

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(1B) dopamine receptor (Homo sapiens (Human)) | BDBM86180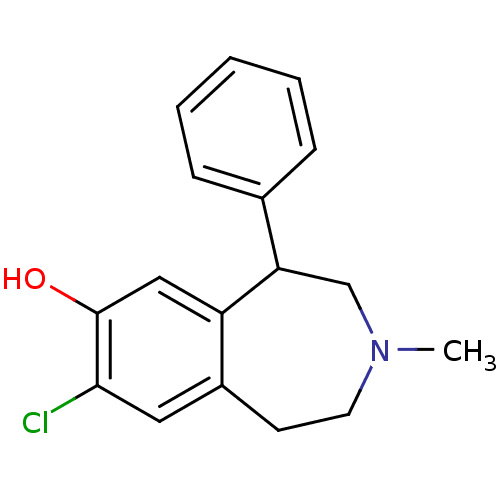 (CAS_87075-17-0 | NSC_5018 | SCH 23390 | SCH23390 |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]SCH 23390 from human recombinant D5 receptor expressed in GH4 cells measured after 60 mins by scintillation counting method | Bioorg Med Chem 25: 471-482 (2017) Article DOI: 10.1016/j.bmc.2016.11.014 BindingDB Entry DOI: 10.7270/Q2CF9S3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1B) dopamine receptor (Homo sapiens (Human)) | BDBM86180 (CAS_87075-17-0 | NSC_5018 | SCH 23390 | SCH23390 |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]SCH23390 from human recombinant Dopamine D5 receptor expressed in GH4 cells | Bioorg Med Chem 24: 1793-810 (2016) Article DOI: 10.1016/j.bmc.2016.03.006 BindingDB Entry DOI: 10.7270/Q2J67JS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1B) dopamine receptor (Homo sapiens (Human)) | BDBM50105105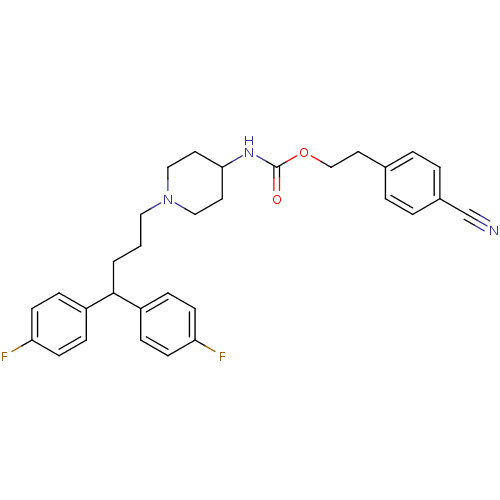 (CHEMBL116463 | {1-[4,4-Bis-(4-fluoro-phenyl)-butyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 513 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille Curated by ChEMBL | Assay Description Inhibition of human dopamine receptor D5 | J Med Chem 44: 3391-401 (2001) BindingDB Entry DOI: 10.7270/Q2057GN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1B) dopamine receptor (Homo sapiens (Human)) | BDBM50105109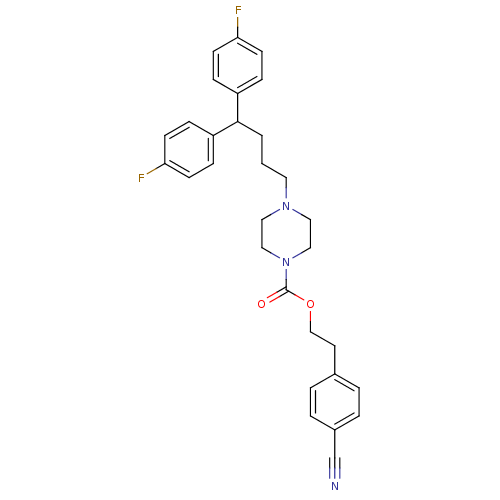 (4-[4,4-Bis-(4-fluoro-phenyl)-butyl]-piperazine-1-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 988 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille Curated by