Med Chem 56: 6954-6966 (2013)
Article DOI: 10.1021/jm4007774
BindingDB Entry DOI: 10.7270/Q28K7D11 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50074960

((R)-2-(3,4-Dimethoxy-5-propyl-phenyl)-4,5-dihydro-...)Show SMILES CCCc1cc(cc(OC)c1OC)C1=N[C@H](CO1)C(=O)NO |t:14| Show InChI InChI=1S/C15H20N2O5/c1-4-5-9-6-10(7-12(20-2)13(9)21-3)15-16-11(8-22-15)14(18)17-19/h6-7,11,19H,4-5,8H2,1-3H3,(H,17,18)/t11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50495314

(CHEMBL3103559)Show SMILES OC[C@@H](OCC(=O)NO)c1ccc(cc1)C#Cc1ccccc1 |r| Show InChI InChI=1S/C18H17NO4/c20-12-17(23-13-18(21)19-22)16-10-8-15(9-11-16)7-6-14-4-2-1-3-5-14/h1-5,8-11,17,20,22H,12-13H2,(H,19,21)/t17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Westf£lischen Wilhelms-Universit£t M£nster
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetylglucosamine as substrate incubated for 30 mins prior to enzyme add... |
Bioorg Med Chem 22: 1016-28 (2014)
Article DOI: 10.1016/j.bmc.2013.12.057
BindingDB Entry DOI: 10.7270/Q2X92F7C |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50495317

(CHEMBL3103561)Show SMILES OC[C@@H](OCC(=O)NO)c1ccc(cc1)C#Cc1ccc(CN2CCOCC2)cc1 |r| Show InChI InChI=1S/C23H26N2O5/c26-16-22(30-17-23(27)24-28)21-9-7-19(8-10-21)2-1-18-3-5-20(6-4-18)15-25-11-13-29-14-12-25/h3-10,22,26,28H,11-17H2,(H,24,27)/t22-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Westf£lischen Wilhelms-Universit£t M£nster
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetylglucosamine as substrate incubated for 30 mins prior to enzyme add... |
Bioorg Med Chem 22: 1016-28 (2014)
Article DOI: 10.1016/j.bmc.2013.12.057
BindingDB Entry DOI: 10.7270/Q2X92F7C |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50495323

(CHEMBL3103548)Show SMILES OC[C@H](O[C@H](CO)c1ccc(cc1)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO |r| Show InChI InChI=1S/C24H28N2O6/c27-16-22(32-23(17-28)24(29)25-30)21-9-7-19(8-10-21)2-1-18-3-5-20(6-4-18)15-26-11-13-31-14-12-26/h3-10,22-23,27-28,30H,11-17H2,(H,25,29)/t22-,23+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 358 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Westf£lischen Wilhelms-Universit£t M£nster
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetylglucosamine as substrate incubated for 30 mins prior to enzyme add... |
Bioorg Med Chem 22: 1016-28 (2014)
Article DOI: 10.1016/j.bmc.2013.12.057
BindingDB Entry DOI: 10.7270/Q2X92F7C |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50483375

(CHEMBL1236446)Show SMILES CCCCCCCCCCCCCC(=O)O[C@@H]1[C@@H](CC(=O)NO)CO[C@H](CO)[C@H]1O |r| Show InChI InChI=1S/C22H41NO7/c1-2-3-4-5-6-7-8-9-10-11-12-13-20(26)30-22-17(14-19(25)23-28)16-29-18(15-24)21(22)27/h17-18,21-22,24,27-28H,2-16H2,1H3,(H,23,25)/t17-,18+,21+,22+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Bioorg Med Chem Lett 21: 1155-61 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.111
BindingDB Entry DOI: 10.7270/Q2Q52SFZ |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50483375

(CHEMBL1236446)Show SMILES CCCCCCCCCCCCCC(=O)O[C@@H]1[C@@H](CC(=O)NO)CO[C@H](CO)[C@H]1O |r| Show InChI InChI=1S/C22H41NO7/c1-2-3-4-5-6-7-8-9-10-11-12-13-20(26)30-22-17(14-19(25)23-28)16-29-18(15-24)21(22)27/h17-18,21-22,24,27-28H,2-16H2,1H3,(H,23,25)/t17-,18+,21+,22+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50495312

