 Found 762 hits of ic50 for UniProtKB: P05771
Found 762 hits of ic50 for UniProtKB: P05771 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50393214

(CHEMBL2151411)Show SMILES CN1CCN(CC1)c1nc(C2=C(C(=O)NC2=O)c2c[nH]c3c(C)cccc23)c2ccccc2n1 |t:11| Show InChI InChI=1S/C26H24N6O2/c1-15-6-5-8-16-18(14-27-22(15)16)20-21(25(34)30-24(20)33)23-17-7-3-4-9-19(17)28-26(29-23)32-12-10-31(2)11-13-32/h3-9,14,27H,10-13H2,1-2H3,(H,30,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCbeta-1 by scintillation proximity assay |
J Med Chem 54: 6028-39 (2011)
Article DOI: 10.1021/jm200469u
BindingDB Entry DOI: 10.7270/Q2K35VR1 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50393218
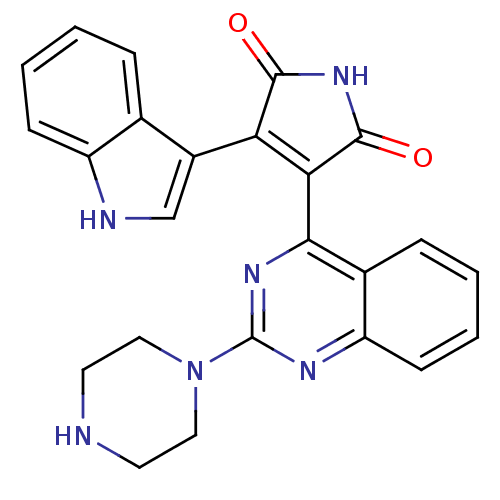
(CHEMBL1996510)Show SMILES O=C1NC(=O)C(=C1c1c[nH]c2ccccc12)c1nc(nc2ccccc12)N1CCNCC1 |c:5| Show InChI InChI=1S/C24H20N6O2/c31-22-19(16-13-26-17-7-3-1-5-14(16)17)20(23(32)29-22)21-15-6-2-4-8-18(15)27-24(28-21)30-11-9-25-10-12-30/h1-8,13,25-26H,9-12H2,(H,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCbeta-1 by scintillation proximity assay |
J Med Chem 54: 6028-39 (2011)
Article DOI: 10.1021/jm200469u
BindingDB Entry DOI: 10.7270/Q2K35VR1 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM33971

(AEB071 | Sotrastaurin | med.21724, Compound 190)Show SMILES CN1CCN(CC1)c1nc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2ccccc2n1 |t:11| Show InChI InChI=1S/C25H22N6O2/c1-30-10-12-31(13-11-30)25-27-19-9-5-3-7-16(19)22(28-25)21-20(23(32)29-24(21)33)17-14-26-18-8-4-2-6-15(17)18/h2-9,14,26H,10-13H2,1H3,(H,29,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01031
BindingDB Entry DOI: 10.7270/Q2FF3XF8 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50235283
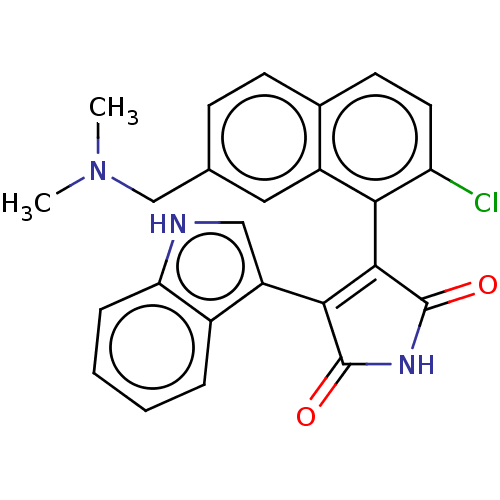
(CHEMBL4102228)Show SMILES CN(C)Cc1ccc2ccc(Cl)c(C3=C(C(=O)NC3=O)c3c[nH]c4ccccc34)c2c1 |t:13| Show InChI InChI=1S/C25H20ClN3O2/c1-29(2)13-14-7-8-15-9-10-19(26)21(17(15)11-14)23-22(24(30)28-25(23)31)18-12-27-20-6-4-3-5-16(18)20/h3-12,27H,13H2,1-2H3,(H,28,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCbeta1 (unknown origin) after 60 mins in presence of [gamma33P]ATP by scintillation proximity assay |
Bioorg Med Chem Lett 27: 781-786 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.038
BindingDB Entry DOI: 10.7270/Q2930WF1 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50393228
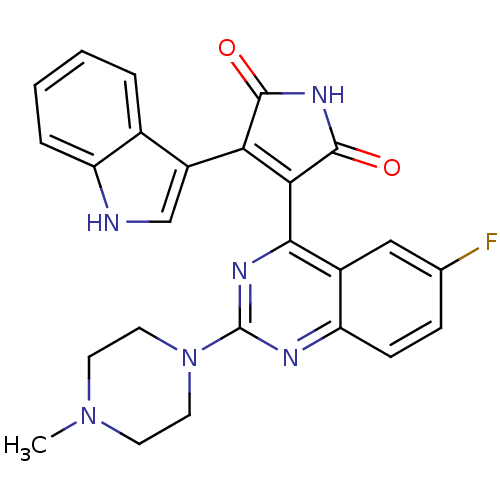
(CHEMBL2153750)Show SMILES CN1CCN(CC1)c1nc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2cc(F)ccc2n1 |t:11| Show InChI InChI=1S/C25H21FN6O2/c1-31-8-10-32(11-9-31)25-28-19-7-6-14(26)12-16(19)22(29-25)21-20(23(33)30-24(21)34)17-13-27-18-5-3-2-4-15(17)18/h2-7,12-13,27H,8-11H2,1H3,(H,30,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCbeta-1 by scintillation proximity assay |
J Med Chem 54: 6028-39 (2011)
Article DOI: 10.1021/jm200469u
BindingDB Entry DOI: 10.7270/Q2K35VR1 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Gachon University
Curated by ChEMBL
| Assay Description
Inhibition of human PKCb2 using Histone H1 as substrate by [gamma-33P]-ATP assay |
Eur J Med Chem 163: 453-480 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.037
BindingDB Entry DOI: 10.7270/Q2TQ650N |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Gachon University
Curated by ChEMBL
| Assay Description
Inhibition of human PKCb2 using Histone H1 as substrate by [gamma-33P]-ATP assay |
Eur J Med Chem 163: 453-480 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.037
BindingDB Entry DOI: 10.7270/Q2TQ650N |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50235288

