

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50061669 ((R)-2-(Benzylamino-methyl)-chroman-7-ol; oxalic ac...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a |
University of Amsterdam Curated by ChEMBL | Assay Description Agonist activity at human dopamine D2L receptor expressed in HEK293 cells assessed as inhibition of forskolin-induced cAMP accumulation after 30 mins... | J Med Chem 57: 391-410 (2014) Article DOI: 10.1021/jm401384w BindingDB Entry DOI: 10.7270/Q2WS8VQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50446924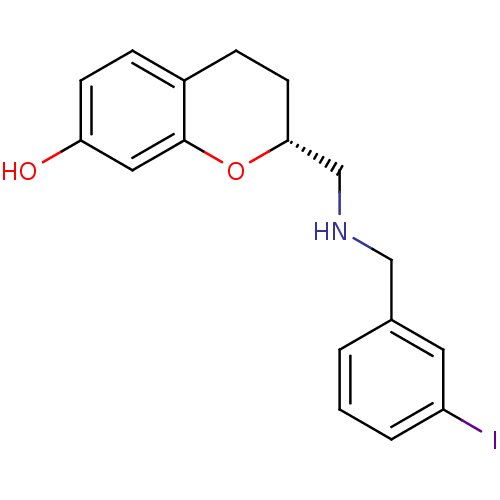 (CHEMBL3115575) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0316 | n/a | n/a | n/a | n/a |
University of Amsterdam Curated by ChEMBL | Assay Description Agonist activity at human dopamine D2L receptor expressed in HEK293 cells assessed as inhibition of forskolin-induced cAMP accumulation after 30 mins... | J Med Chem 57: 391-410 (2014) Article DOI: 10.1021/jm401384w BindingDB Entry DOI: 10.7270/Q2WS8VQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50007422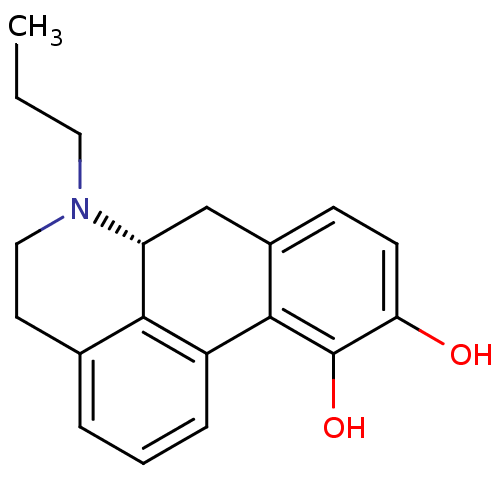 ((+)-6-Propyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]q...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0398 | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Agonist activity at D2 long receptor (unknown origin) transfected in human HEK293T cells assessed as increase in cAMP accumulation incubated for 2 hr... | ACS Med Chem Lett 11: 385-392 (2020) Article DOI: 10.1021/acsmedchemlett.9b00575 BindingDB Entry DOI: 10.7270/Q25X2D8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50007422 ((+)-6-Propyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]q...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Agonist activity at D2 long receptor (unknown origin) transfected in human HEK293T cells assessed as increase in cAMP accumulation incubated for 2 hr... | ACS Med Chem Lett 11: 385-392 (2020) Article DOI: 10.1021/acsmedchemlett.9b00575 BindingDB Entry DOI: 10.7270/Q25X2D8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50446928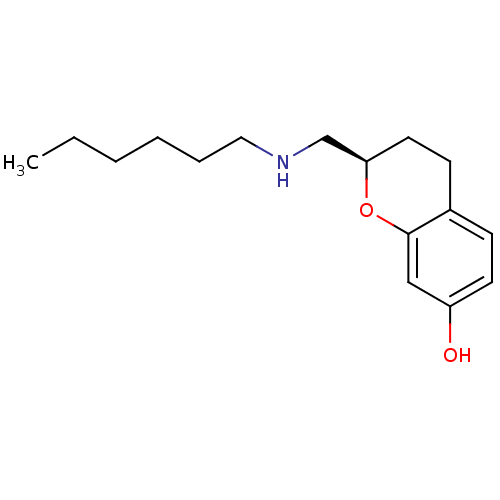 (CHEMBL3115585) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0501 | n/a | n/a | n/a | n/a |