(CHEMBL3103550)Show InChI InChI=1S/C22H24N2O4/c25-22(23-26)17-28-16-21-9-5-19(6-10-21)2-1-18-3-7-20(8-4-18)15-24-11-13-27-14-12-24/h3-10,26H,11-17H2,(H,23,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Westf£lischen Wilhelms-Universit£t M£nster
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetylglucosamine as substrate incubated for 30 mins prior to enzyme add... |
Bioorg Med Chem 22: 1016-28 (2014)
Article DOI: 10.1016/j.bmc.2013.12.057
BindingDB Entry DOI: 10.7270/Q2X92F7C |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50495316
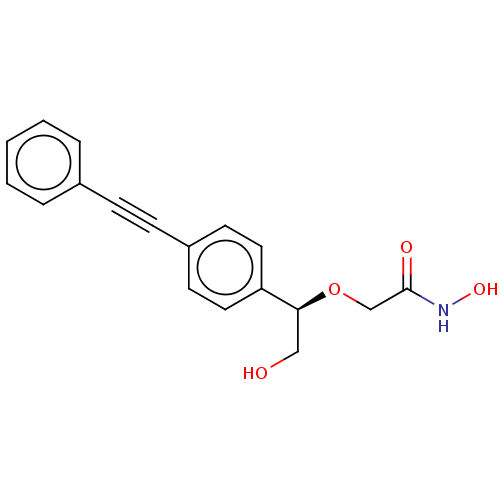
(CHEMBL3103560)Show SMILES OC[C@H](OCC(=O)NO)c1ccc(cc1)C#Cc1ccccc1 |r| Show InChI InChI=1S/C18H17NO4/c20-12-17(23-13-18(21)19-22)16-10-8-15(9-11-16)7-6-14-4-2-1-3-5-14/h1-5,8-11,17,20,22H,12-13H2,(H,19,21)/t17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Westf£lischen Wilhelms-Universit£t M£nster
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetylglucosamine as substrate incubated for 30 mins prior to enzyme add... |
Bioorg Med Chem 22: 1016-28 (2014)
Article DOI: 10.1016/j.bmc.2013.12.057
BindingDB Entry DOI: 10.7270/Q2X92F7C |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50495311
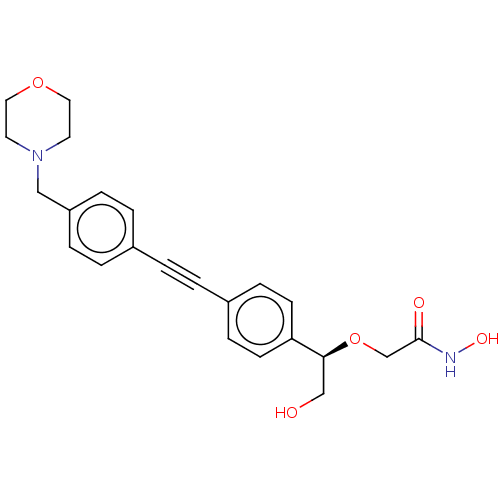
(CHEMBL3103562)Show SMILES OC[C@H](OCC(=O)NO)c1ccc(cc1)C#Cc1ccc(CN2CCOCC2)cc1 |r| Show InChI InChI=1S/C23H26N2O5/c26-16-22(30-17-23(27)24-28)21-9-7-19(8-10-21)2-1-18-3-5-20(6-4-18)15-25-11-13-29-14-12-25/h3-10,22,26,28H,11-17H2,(H,24,27)/t22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Westf£lischen Wilhelms-Universit£t M£nster
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetylglucosamine as substrate incubated for 30 mins prior to enzyme add... |
Bioorg Med Chem 22: 1016-28 (2014)
Article DOI: 10.1016/j.bmc.2013.12.057
BindingDB Entry DOI: 10.7270/Q2X92F7C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data