(CHEMBL4072879)Show SMILES CNCc1ccc2ccc(Cl)c(C3=C(C(=O)NC3=O)c3cn(C)c4ccccc34)c2c1 |t:12| Show InChI InChI=1S/C25H20ClN3O2/c1-27-12-14-7-8-15-9-10-19(26)21(17(15)11-14)23-22(24(30)28-25(23)31)18-13-29(2)20-6-4-3-5-16(18)20/h3-11,13,27H,12H2,1-2H3,(H,28,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCbeta1 (unknown origin) after 60 mins in presence of [gamma33P]ATP by scintillation proximity assay |
Bioorg Med Chem Lett 27: 781-786 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.038
BindingDB Entry DOI: 10.7270/Q2930WF1 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50393219
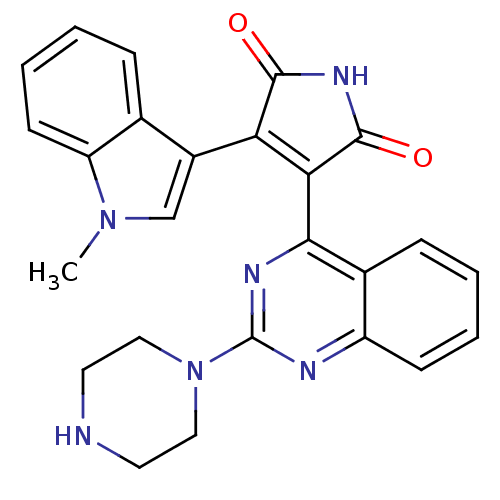
(CHEMBL2151415)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2nc(nc3ccccc23)N2CCNCC2)c2ccccc12 |t:4| Show InChI InChI=1S/C25H22N6O2/c1-30-14-17(15-6-3-5-9-19(15)30)20-21(24(33)29-23(20)32)22-16-7-2-4-8-18(16)27-25(28-22)31-12-10-26-11-13-31/h2-9,14,26H,10-13H2,1H3,(H,29,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCbeta-1 by scintillation proximity assay |
J Med Chem 54: 6028-39 (2011)
Article DOI: 10.1021/jm200469u
BindingDB Entry DOI: 10.7270/Q2K35VR1 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50486405

(CHEMBL2236794)Show SMILES NC[C@H]1Cc2c(C3=C(Nc4ccccc4)C(=O)NC3=O)c3ccccc3n2C1 |r,c:6| Show InChI InChI=1S/C22H20N4O2/c23-11-13-10-17-18(15-8-4-5-9-16(15)26(17)12-13)19-20(22(28)25-21(19)27)24-14-6-2-1-3-7-14/h1-9,13H,10-12,23H2,(H2,24,25,27,28)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PKCbeta2 (unknown origin) |
Citation and Details
Article DOI: 10.1007/s00044-010-9439-6
BindingDB Entry DOI: 10.7270/Q2862KBF |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50486405

(CHEMBL2236794)Show SMILES NC[C@H]1Cc2c(C3=C(Nc4ccccc4)C(=O)NC3=O)c3ccccc3n2C1 |r,c:6| Show InChI InChI=1S/C22H20N4O2/c23-11-13-10-17-18(15-8-4-5-9-16(15)26(17)12-13)19-20(22(28)25-21(19)27)24-14-6-2-1-3-7-14/h1-9,13H,10-12,23H2,(H2,24,25,27,28)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PKCbeta2 (unknown origin) |
Citation and Details
Article DOI: 10.1007/s00044-010-9439-6
BindingDB Entry DOI: 10.7270/Q2862KBF |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM2683
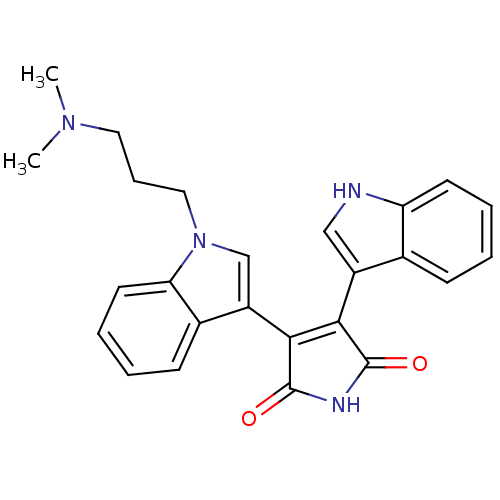
(2-[1-(3-dimethylaminopropyl)-indol-3-yl]-3-(indol-...)Show SMILES CN(C)CCCn1cc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C25H24N4O2/c1-28(2)12-7-13-29-15-19(17-9-4-6-11-21(17)29)23-22(24(30)27-25(23)31)18-14-26-20-10-5-3-8-16(18)20/h3-6,8-11,14-15,26H,7,12-13H2,1-2H3,(H,27,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Novartis
| Assay Description
Classical and novel PKC isotypes were assayed by scintillation proximity assay technology. In brief, the assay was performed in reaction buffer by in... |
J Med Chem 52: 6193-6 (2009)
Article DOI: 10.1021/jm901108b
BindingDB Entry DOI: 10.7270/Q25X278Q |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM33970
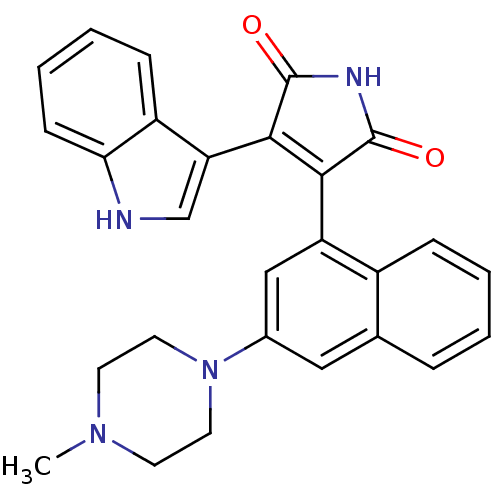
(maleimide derivative, 12)Show SMILES CN1CCN(CC1)c1cc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2ccccc2c1 |t:11| Show InChI InChI=1S/C27H24N4O2/c1-30-10-12-31(13-11-30)18-14-17-6-2-3-7-19(17)21(15-18)24-25(27(33)29-26(24)32)22-16-28-23-9-5-4-8-20(22)23/h2-9,14-16,28H,10-13H2,1H3,(H,29,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Novartis
| Assay Description
Classical and novel PKC isotypes were assayed by scintillation proximity assay technology. In brief, the assay was performed in reaction buffer by in... |
J Med Chem 52: 6193-6 (2009)
Article DOI: 10.1021/jm901108b
BindingDB Entry DOI: 10.7270/Q25X278Q |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM33970