University of Amsterdam Curated by ChEMBL | Assay Description Agonist activity at human dopamine D2L receptor expressed in HEK293 cells assessed as inhibition of forskolin-induced cAMP accumulation after 30 mins... | J Med Chem 57: 391-410 (2014) Article DOI: 10.1021/jm401384w BindingDB Entry DOI: 10.7270/Q2WS8VQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50233623 (CHEMBL4104013) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0955 | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Agonist activity at human dopamine D2L receptor expressed in F1pIn CHO cells assessed as increase in forskolin-mediated cAMP accumulation incubated f... | J Med Chem 58: 1550-5 (2015) Article DOI: 10.1021/jm5013243 BindingDB Entry DOI: 10.7270/Q2F76FTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50233623 (CHEMBL4104013) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0960 | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Agonist activity at human dopamine D2L receptor expressed in F1pIn CHO cells assessed as increase in forskolin-mediated cAMP accumulation incubated f... | J Med Chem 58: 1550-5 (2015) Article DOI: 10.1021/jm5013243 BindingDB Entry DOI: 10.7270/Q2F76FTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM185688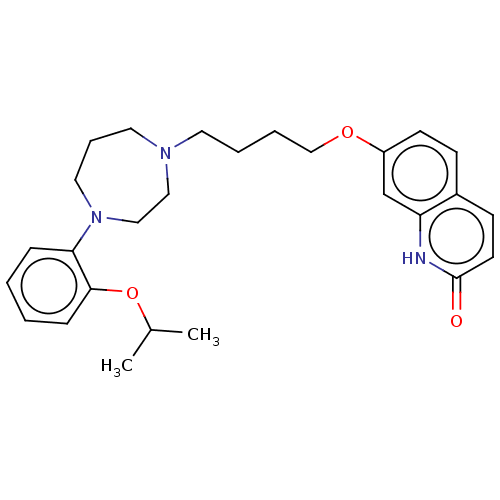 (UNC10107969 | US9156822, 18) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | 7.4 | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description HEK293T cells co-expressing the cAMP biosensor GloSensor-22F (Promega) and hD2 receptors were seeded (10,000 cells/20 ul/well) into white, clear-bott... | US Patent US9156822 (2015) BindingDB Entry DOI: 10.7270/Q2H41Q6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50237166 (CHEMBL4097330) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Agonist activity at N-terminal flag-tagged D2S receptor (unknown origin) expressed in HEK293 cells coexpressing renilla luciferase 2-tagged GalphaoB ... | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50237162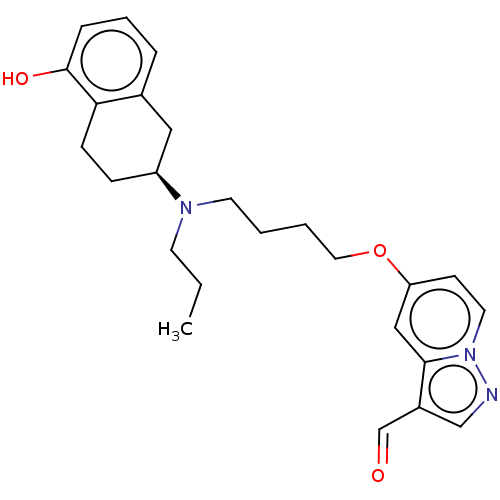 (CHEMBL4090208) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Agonist activity at N-terminal flag-tagged D2S receptor (unknown origin) expressed in HEK293 cells coexpressing renilla luciferase 2-tagged GalphaoA ... | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50237162 (CHEMBL4090208) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Agonist activity at N-terminal flag-tagged D2S receptor (unknown origin) expressed in HEK293 cells coexpressing renilla luciferase 2-tagged GalphaoB ... | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50237169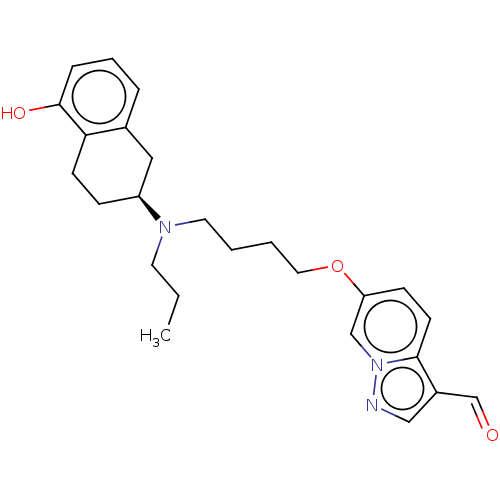 (CHEMBL4071185) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Agonist activity at N-terminal flag-tagged D2S receptor (unknown origin) expressed in HEK293 cells coexpressing renilla luciferase 2-tagged GalphaoB ... | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50236740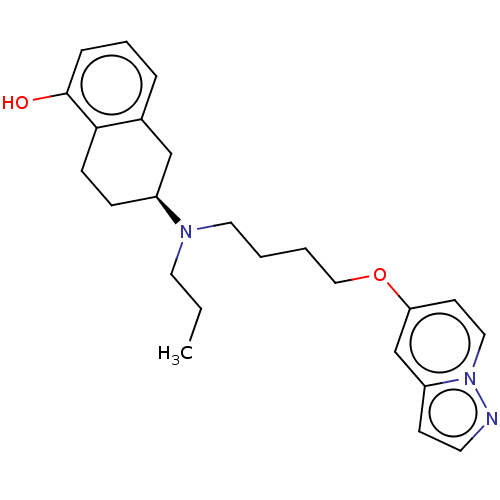 (CHEMBL4078348) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Agonist activity at N-terminal flag-tagged D2S receptor (unknown origin) expressed in HEK293 cells coexpressing renilla luciferase 2-tagged GalphaoA ... | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50236740 (CHEMBL4078348) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Agonist activity at N-terminal flag-tagged D2S receptor (unknown origin) expressed in HEK293 cells coexpressing renilla luciferase 2-tagged GalphaoB ... | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50237169 (CHEMBL4071185) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Concentration required for the inhibitory activity against human Farnesyltransferase | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50237166 (CHEMBL4097330) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Agonist activity at N-terminal flag-tagged D2S receptor (unknown origin) expressed in HEK293 cells coexpressing renilla luciferase 2-tagged GalphaoA ... | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50444830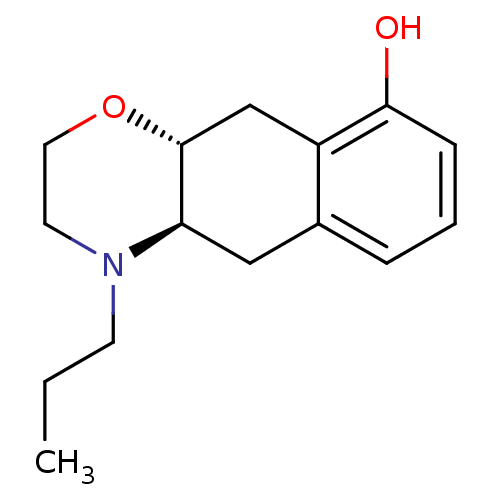 (CHEMBL3099226) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Agonist activity at human dopamine D2 receptor expressed in CHO cells assessed as increase of forskolin-induced cAMP production after 20 mins | Bioorg Med Chem 22: 381-92 (2013) Article DOI: 10.1016/j.bmc.2013.11.012 BindingDB Entry DOI: 10.7270/Q26T0P3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50446926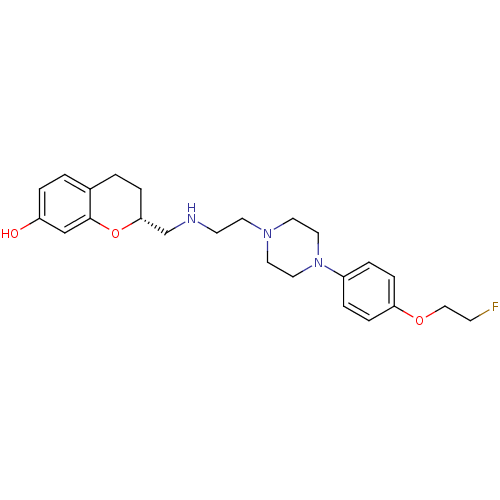 (CHEMBL3115582) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.158 | n/a | n/a | n/a | n/a |
University of Amsterdam Curated by ChEMBL | Assay Description Agonist activity at human dopamine D2L receptor expressed in HEK293 cells assessed as inhibition of forskolin-induced cAMP accumulation after 30 mins... | J Med Chem 57: 391-410 (2014) Article DOI: 10.1021/jm401384w BindingDB Entry DOI: 10.7270/Q2WS8VQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50237158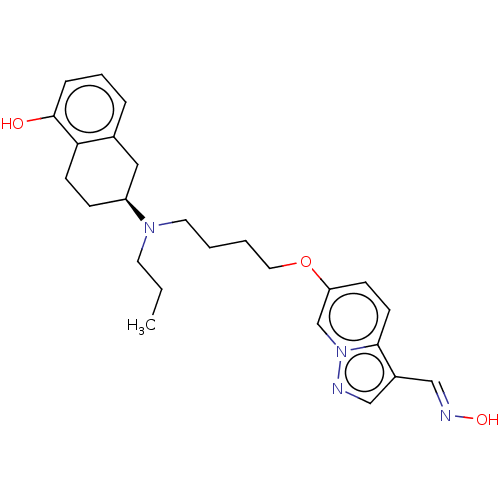 (CHEMBL4071868) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Agonist