

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM39347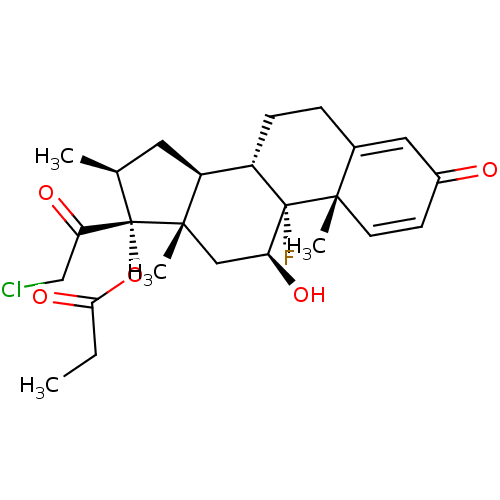 (CLOBETASOL PROPIONATE | MLS000028708 | SMR00005874...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of CYP3A5 in doxycycline-induced CYP3A5 overexpressing wild type human AsPC1 cells assessed as decrease in 1-hydroxymidazolam formation us... | J Med Chem 63: 1415-1433 (2020) Article DOI: 10.1021/acs.jmedchem.9b02067 BindingDB Entry DOI: 10.7270/Q2NS0Z6Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM39347 (CLOBETASOL PROPIONATE | MLS000028708 | SMR00005874...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of CYP3A5 in wild type human AsPC1 cells assessed as decrease in 1-hydroxymidazolam formation using midazolam as substrate after 24 hrs by... | J Med Chem 63: 1415-1433 (2020) Article DOI: 10.1021/acs.jmedchem.9b02067 BindingDB Entry DOI: 10.7270/Q2NS0Z6Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50123453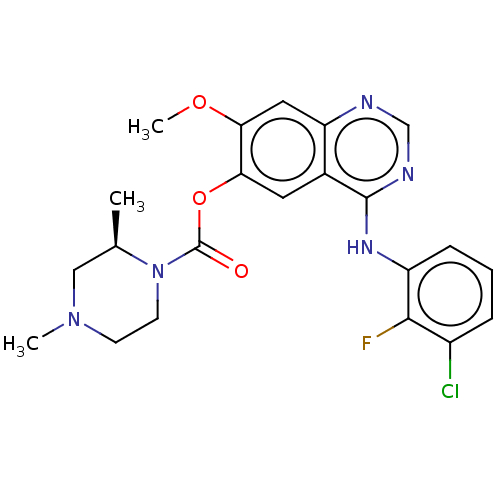 (CHEMBL3623290) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >50 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of CYP3A5 (unknown origin) | J Med Chem 58: 8200-15 (2015) Article DOI: 10.1021/acs.jmedchem.5b01073 BindingDB Entry DOI: 10.7270/Q29P33FH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM39347 (CLOBETASOL PROPIONATE | MLS000028708 | SMR00005874...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of CYP3A5 in lentiviral pLVX-TRE3G-ZsGreen1-CYP3A5 transduced wild type human AsPC1 cells overexpressing CYP3A5 assessed as decrease in 1-... | J Med Chem 63: 1415-1433 (2020) Article DOI: 10.1021/acs.jmedchem.9b02067 BindingDB Entry DOI: 10.7270/Q2NS0Z6Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM39347 (CLOBETASOL PROPIONATE | MLS000028708 | SMR00005874...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 103 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of CYP3A5 in CRISPR/Cas9-mediated CYP3A5 knock-out and doxycycline-induced CYP3A5 overexpressing human AsPC1 cells assessed as decrease in... | J Med Chem 63: 1415-1433 (2020) Article DOI: 10.1021/acs.jmedchem.9b02067 BindingDB Entry DOI: 10.7270/Q2NS0Z6Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM151585 (US11739089, Compound Ketoconazole | US8987315, Ket...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | 37 |
Telormedix SA US Patent | Assay Description The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w... | US Patent US9180183 (2015) BindingDB Entry DOI: 10.7270/Q2B27T2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM151585 (US11739089, Compound Ketoconazole | US8987315, Ket...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Telormedix SA US Patent | Assay Description The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w... | US Patent US9173935 (2015) BindingDB Entry DOI: 10.7270/Q2JS9P8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50030448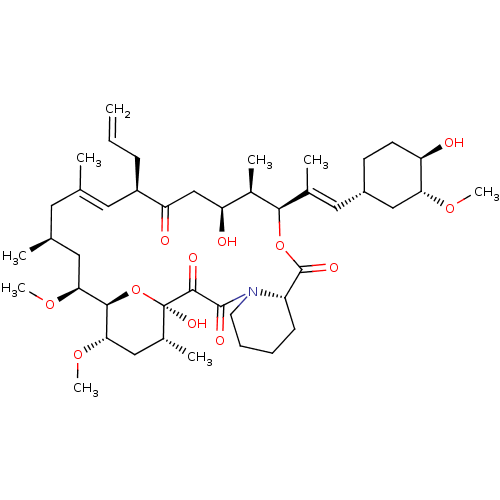 (8-DEETHYL-8-[BUT-3-ENYL]-ASCOMYCIN | CHEMBL269732 ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oslo Curated by ChEMBL | Assay Description Inhibition of human CYP3A5 expressed in supersomes assessed inhibition of 1'-OH midazolam formation preincubated with compound before substrate addit... | Drug Metab Dispos 40: 655-61 (2012) Article DOI: 10.1124/dmd.111.043018 BindingDB Entry DOI: 10.7270/Q22Z1796 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 141 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of CYP3A5 in doxycycline-induced CYP3A5 overexpressing wild type human AsPC1 cells assessed as decrease in 1-hydroxymidazolam formation us... | J Med Chem 63: 1415-1433 (2020) Article DOI: 10.1021/acs.jmedchem.9b02067 BindingDB Entry DOI: 10.7270/Q2NS0Z6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 162 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of CYP3A5 in CRISPR/Cas9-mediated CYP3A5 knock-out and