(maleimide derivative, 12)Show SMILES CN1CCN(CC1)c1cc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2ccccc2c1 |t:11| Show InChI InChI=1S/C27H24N4O2/c1-30-10-12-31(13-11-30)18-14-17-6-2-3-7-19(17)21(15-18)24-25(27(33)29-26(24)32)22-16-28-23-9-5-4-8-20(22)23/h2-9,14-16,28H,10-13H2,1H3,(H,29,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCbeta-1 by scintillation proximity assay |
J Med Chem 54: 6028-39 (2011)
Article DOI: 10.1021/jm200469u
BindingDB Entry DOI: 10.7270/Q2K35VR1 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50486401
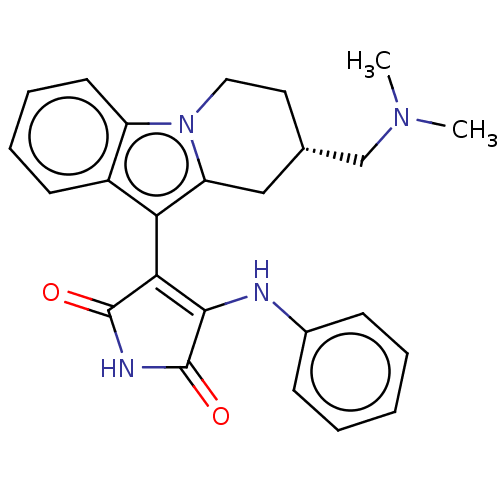
(CHEMBL2236799)Show SMILES CN(C)C[C@H]1CCn2c(C1)c(C1=C(Nc3ccccc3)C(=O)NC1=O)c1ccccc21 |r,c:12| Show InChI InChI=1S/C25H26N4O2/c1-28(2)15-16-12-13-29-19-11-7-6-10-18(19)21(20(29)14-16)22-23(25(31)27-24(22)30)26-17-8-4-3-5-9-17/h3-11,16H,12-15H2,1-2H3,(H2,26,27,30,31)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PKCbeta2 (unknown origin) |
Citation and Details
Article DOI: 10.1007/s00044-010-9439-6
BindingDB Entry DOI: 10.7270/Q2862KBF |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM33971

(AEB071 | Sotrastaurin | med.21724, Compound 190)Show SMILES CN1CCN(CC1)c1nc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2ccccc2n1 |t:11| Show InChI InChI=1S/C25H22N6O2/c1-30-10-12-31(13-11-30)25-27-19-9-5-3-7-16(19)22(28-25)21-20(23(32)29-24(21)33)17-14-26-18-8-4-2-6-15(17)18/h2-9,14,26H,10-13H2,1H3,(H,29,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Novartis
| Assay Description
Classical and novel PKC isotypes were assayed by scintillation proximity assay technology. In brief, the assay was performed in reaction buffer by in... |
J Med Chem 52: 6193-6 (2009)
Article DOI: 10.1021/jm901108b
BindingDB Entry DOI: 10.7270/Q25X278Q |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50393226
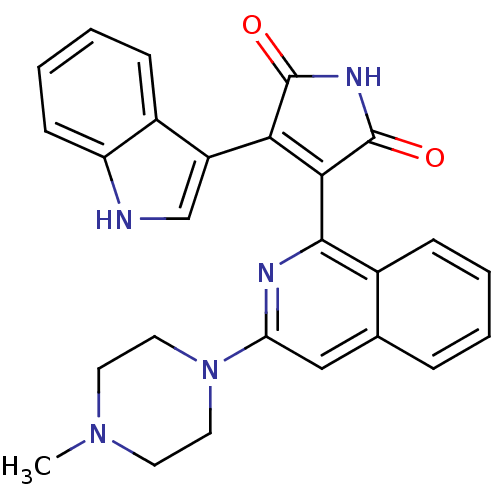
(CHEMBL2153748)Show SMILES CN1CCN(CC1)c1cc2ccccc2c(n1)C1=C(C(=O)NC1=O)c1c[nH]c2ccccc12 |t:20| Show InChI InChI=1S/C26H23N5O2/c1-30-10-12-31(13-11-30)21-14-16-6-2-3-7-17(16)24(28-21)23-22(25(32)29-26(23)33)19-15-27-20-9-5-4-8-18(19)20/h2-9,14-15,27H,10-13H2,1H3,(H,29,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCbeta-1 by scintillation proximity assay |
J Med Chem 54: 6028-39 (2011)
Article DOI: 10.1021/jm200469u
BindingDB Entry DOI: 10.7270/Q2K35VR1 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM33971

(AEB071 | Sotrastaurin | med.21724, Compound 190)Show SMILES CN1CCN(CC1)c1nc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2ccccc2n1 |t:11| Show InChI InChI=1S/C25H22N6O2/c1-30-10-12-31(13-11-30)25-27-19-9-5-3-7-16(19)22(28-25)21-20(23(32)29-24(21)33)17-14-26-18-8-4-2-6-15(17)18/h2-9,14,26H,10-13H2,1H3,(H,29,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCbeta-1 by scintillation proximity assay |
J Med Chem 54: 6028-39 (2011)
Article DOI: 10.1021/jm200469u
BindingDB Entry DOI: 10.7270/Q2K35VR1 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50153465

(2-{3-[3-(2,5-Dioxo-4-phenylamino-2,5-dihydro-1H-py...)Show SMILES NC(=N)SCCCn1cc(C2=C(Nc3ccccc3)C(=O)NC2=O)c2ccccc12 |c:10| Show InChI InChI=1S/C22H21N5O2S/c23-22(24)30-12-6-11-27-13-16(15-9-4-5-10-17(15)27)18-19(21(29)26-20(18)28)25-14-7-2-1-3-8-14/h1-5,7-10,13H,6,11-12H2,(H3,23,24)(H2,25,26,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human Protein kinase C beta 2 |
Bioorg Med Chem Lett 14: 5171-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.061
BindingDB Entry DOI: 10.7270/Q2K35VDJ |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50153465

(2-{3-[3-(2,5-Dioxo-4-phenylamino-2,5-dihydro-1H-py...)Show SMILES NC(=N)SCCCn1cc(C2=C(Nc3ccccc3)C(=O)NC2=O)c2ccccc12 |c:10| Show InChI InChI=1S/C22H21N5O2S/c23-22(24)30-12-6-11-27-13-16(15-9-4-5-10-17(15)27)18-19(21(29)26-20(18)28)25-14-7-2-1-3-8-14/h1-5,7-10,13H,6,11-12H2,(H3,23,24)(H2,25,26,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PKCbeta2 (unknown origin) |
Citation and Details
Article DOI: 10.1007/s00044-010-9439-6
BindingDB Entry DOI: 10.7270/Q2862KBF |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50153465