activity at N-terminal flag-tagged D2S receptor (unknown origin) expressed in HEK293 cells coexpressing renilla luciferase 2-tagged GalphaoB ... | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50237165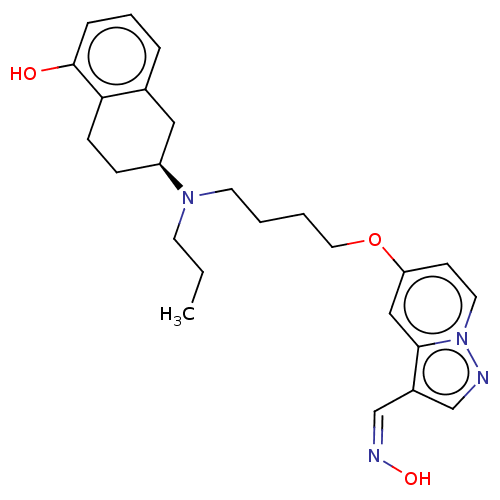 (CHEMBL4098859) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Agonist activity at N-terminal flag-tagged D2S receptor (unknown origin) expressed in HEK293 cells coexpressing renilla luciferase 2-tagged GalphaoB ... | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50273465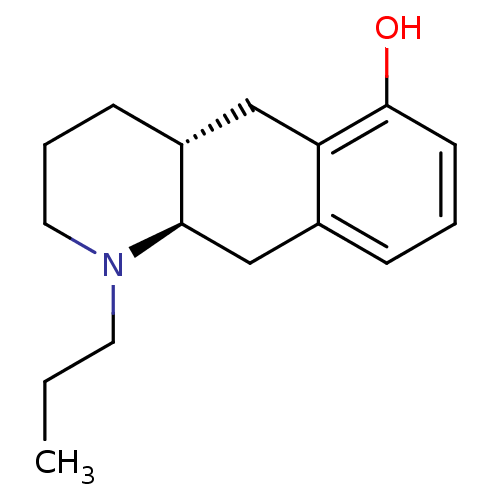 ((+/-)-trans-1-Propyl-1,2,3,4,4a,5,10,10a-octahydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Agonist activity at human dopamine D2 receptor expressed in CHO cells assessed as increase of forskolin-induced cAMP production after 20 mins | Bioorg Med Chem 22: 381-92 (2013) Article DOI: 10.1016/j.bmc.2013.11.012 BindingDB Entry DOI: 10.7270/Q26T0P3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50443101 (Cariprazine | RGH-188) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | DrugBank | n/a | n/a | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50237158 (CHEMBL4071868) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Concentration required for the inhibitory activity against human Farnesyltransferase | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM185687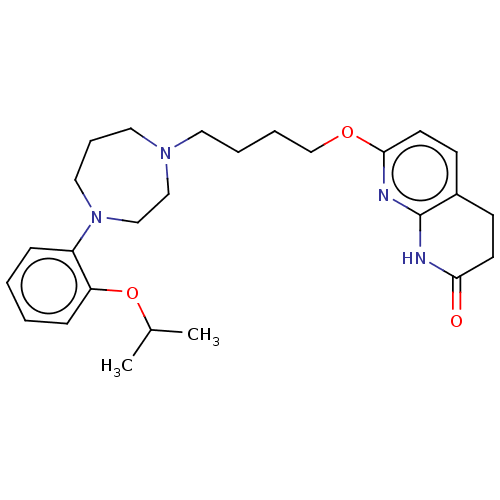 (UNC10107968 | US9156822, 17) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 0.200 | n/a | n/a | 7.4 | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description HEK293T cells co-expressing the cAMP biosensor GloSensor-22F (Promega) and hD2 receptors were seeded (10,000 cells/20 ul/well) into white, clear-bott... | US Patent US9156822 (2015) BindingDB Entry DOI: 10.7270/Q2H41Q6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50237165 (CHEMBL4098859) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Agonist activity at N-terminal flag-tagged D2S receptor (unknown origin) expressed in HEK293 cells coexpressing renilla luciferase 2-tagged GalphaoA ... | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50184795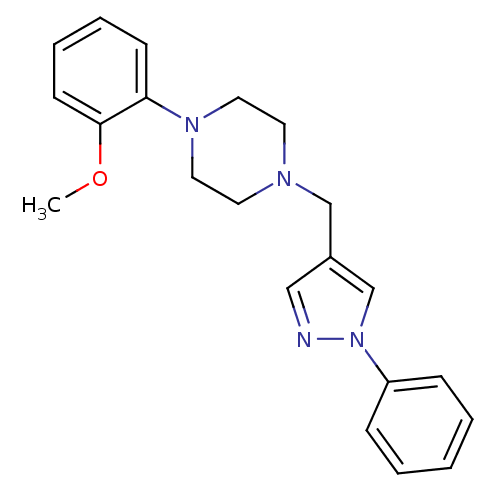 (1-(2-methoxyphenyl)-4-((1-phenyl-1H-pyrazol-4-yl)m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Curated by ChEMBL | Assay Description Intrinsic activity against dopamine D2(short) receptor assessed as [3H]thymidine