ChEMBL | Assay Description Inhibition of human dopamine receptor D5 | J Med Chem 44: 3391-401 (2001) BindingDB Entry DOI: 10.7270/Q2057GN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1B) dopamine receptor (Homo sapiens (Human)) | BDBM50382290 (CARIPRAZINE HYDROCHLORIDE | RGH-188 HCL) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc Curated by ChEMBL | Assay Description Binding affinity to human dopamine D5 receptor | Bioorg Med Chem Lett 22: 3437-40 (2012) Article DOI: 10.1016/j.bmcl.2012.03.104 BindingDB Entry DOI: 10.7270/Q2ZW1MZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1B) dopamine receptor (Homo sapiens (Human)) | BDBM50105102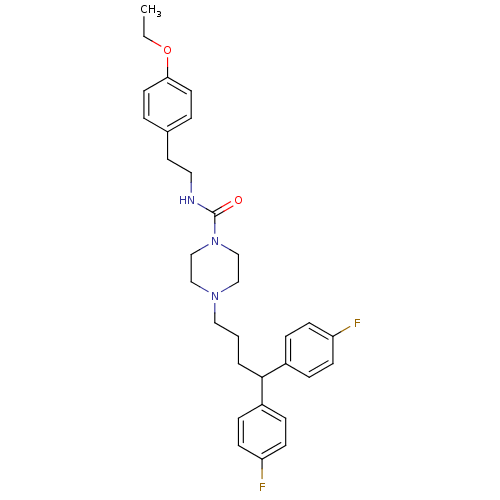 (4-[4,4-Bis-(4-fluoro-phenyl)-butyl]-piperazine-1-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille Curated by ChEMBL | Assay Description Inhibition of human dopamine receptor D5 | J Med Chem 44: 3391-401 (2001) BindingDB Entry DOI: 10.7270/Q2057GN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1B) dopamine receptor (Homo sapiens (Human)) | BDBM50105101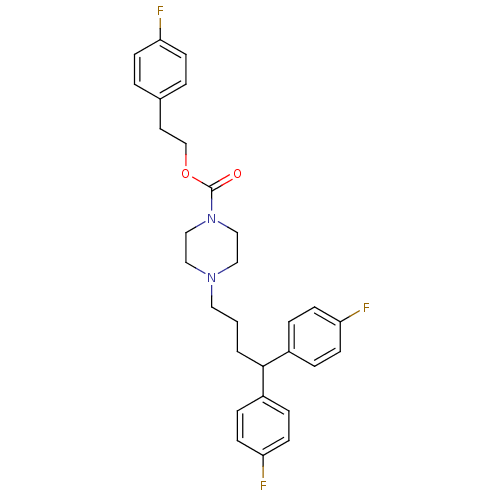 (4-[4,4-Bis-(4-fluoro-phenyl)-butyl]-piperazine-1-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille Curated by ChEMBL | Assay Description Inhibition of human dopamine receptor D5 | J Med Chem 44: 3391-401 (2001) BindingDB Entry DOI: 10.7270/Q2057GN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1B) dopamine receptor (Homo sapiens (Human)) | BDBM50105107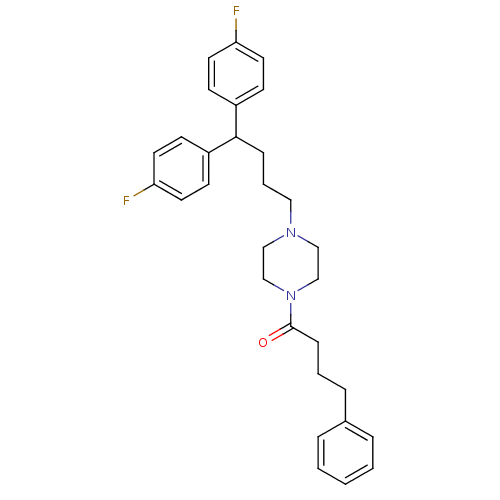 (1-{4-[4,4-Bis-(4-fluoro-phenyl)-butyl]-piperazin-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille Curated by ChEMBL | Assay Description Inhibition of human dopamine