(2-{3-[3-(2,5-Dioxo-4-phenylamino-2,5-dihydro-1H-py...)Show SMILES NC(=N)SCCCn1cc(C2=C(Nc3ccccc3)C(=O)NC2=O)c2ccccc12 |c:10| Show InChI InChI=1S/C22H21N5O2S/c23-22(24)30-12-6-11-27-13-16(15-9-4-5-10-17(15)27)18-19(21(29)26-20(18)28)25-14-7-2-1-3-8-14/h1-5,7-10,13H,6,11-12H2,(H3,23,24)(H2,25,26,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PKCbeta2 (unknown origin) |
Citation and Details
Article DOI: 10.1007/s00044-010-9439-6
BindingDB Entry DOI: 10.7270/Q2862KBF |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50486401

(CHEMBL2236799)Show SMILES CN(C)C[C@H]1CCn2c(C1)c(C1=C(Nc3ccccc3)C(=O)NC1=O)c1ccccc21 |r,c:12| Show InChI InChI=1S/C25H26N4O2/c1-28(2)15-16-12-13-29-19-11-7-6-10-18(19)21(20(29)14-16)22-23(25(31)27-24(22)30)26-17-8-4-3-5-9-17/h3-11,16H,12-15H2,1-2H3,(H2,26,27,30,31)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PKCbeta2 (unknown origin) |
Citation and Details
Article DOI: 10.1007/s00044-010-9439-6
BindingDB Entry DOI: 10.7270/Q2862KBF |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50285240
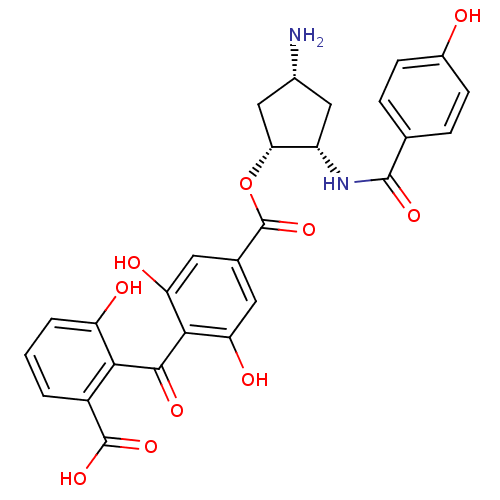
(4-(2-Carboxy-6-hydroxy-benzoyl)-3,5-dihydroxy-benz...)Show SMILES N[C@@H]1C[C@H](NC(=O)c2ccc(O)cc2)[C@@H](C1)OC(=O)c1cc(O)c(C(=O)c2c(O)cccc2C(O)=O)c(O)c1 Show InChI InChI=1S/C27H24N2O10/c28-14-10-17(29-25(35)12-4-6-15(30)7-5-12)21(11-14)39-27(38)13-8-19(32)23(20(33)9-13)24(34)22-16(26(36)37)2-1-3-18(22)31/h1-9,14,17,21,30-33H,10-11,28H2,(H,29,35)(H,36,37)/t14-,17+,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human Protein kinase C beta 1 |
Bioorg Med Chem Lett 5: 2155-2160 (1995)
Article DOI: 10.1016/0960-894X(95)00367-3
BindingDB Entry DOI: 10.7270/Q22N52RC |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM3033
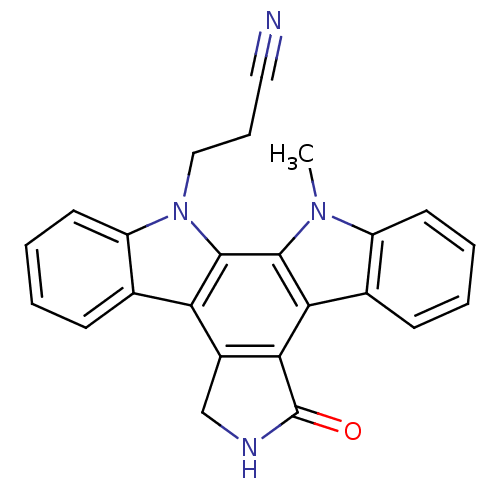
(3-{23-methyl-14-oxo-3,13,23-triazahexacyclo[14.7.0...)Show SMILES Cn1c2ccccc2c2c3C(=O)NCc3c3c4ccccc4n(CCC#N)c3c12 Show InChI InChI=1S/C24H18N4O/c1-27-17-9-4-2-7-14(17)20-21-16(13-26-24(21)29)19-15-8-3-5-10-18(15)28(12-6-11-25)23(19)22(20)27/h2-5,7-10H,6,12-13H2,1H3,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of PKCbeta |
Proc Natl Acad Sci USA 104: 20523-8 (2007)
Checked by Author
Article DOI: 10.1073/pnas.0708800104
BindingDB Entry DOI: 10.7270/Q2DB82RH |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM33971