uptake in CHO cells by mitogenesis assay | Bioorg Med Chem Lett 16: 2955-9 (2006) Article DOI: 10.1016/j.bmcl.2006.02.075 BindingDB Entry DOI: 10.7270/Q21N80QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50595104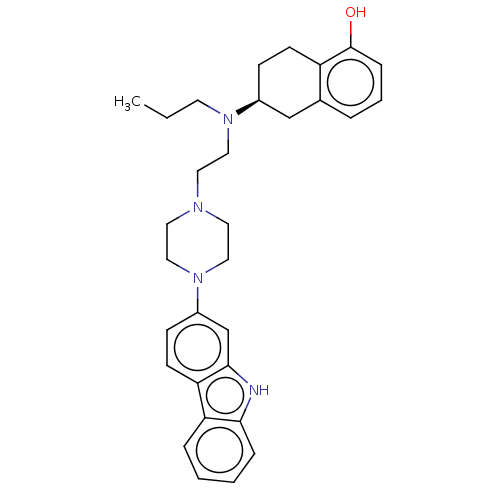 (CHEMBL5192367) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114378 BindingDB Entry DOI: 10.7270/Q2377DQ0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50533790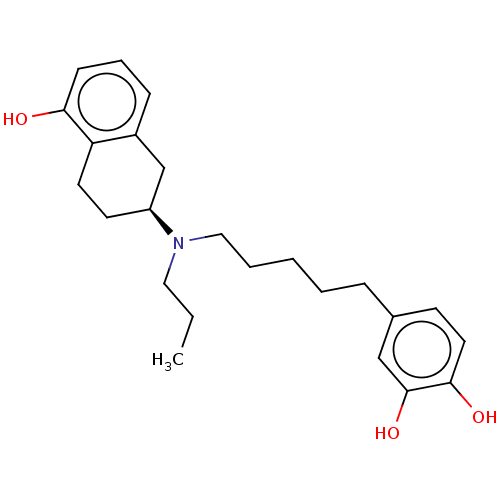 (CHEMBL4538230) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Agonist activity at human D2 dopamine receptor expressed in CHO cells by [35S]GTPgammaS binding assay | Bioorg Med Chem 24: 5088-5102 (2016) Article DOI: 10.1016/j.bmc.2016.08.021 BindingDB Entry DOI: 10.7270/Q2B85CNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50184795 (1-(2-methoxyphenyl)-4-((1-phenyl-1H-pyrazol-4-yl)m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Curated by ChEMBL | Assay Description Intrinsic activity against dopamine D2(long) receptor assessed as [3H]thymidine uptake in CHO cells by mitogenesis assay | Bioorg Med Chem Lett 16: 2955-9 (2006) Article DOI: 10.1016/j.bmcl.2006.02.075 BindingDB Entry DOI: 10.7270/Q21N80QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50237166 (CHEMBL4097330) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Agonist activity at N-terminal flag-tagged D2S receptor (unknown origin) expressed in HEK293 cells coexpressing renilla luciferase 2-tagged Galphai1 ... | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50164848 (CHEMBL3798179) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Agonistic activity against progesterone receptor in alkaline phosphatase assay using human T47D breast carcinoma cell line | J Med Chem 60: 2890-2907 (2017) Article DOI: 10.1021/acs.jmedchem.6b01875 BindingDB Entry DOI: 10.7270/Q28P62SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50395564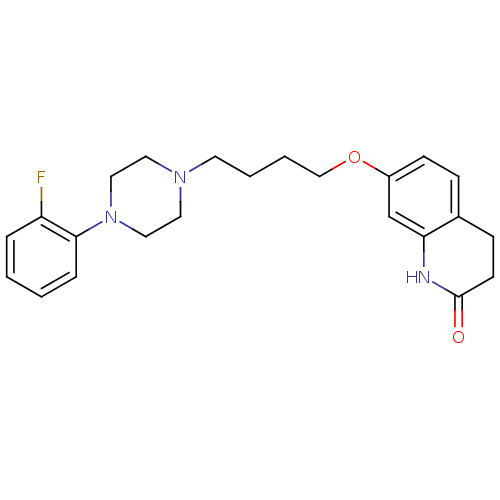 (CHEMBL160630) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Agonist activity at human D2L receptor expressed in HEK293T cells coexpressing Gi subunit assessed as inhibition of isoproterenol-stimulated cAMP pro... | J Med Chem 55: 7141-53 (2012) Article DOI: 10.1021/jm300603y BindingDB Entry DOI: 10.7270/Q2JD4XXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50511513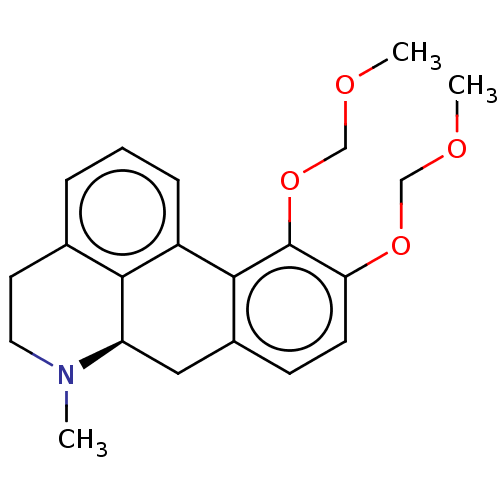 (CHEMBL4591021) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Agonist