receptor D5 | J Med Chem 44: 3391-401 (2001) BindingDB Entry DOI: 10.7270/Q2057GN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1B) dopamine receptor (Homo sapiens (Human)) | BDBM50105103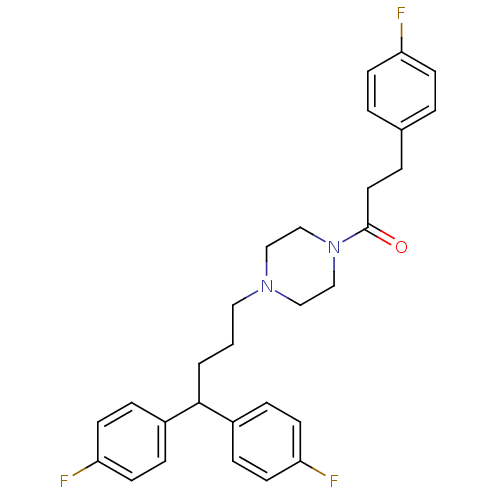 (1-{4-[4,4-Bis-(4-fluoro-phenyl)-butyl]-piperazin-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille Curated by ChEMBL | Assay Description Inhibition of human dopamine receptor D5 | J Med Chem 44: 3391-401 (2001) BindingDB Entry DOI: 10.7270/Q2057GN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1B) dopamine receptor (Homo sapiens (Human)) | BDBM50105090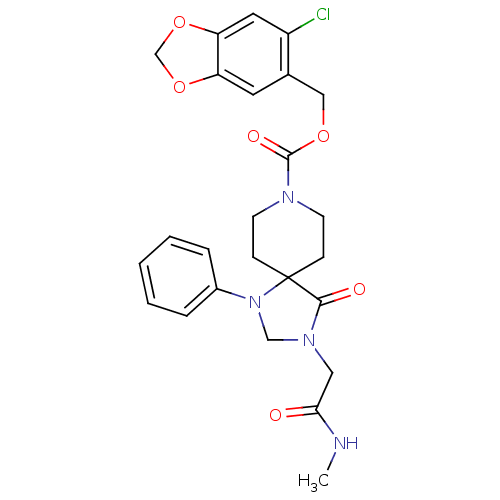 (3-Methylcarbamoylmethyl-4-oxo-1-phenyl-1,3,8-triaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille Curated by ChEMBL | Assay Description Inhibition of human dopamine receptor D5 | J Med Chem 44: 3391-401 (2001) BindingDB Entry DOI: 10.7270/Q2057GN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1B) dopamine receptor (Homo sapiens (Human)) | BDBM50105061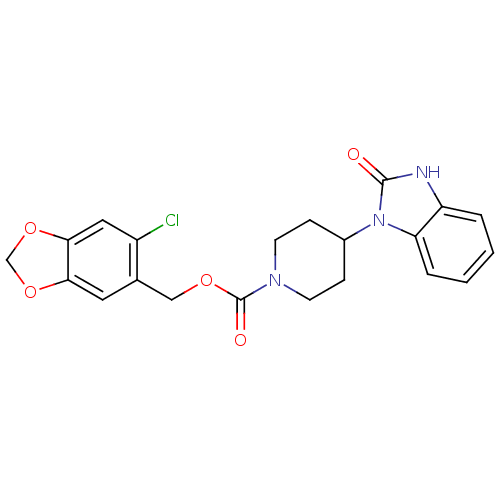 (4-(2-Oxo-2,3-dihydro-benzoimidazol-1-yl)-piperidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille Curated by ChEMBL | Assay Description Inhibition of human dopamine receptor D5 | J Med Chem 44: 3391-401 (2001) BindingDB Entry DOI: 10.7270/Q2057GN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1B) dopamine receptor (Homo sapiens (Human)) | BDBM50304075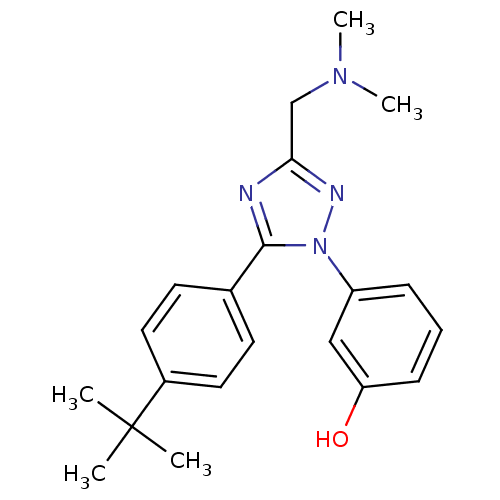 (3-(5-(4-tert-butylphenyl)-3-((dimethylamino)methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Inhibition of dopamine D5 receptor | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1B) dopamine receptor (Homo sapiens (Human)) | BDBM50212239 (5-(4-chlorophenyl)-N-(3,5-dimethoxyphenyl)furan-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCH23390 from dopamine D5 receptor | Proc Natl Acad Sci USA 104: 8520-5 (2007) Article DOI: 10.1073/pnas.0611364104 BindingDB Entry DOI: 10.7270/Q2J10411 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1B) dopamine receptor (Homo sapiens (Human)) | BDBM50226008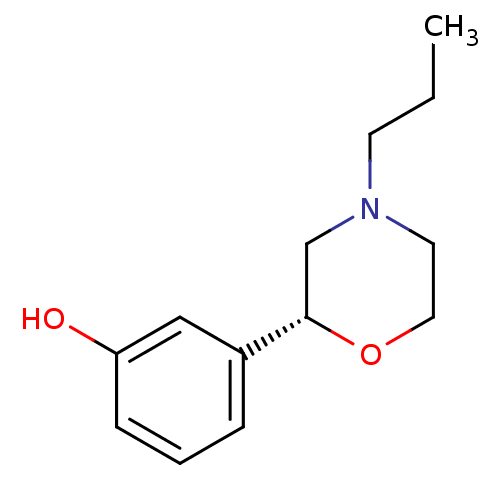 ((R)-3-(4-propylmorpholin-2-yl)phenol | CHEMBL25040...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Binding affinity to dopamine D5 receptor | Bioorg Med Chem Lett 17: 6691-6 (2007) Article DOI: 10.1016/j.bmcl.2007.10.059 BindingDB Entry DOI: 10.7270/Q23J3CQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1B) dopamine receptor (Homo sapiens (Human)) | BDBM50176440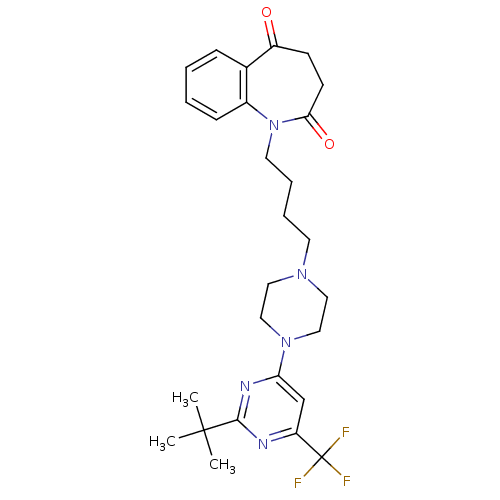 (1-(4-(4-(2-tert-butyl-6-(trifluoromethyl)pyrimidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibitory activity against dopamine D5 receptor | Bioorg Med Chem Lett 16: 658-62 (2005) Article DOI: 10.1016/j.bmcl.2005.10.035 BindingDB Entry DOI: 10.7270/Q2K64HN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1B) dopamine receptor (Homo sapiens (Human)) | BDBM50527972 (CHEMBL4476784 | US11634404, Compound 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Neurological Disorders and Stroke Curated by ChEMBL | Assay Description Antagonist activity at Gs-coupled human D5R expressed in CHOK1 cells assessed as inhibition of dopamine-induced beta-arrestin recruitment measured af... | J Med Chem 63: 5526-5567 (2020) Article DOI: 10.1021/acs.jmedchem.0c00424 BindingDB Entry DOI: 10.7270/Q28D00QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||