(AEB071 | Sotrastaurin | med.21724, Compound 190)Show SMILES CN1CCN(CC1)c1nc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2ccccc2n1 |t:11| Show InChI InChI=1S/C25H22N6O2/c1-30-10-12-31(13-11-30)25-27-19-9-5-3-7-16(19)22(28-25)21-20(23(32)29-24(21)33)17-14-26-18-8-4-2-6-15(17)18/h2-9,14,26H,10-13H2,1H3,(H,29,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCbeta1 (unknown origin) after 60 mins in presence of [gamma33P]ATP by scintillation proximity assay |
Bioorg Med Chem Lett 27: 781-786 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.038
BindingDB Entry DOI: 10.7270/Q2930WF1 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50235287
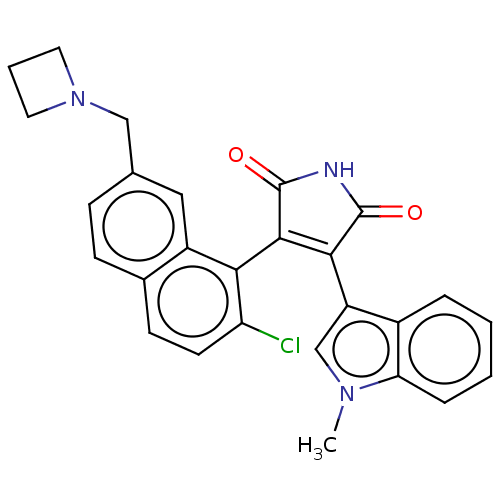
(CHEMBL4089217)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2c(Cl)ccc3ccc(CN4CCC4)cc23)c2ccccc12 |t:4| Show InChI InChI=1S/C27H22ClN3O2/c1-30-15-20(18-5-2-3-6-22(18)30)24-25(27(33)29-26(24)32)23-19-13-16(14-31-11-4-12-31)7-8-17(19)9-10-21(23)28/h2-3,5-10,13,15H,4,11-12,14H2,1H3,(H,29,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCbeta1 (unknown origin) after 60 mins in presence of [gamma33P]ATP by scintillation proximity assay |
Bioorg Med Chem Lett 27: 781-786 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.038
BindingDB Entry DOI: 10.7270/Q2930WF1 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50055668
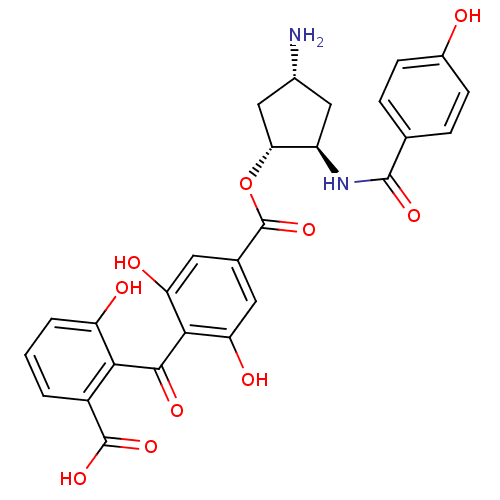
(4-(2-Carboxy-6-hydroxy-benzoyl)-3,5-dihydroxy-benz...)Show SMILES N[C@@H]1C[C@@H](NC(=O)c2ccc(O)cc2)[C@@H](C1)OC(=O)c1cc(O)c(C(=O)c2c(O)cccc2C(O)=O)c(O)c1 Show InChI InChI=1S/C27H24N2O10/c28-14-10-17(29-25(35)12-4-6-15(30)7-5-12)21(11-14)39-27(38)13-8-19(32)23(20(33)9-13)24(34)22-16(26(36)37)2-1-3-18(22)31/h1-9,14,17,21,30-33H,10-11,28H2,(H,29,35)(H,36,37)/t14-,17-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
A Division of Eli Lilly & Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human Protein kinase C beta 2 isozyme |
J Med Chem 40: 226-35 (1997)
Article DOI: 10.1021/jm960497g
BindingDB Entry DOI: 10.7270/Q2J965HR |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM33968
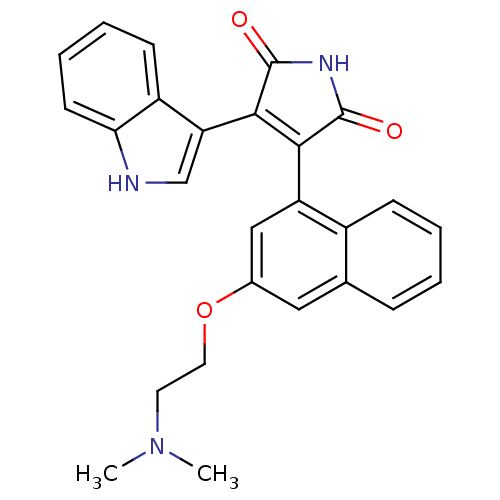
(maleimide derivative, 10)Show SMILES CN(C)CCOc1cc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2ccccc2c1 |t:9| Show InChI InChI=1S/C26H23N3O3/c1-29(2)11-12-32-17-13-16-7-3-4-8-18(16)20(14-17)23-24(26(31)28-25(23)30)21-15-27-22-10-6-5-9-19(21)22/h3-10,13-15,27H,11-12H2,1-2H3,(H,28,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Novartis
| Assay Description
Classical and novel PKC isotypes were assayed by scintillation proximity assay technology. In brief, the assay was performed in reaction buffer by in... |
J Med Chem 52: 6193-6 (2009)
Article DOI: 10.1021/jm901108b
BindingDB Entry DOI: 10.7270/Q25X278Q |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50486402

(CHEMBL2236809)Show SMILES CNC[C@H]1Cc2c(C3=C(Nc4ccccc4)C(=O)NC3=O)c3ccccc3n2C1 |r,c:7| Show InChI InChI=1S/C23H22N4O2/c1-24-12-14-11-18-19(16-9-5-6-10-17(16)27(18)13-14)20-21(23(29)26-22(20)28)25-15-7-3-2-4-8-15/h2-10,14,24H,11-13H2,1H3,(H2,25,26,28,29)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PKCbeta2 (unknown origin) |
Citation and Details
Article DOI: 10.1007/s00044-010-9439-6
BindingDB Entry DOI: 10.7270/Q2862KBF |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50486402

(CHEMBL2236809)Show SMILES CNC[C@H]1Cc2c(C3=C(Nc4ccccc4)C(=O)NC3=O)c3ccccc3n2C1 |r,c:7| Show InChI InChI=1S/C23H22N4O2/c1-24-12-14-11-18-19(16-9-5-6-10-17(16)27(18)13-14)20-21(23(29)26-22(20)28)25-15-7-3-2-4-8-15/h2-10,14,24H,11-13H2,1H3,(H2,25,26,28,29)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PKCbeta2 (unknown origin) |
Citation and Details
Article DOI: 10.1007/s00044-010-9439-6
BindingDB Entry DOI: 10.7270/Q2862KBF |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50486410
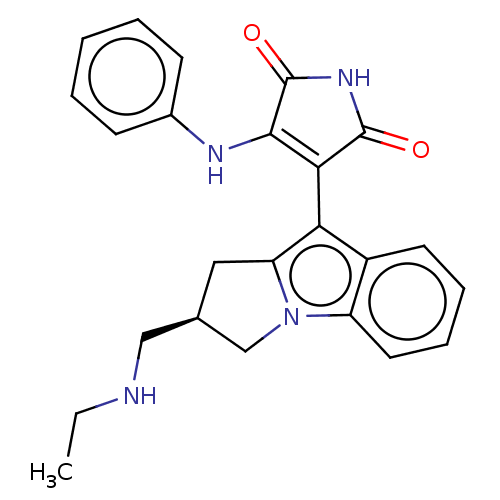
(CHEMBL2236793)Show SMILES CCNC[C@H]1Cc2c(C3=C(Nc4ccccc4)C(=O)NC3=O)c3ccccc3n2C1 |r,c:8| Show InChI InChI=1S/C24H24N4O2/c1-2-25-13-15-12-19-20(17-10-6-7-11-18(17)28(19)14-15)21-22(24(30)27-23(21)29)26-16-8-4-3-5-9-16/h3-11,15,25H,2,12-14H2,1H3,(H2,26,27,29,30)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PKCbeta2 (unknown origin) |
Citation and Details
Article DOI: 10.1007/s00044-010-9439-6
BindingDB Entry DOI: 10.7270/Q2862KBF |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PKCb2 using Histone H1 as substrate by [gamma-33P]-ATP assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2018.02.022
BindingDB Entry DOI: 10.7270/Q2DJ5KB8 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50486410

(CHEMBL2236793)Show SMILES CCNC[C@H]1Cc2c(C3=C(Nc4ccccc4)C(=O)NC3=O)c3ccccc3n2C1 |r,c:8| Show InChI InChI=1S/C24H24N4O2/c1-2-25-13-15-12-19-20(17-10-6-7-11-18(17)28(19)14-15)21-22(24(30)27-23(21)29)26-16-8-4-3-5-9-16/h3-11,15,25H,2,12-14H2,1H3,(H2,26,27,29,30)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PKCbeta2 (unknown origin) |
Citation and Details
Article DOI: 10.1007/s00044-010-9439-6
BindingDB Entry DOI: 10.7270/Q2862KBF |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of human PKCb2 using Histone H1 as substrate by [gamma-33P]-ATP assay |
Eur J Med Chem 161: 456-467 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.052
BindingDB Entry DOI: 10.7270/Q2W380MT |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50235286