activity at C-terminal RLuc8-fused D2 long receptor (unknown origin) transfected in human HEK293T cells co-expressing N-terminal Venus-tagged... | ACS Med Chem Lett 11: 385-392 (2020) Article DOI: 10.1021/acsmedchemlett.9b00575 BindingDB Entry DOI: 10.7270/Q25X2D8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50021770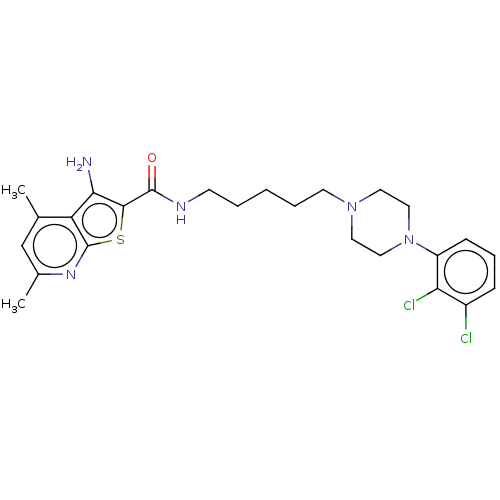 (CHEMBL3298895) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Agonist activity at human D2L receptor expressed in FlpIn CHO cells assessed as inhibition of forskolin-stimulated cAMP production treated for 30 min... | J Med Chem 57: 4924-39 (2014) Article DOI: 10.1021/jm500457x BindingDB Entry DOI: 10.7270/Q29Z96FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50164848 (CHEMBL3798179) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at dopamine D2 receptor (unknown origin) by cAMP accumulation assay | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50237169 (CHEMBL4071185) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Concentration required for the inhibitory activity against human Farnesyltransferase | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50511513 (CHEMBL4591021) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.316 | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Agonist activity at C-terminal RLuc8-fused D2 long receptor (unknown origin) transfected in human HEK293T cells co-expressing N-terminal Venus-tagged... | ACS Med Chem Lett 11: 385-392 (2020) Article DOI: 10.1021/acsmedchemlett.9b00575 BindingDB Entry DOI: 10.7270/Q25X2D8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50002173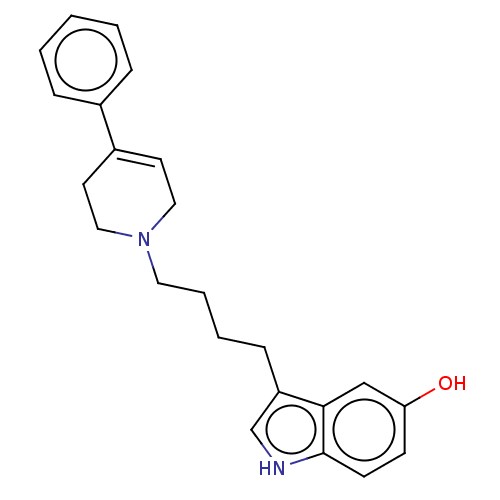 (3-(4-(3,6-dihydro-4-phenyl-1(2H)-pyridinyl)butyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Compound was evaluated for effective concentration in vivo for Dopamine receptor D2 mitogenesis. (95% confidence intervals) | J Med Chem 39: 3179-87 (1996) Article DOI: 10.1021/jm950721m BindingDB Entry DOI: 10.7270/Q23N22GZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50511516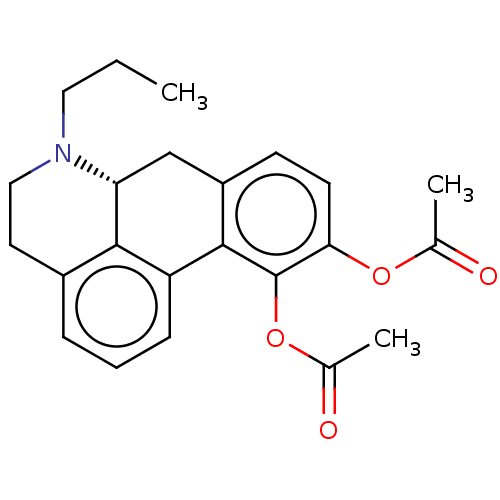 (CHEMBL4555547) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.372 | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Agonist activity at D2 long receptor (unknown origin) transfected in human HEK293T cells assessed as increase in cAMP accumulation incubated for 2 hr... | ACS Med Chem Lett 11: 385-392 (2020) Article DOI: 