(CHEMBL4083319)Show SMILES CC(C)NCc1ccc2ccc(Cl)c(C3=C(C(=O)NC3=O)c3cn(C)c4ccccc34)c2c1 |t:14| Show InChI InChI=1S/C27H24ClN3O2/c1-15(2)29-13-16-8-9-17-10-11-21(28)23(19(17)12-16)25-24(26(32)30-27(25)33)20-14-31(3)22-7-5-4-6-18(20)22/h4-12,14-15,29H,13H2,1-3H3,(H,30,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCbeta1 (unknown origin) after 60 mins in presence of [gamma33P]ATP by scintillation proximity assay |
Bioorg Med Chem Lett 27: 781-786 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.038
BindingDB Entry DOI: 10.7270/Q2930WF1 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM3199
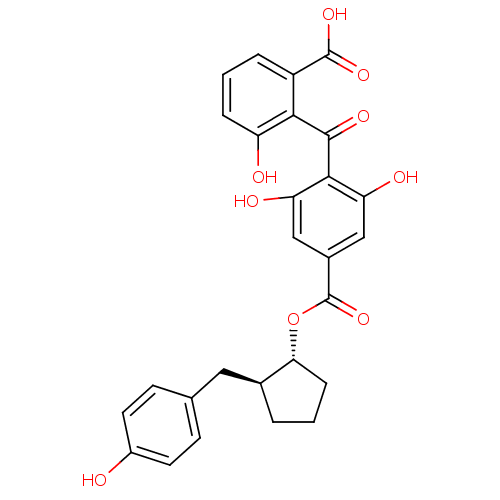
((+-)-anti-2-[[2,6-Dihydroxy-4-[[[2-(4-hydroxybenzy...)Show SMILES OC(=O)c1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CCC[C@H]1Cc1ccc(O)cc1 |r| Show InChI InChI=1S/C27H24O9/c28-17-9-7-14(8-10-17)11-15-3-1-6-22(15)36-27(35)16-12-20(30)24(21(31)13-16)25(32)23-18(26(33)34)4-2-5-19(23)29/h2,4-5,7-10,12-13,15,22,28-31H,1,3,6,11H2,(H,33,34)/t15-,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of protein kinase C epsilon |
Bioorg Med Chem Lett 6: 1759-1764 (1996)
Article DOI: 10.1016/0960-894X(96)00311-3
BindingDB Entry DOI: 10.7270/Q2QR4X3R |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM3199

((+-)-anti-2-[[2,6-Dihydroxy-4-[[[2-(4-hydroxybenzy...)Show SMILES OC(=O)c1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CCC[C@H]1Cc1ccc(O)cc1 |r| Show InChI InChI=1S/C27H24O9/c28-17-9-7-14(8-10-17)11-15-3-1-6-22(15)36-27(35)16-12-20(30)24(21(31)13-16)25(32)23-18(26(33)34)4-2-5-19(23)29/h2,4-5,7-10,12-13,15,22,28-31H,1,3,6,11H2,(H,33,34)/t15-,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of protein kinase C beta II |
Bioorg Med Chem Lett 6: 1759-1764 (1996)
Article DOI: 10.1016/0960-894X(96)00311-3
BindingDB Entry DOI: 10.7270/Q2QR4X3R |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50285239
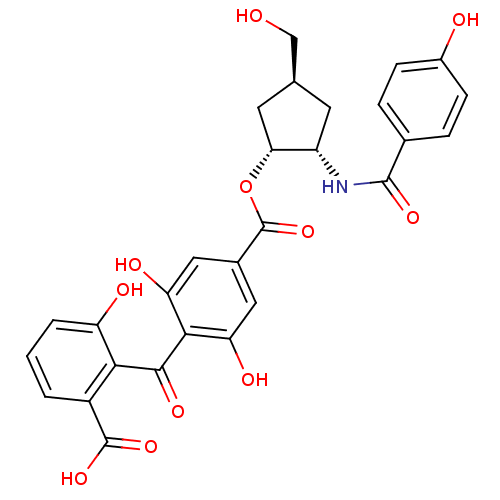
(4-(2-Carboxy-6-hydroxy-benzoyl)-3,5-dihydroxy-benz...)Show SMILES OC[C@H]1C[C@H](NC(=O)c2ccc(O)cc2)[C@@H](C1)OC(=O)c1cc(O)c(C(=O)c2c(O)cccc2C(O)=O)c(O)c1 Show InChI InChI=1S/C28H25NO11/c30-12-13-8-18(29-26(36)14-4-6-16(31)7-5-14)22(9-13)40-28(39)15-10-20(33)24(21(34)11-15)25(35)23-17(27(37)38)2-1-3-19(23)32/h1-7,10-11,13,18,22,30-34H,8-9,12H2,(H,29,36)(H,37,38)/t13-,18-,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human Protein kinase C beta 2 |
Bioorg Med Chem Lett 5: 2155-2160 (1995)
Article DOI: 10.1016/0960-894X(95)00367-3
BindingDB Entry DOI: 10.7270/Q22N52RC |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50284330
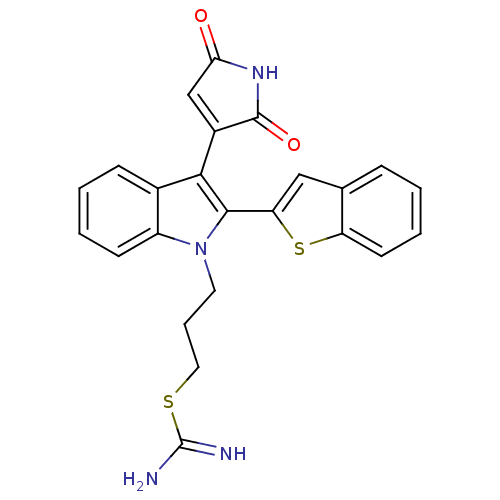
(2-{3-[2-Benzo[b]thiophen-2-yl-3-(2,5-dioxo-2,5-dih...)Show SMILES NC(=N)SCCCn1c(-c2cc3ccccc3s2)c(C2=CC(=O)NC2=O)c2ccccc12 |t:21| Show InChI InChI=1S/C24H20N4O2S2/c25-24(26)31-11-5-10-28-17-8-3-2-7-15(17)21(16-13-20(29)27-23(16)30)22(28)19-12-14-6-1-4-9-18(14)32-19/h1-4,6-9,12-13H,5,10-11H2,(H3,25,26)(H,27,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of protein kinase C beta |
Bioorg Med Chem Lett 5: 67-72 (1995)
Article DOI: 10.1016/0960-894X(94)00460-W
BindingDB Entry DOI: 10.7270/Q2ZG6S72 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM3199

((+-)-anti-2-[[2,6-Dihydroxy-4-[[[2-(4-hydroxybenzy...)Show SMILES OC(=O)c1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CCC[C@H]1Cc1ccc(O)cc1 |r| Show InChI InChI=1S/C27H24O9/c28-17-9-7-14(8-10-17)11-15-3-1-6-22(15)36-27(35)16-12-20(30)24(21(31)13-16)25(32)23-18(26(33)34)4-2-5-19(23)29/h2,4-5,7-10,12-13,15,22,28-31H,1,3,6,11H2,(H,33,34)/t15-,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. |
J Med Chem 39: 5215-27 (1996)
Article DOI: 10.1021/jm960581w
BindingDB Entry DOI: 10.7270/Q2G73BVV |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50055672
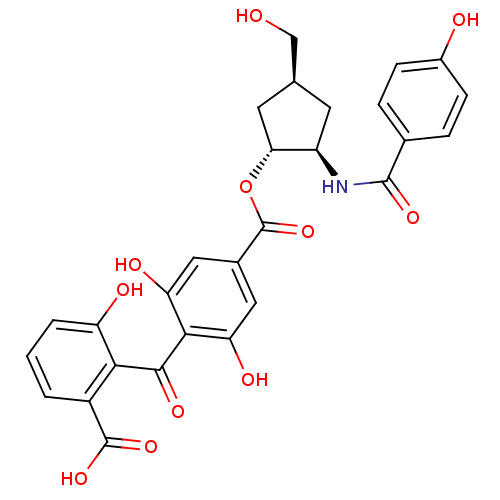
(4-(2-Carboxy-6-hydroxy-benzoyl)-3,5-dihydroxy-benz...)Show SMILES OC[C@H]1C[C@@H](NC(=O)c2ccc(O)cc2)[C@@H](C1)OC(=O)c1cc(O)c(C(=O)c2c(O)cccc2C(O)=O)c(O)c1 Show InChI InChI=1S/C28H25NO11/c30-12-13-8-18(29-26(36)14-4-6-16(31)7-5-14)22(9-13)40-28(39)15-10-20(33)24(21(34)11-15)25(35)23-17(27(37)38)2-1-3-19(23)32/h1-7,10-11,13,18,22,30-34H,8-9,12H2,(H,29,36)(H,37,38)/t13-,18+,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
A Division of Eli Lilly & Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human Protein kinase C beta 2 isozyme |
J Med Chem 40: 226-35 (1997)
Article DOI: 10.1021/jm960497g
BindingDB Entry DOI: 10.7270/Q2J965HR |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50235285

(CHEMBL4101316)Show SMILES COCCNCc1ccc2ccc(Cl)c(C3=C(C(=O)NC3=O)c3cn(C)c4ccccc34)c2c1 |t:15| Show InChI InChI=1S/C27H24ClN3O3/c1-31-15-20(18-5-3-4-6-22(18)31)24-25(27(33)30-26(24)32)23-19-13-16(14-29-11-12-34-2)7-8-17(19)9-10-21(23)28/h3-10,13,15,29H,11-12,14H2,1-2H3,(H,30,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCbeta1 (unknown origin) after 60 mins in presence of [gamma33P]ATP by scintillation proximity assay |
Bioorg Med Chem Lett 27: 781-786 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.038
BindingDB Entry DOI: 10.7270/Q2930WF1 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50235281
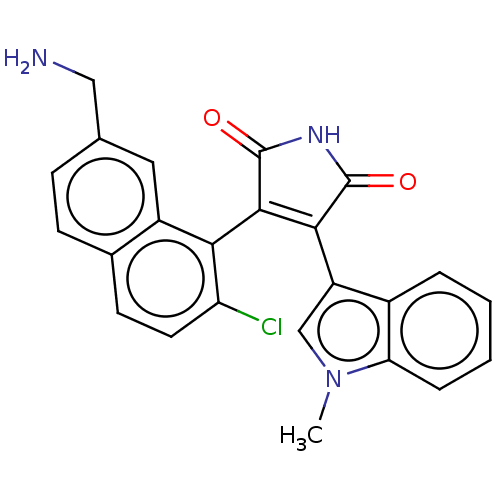
(CHEMBL4089665)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2c(Cl)ccc3ccc(CN)cc23)c2ccccc12 |t:4| Show InChI InChI=1S/C24H18ClN3O2/c1-28-12-17(15-4-2-3-5-19(15)28)21-22(24(30)27-23(21)29)20-16-10-13(11-26)6-7-14(16)8-9-18(20)25/h2-10,12H,11,26H2,1H3,(H,27,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]mazindol from dopamine transporter |
Bioorg Med Chem Lett 27: 781-786 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.038
BindingDB Entry DOI: 10.7270/Q2930WF1 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50235292

(CHEMBL4085837)Show SMILES CN(C)Cc1ccc2c(C3=C(C(=O)NC3=O)c3c[nH]c4ccccc34)c(Cl)ccc2c1 |t:9| Show InChI InChI=1S/C25H20ClN3O2/c1-29(2)13-14-7-9-16-15(11-14)8-10-19(26)21(16)23-22(24(30)28-25(23)31)18-12-27-20-6-4-3-5-17(18)20/h3-12,27H,13H2,1-2H3,(H,28,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCbeta1 (unknown origin) after 60 mins in presence of [gamma33P]ATP by scintillation proximity assay |
Bioorg Med Chem Lett 27: 781-786 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.038
BindingDB Entry DOI: 10.7270/Q2930WF1 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50235282