10.1021/acsmedchemlett.9b00575 BindingDB Entry DOI: 10.7270/Q25X2D8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50511516 (CHEMBL4555547) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.373 | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Agonist activity at D2 long receptor (unknown origin) transfected in human HEK293T cells assessed as increase in cAMP accumulation incubated for 2 hr... | ACS Med Chem Lett 11: 385-392 (2020) Article DOI: 10.1021/acsmedchemlett.9b00575 BindingDB Entry DOI: 10.7270/Q25X2D8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50237158 (CHEMBL4071868) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Agonist activity at N-terminal flag-tagged D2S receptor (unknown origin) expressed in HEK293 cells coexpressing renilla luciferase 2-tagged Galphai1 ... | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50237162 (CHEMBL4090208) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Agonist activity at N-terminal flag-tagged D2S receptor (unknown origin) expressed in HEK293 cells coexpressing renilla luciferase 2-tagged Galphai1 ... | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50021796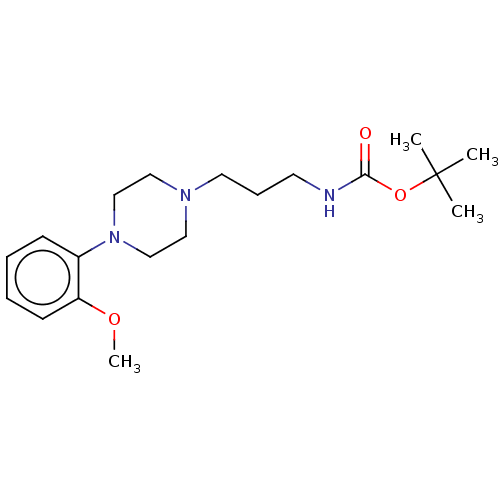 (CHEMBL3299094) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Partial agonist activity at human D2L receptor expressed in FlpIn CHO cells assessed as induction of ERK1/2 phosphorylation by AlphaScreen assay | J Med Chem 57: 4924-39 (2014) Article DOI: 10.1021/jm500457x BindingDB Entry DOI: 10.7270/Q29Z96FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50446927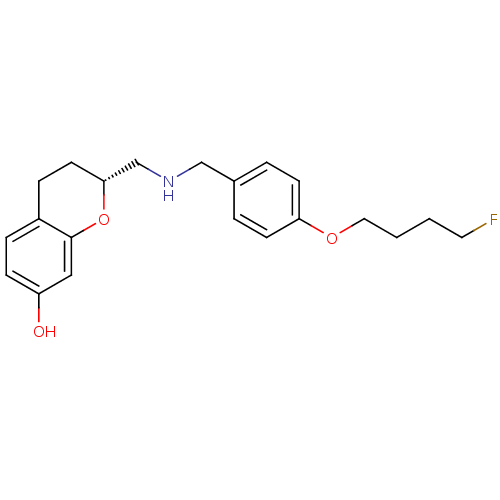 (CHEMBL3115579) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.398 | n/a | n/a | n/a | n/a |
University of Amsterdam Curated by ChEMBL | Assay Description Agonist activity at human dopamine D2L receptor expressed in HEK293 cells assessed as inhibition of forskolin-induced cAMP accumulation after 30 mins... | J Med Chem 57: 391-410 (2014) Article DOI: 10.1021/jm401384w BindingDB Entry DOI: 10.7270/Q2WS8VQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50443101 (Cariprazine | RGH-188) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | DrugBank Article PubMed | n/a | n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Partial agonist activity at human Gi/o-coupled D2R expressed in HEK293T cells assessed as inhibition of isoproterenol-induced cAMP accumulation prein... | J Med Chem 62: 4755-4771 (2019) Article DOI: 10.1021/acs.jmedchem.9b00508 BindingDB Entry DOI: 10.7270/Q2125X2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50443101 (Cariprazine | RGH-188) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | DrugBank Article PubMed | n/a | n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Partial agonist activity at human Gi/o-coupled D2R expressed in HEK293T cells assessed as inhibition of isoproterenol-induced cAMP accumulation prein... | J Med Chem 62: 4755-4771 (2019) Article DOI: 10.1021/acs.jmedchem.9b00508 BindingDB Entry DOI: 10.7270/Q2125X2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50395565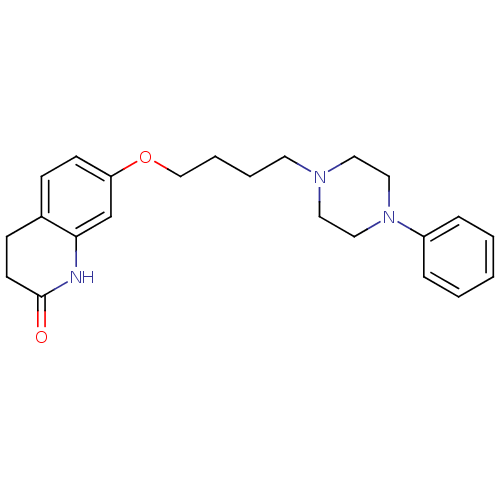 (CHEMBL160357) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Agonist activity at D2L receptor in human HTLA cells assessed as beta arrestin recruitment at 6 uM after 18 hrs by luminescence assay | J Med Chem 55: 7141-53 (2012) Article DOI: 10.1021/jm300603y BindingDB Entry DOI: 10.7270/Q2JD4XXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM185697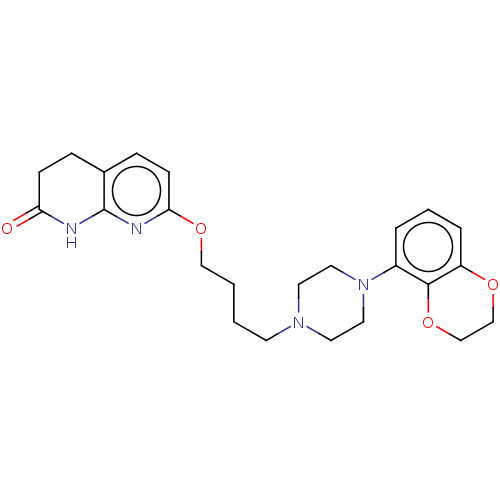 (UNC10108018 | US9156822, 41) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 0.400 | n/a | n/a | 7.4 | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description HEK293T cells co-expressing the cAMP biosensor GloSensor-22F (Promega) and hD2 receptors were seeded (10,000 cells/20 ul/well) into white, clear-bott... | US Patent US9156822 (2015) BindingDB Entry DOI: 10.7270/Q2H41Q6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM185695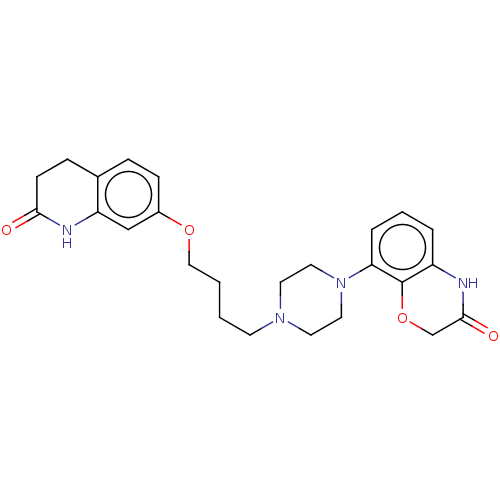 (UNC10108010 | US9156822, 38) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 0.400 | n/a | n/a | 7.4 | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description Recruitment of β-arrestin to agonist-stimulated D2 receptors was performed using a previously described Tango-type assay (Barnea et al., Proc. N... | US Patent US9156822 (2015) BindingDB Entry DOI: 10.7270/Q2H41Q6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50524256 (CHEMBL4451797) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Partial agonist activity at human D2L receptor expressed in HEK293T cells co-expressing Galpahi1/Gbeta1/GFP2-Ggamma2 assessed as Galphai1 dissociatio... | J Med Chem 62: 4755-4771 (2019) Article DOI: 10.1021/acs.jmedchem.9b00508 BindingDB Entry DOI: 10.7270/Q2125X2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1622 total ) | Next | Last >> |