(CHEMBL4077967)Show SMILES CN(C)Cc1ccc2ccc(Cl)c(C3=C(C(=O)NC3=O)c3cn(C)c4ccccc34)c2c1 |t:13| Show InChI InChI=1S/C26H22ClN3O2/c1-29(2)13-15-8-9-16-10-11-20(27)22(18(16)12-15)24-23(25(31)28-26(24)32)19-14-30(3)21-7-5-4-6-17(19)21/h4-12,14H,13H2,1-3H3,(H,28,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCbeta1 (unknown origin) after 60 mins in presence of [gamma33P]ATP by scintillation proximity assay |
Bioorg Med Chem Lett 27: 781-786 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.038
BindingDB Entry DOI: 10.7270/Q2930WF1 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50393227
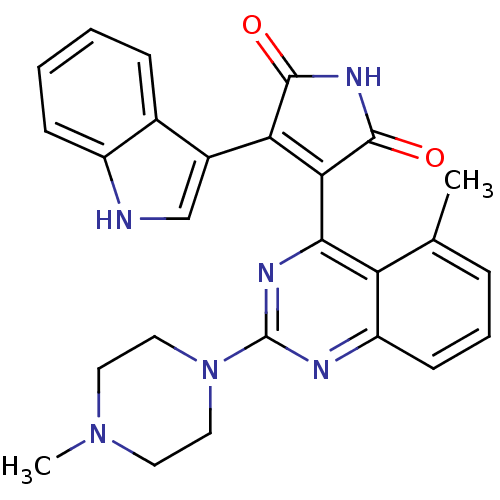
(CHEMBL2153749)Show SMILES CN1CCN(CC1)c1nc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2c(C)cccc2n1 |t:11| Show InChI InChI=1S/C26H24N6O2/c1-15-6-5-9-19-20(15)23(29-26(28-19)32-12-10-31(2)11-13-32)22-21(24(33)30-25(22)34)17-14-27-18-8-4-3-7-16(17)18/h3-9,14,27H,10-13H2,1-2H3,(H,30,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCbeta-1 by scintillation proximity assay |
J Med Chem 54: 6028-39 (2011)
Article DOI: 10.1021/jm200469u
BindingDB Entry DOI: 10.7270/Q2K35VR1 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM3199

((+-)-anti-2-[[2,6-Dihydroxy-4-[[[2-(4-hydroxybenzy...)Show SMILES OC(=O)c1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CCC[C@H]1Cc1ccc(O)cc1 |r| Show InChI InChI=1S/C27H24O9/c28-17-9-7-14(8-10-17)11-15-3-1-6-22(15)36-27(35)16-12-20(30)24(21(31)13-16)25(32)23-18(26(33)34)4-2-5-19(23)29/h2,4-5,7-10,12-13,15,22,28-31H,1,3,6,11H2,(H,33,34)/t15-,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. |
J Med Chem 39: 5215-27 (1996)
Article DOI: 10.1021/jm960581w
BindingDB Entry DOI: 10.7270/Q2G73BVV |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50285241
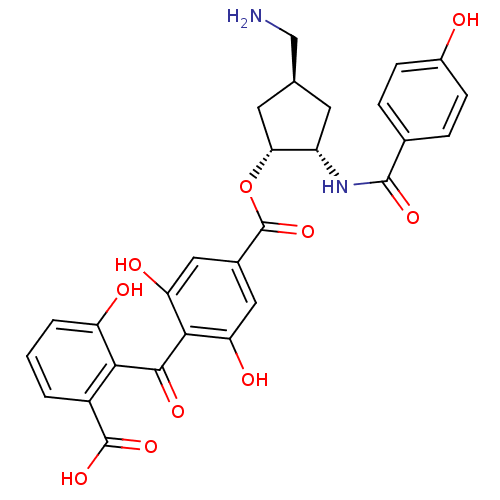
(4-(2-Carboxy-6-hydroxy-benzoyl)-3,5-dihydroxy-benz...)Show SMILES NC[C@H]1C[C@H](NC(=O)c2ccc(O)cc2)[C@@H](C1)OC(=O)c1cc(O)c(C(=O)c2c(O)cccc2C(O)=O)c(O)c1 Show InChI InChI=1S/C28H26N2O10/c29-12-13-8-18(30-26(36)14-4-6-16(31)7-5-14)22(9-13)40-28(39)15-10-20(33)24(21(34)11-15)25(35)23-17(27(37)38)2-1-3-19(23)32/h1-7,10-11,13,18,22,31-34H,8-9,12,29H2,(H,30,36)(H,37,38)/t13-,18-,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human Protein kinase C beta 1 |
Bioorg Med Chem Lett 5: 2155-2160 (1995)
Article DOI: 10.1016/0960-894X(95)00367-3
BindingDB Entry DOI: 10.7270/Q22N52RC |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50486409
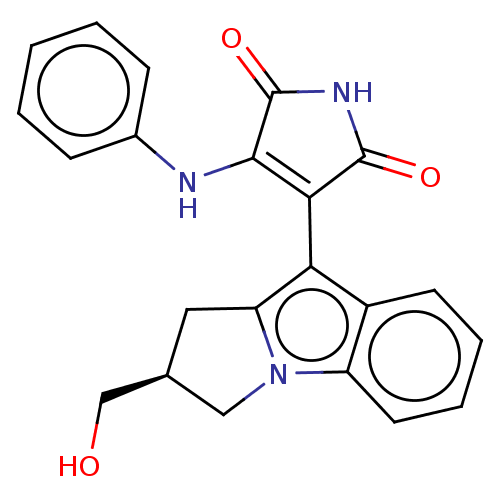
(CHEMBL2236804)Show SMILES OC[C@H]1Cc2c(C3=C(Nc4ccccc4)C(=O)NC3=O)c3ccccc3n2C1 |r,c:6| Show InChI InChI=1S/C22H19N3O3/c26-12-13-10-17-18(15-8-4-5-9-16(15)25(17)11-13)19-20(22(28)24-21(19)27)23-14-6-2-1-3-7-14/h1-9,13,26H,10-12H2,(H2,23,24,27,28)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PKCbeta2 (unknown origin) |
Citation and Details
Article DOI: 10.1007/s00044-010-9439-6
BindingDB Entry DOI: 10.7270/Q2862KBF |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50486411
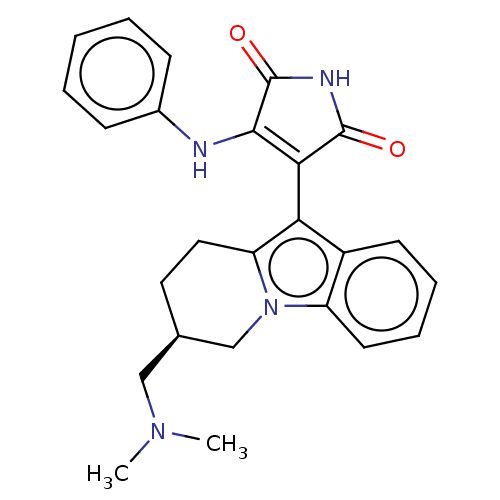
(CHEMBL2236802)Show SMILES CN(C)C[C@H]1CCc2c(C3=C(Nc4ccccc4)C(=O)NC3=O)c3ccccc3n2C1 |r,c:9| Show InChI InChI=1S/C25H26N4O2/c1-28(2)14-16-12-13-20-21(18-10-6-7-11-19(18)29(20)15-16)22-23(25(31)27-24(22)30)26-17-8-4-3-5-9-17/h3-11,16H,12-15H2,1-2H3,(H2,26,27,30,31)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PKCbeta2 (unknown origin) |
Citation and Details
Article DOI: 10.1007/s00044-010-9439-6
BindingDB Entry DOI: 10.7270/Q2862KBF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data