doxycycline-induced CYP3A5 overexpressing human AsPC1 cells assessed as decrease in... | J Med Chem 63: 1415-1433 (2020) Article DOI: 10.1021/acs.jmedchem.9b02067 BindingDB Entry DOI: 10.7270/Q2NS0Z6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 439 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of CYP3A5 in lentiviral pLVX-TRE3G-ZsGreen1-CYP3A5 transduced wild type human AsPC1 cells overexpressing CYP3A5 assessed as decrease in 1-... | J Med Chem 63: 1415-1433 (2020) Article DOI: 10.1021/acs.jmedchem.9b02067 BindingDB Entry DOI: 10.7270/Q2NS0Z6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 513 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of CYP3A5 in wild type human AsPC1 cells assessed as decrease in 1-hydroxymidazolam formation using midazolam as substrate after 24 hrs by... | J Med Chem 63: 1415-1433 (2020) Article DOI: 10.1021/acs.jmedchem.9b02067 BindingDB Entry DOI: 10.7270/Q2NS0Z6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50081468 (CHEMBL3422028) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 556 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A5 assessed as metabolism of 7-benzyloxy-4-trifluoromethylcoumarin to HFC after 30 mins by fluorescence assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50030448 (8-DEETHYL-8-[BUT-3-ENYL]-ASCOMYCIN | CHEMBL269732 ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oslo Curated by ChEMBL | Assay Description Inhibition of human CYP3A5 expressed in supersomes assessed inhibition of 1'-OH midazolam formation by LC-MS method | Drug Metab Dispos 40: 655-61 (2012) Article DOI: 10.1124/dmd.111.043018 BindingDB Entry DOI: 10.7270/Q22Z1796 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50435005 (CHEMBL2386285) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 723 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A5 assessed as metabolism of 7-benzyloxy-4-trifluoromethylcoumarin to HFC after 30 mins by fluorescence assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50113995 (CHEMBL3605542) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of CYP3A5 in human liver microsomes incubated for 5 mins in presence of NADPH and specific substrates by LC/MS/MS method | J Med Chem 58: 7057-75 (2015) Article DOI: 10.1021/acs.jmedchem.5b01078 BindingDB Entry DOI: 10.7270/Q2Z89F6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50364653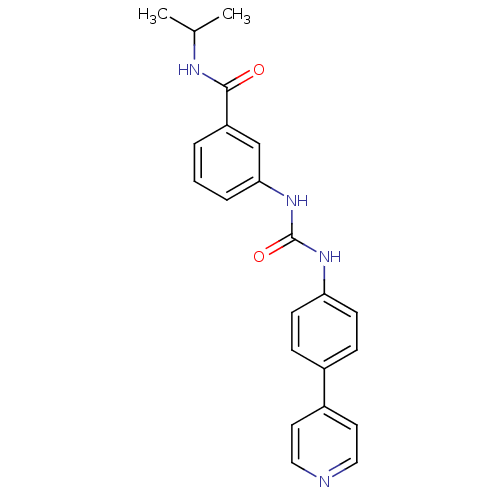 (CHEMBL1951443) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP3A5 in supersomes using midazolam as substrate after 5 mins by LC-MS/MS analysis | Bioorg Med Chem Lett 22: 1611-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.125 BindingDB Entry DOI: 10.7270/Q2T72HXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50364661 (CHEMBL1951339) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP3A5 in supersomes using midazolam as substrate after 5 mins by LC-MS/MS analysis | Bioorg Med Chem Lett 22: 1611-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.125 BindingDB Entry DOI: 10.7270/Q2T72HXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50584760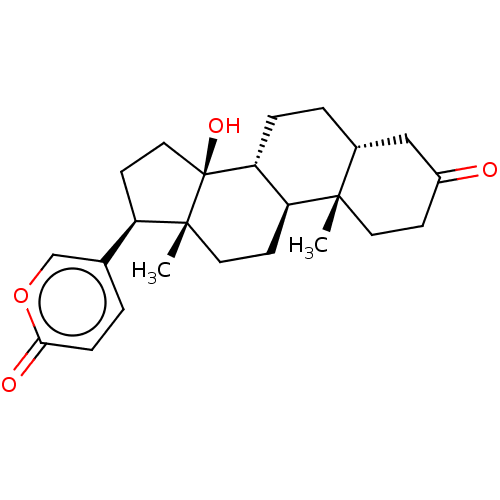 (CHEMBL2068968) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Time dependent inhibition of recombinant human CYP3A5 using midazolam as substrate preincubated for 30 mins in presence of NADPH generating system fo... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01875 BindingDB Entry DOI: 10.7270/Q2PZ5DQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50584761 (BUFALIN | Bufalin | CHEBI:517248) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Time dependent inhibition of recombinant human CYP3A5 incubated for 30 mins in presence of NADPH generating system by LC-MS/MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01875 BindingDB Entry DOI: 10.7270/Q2PZ5DQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50265673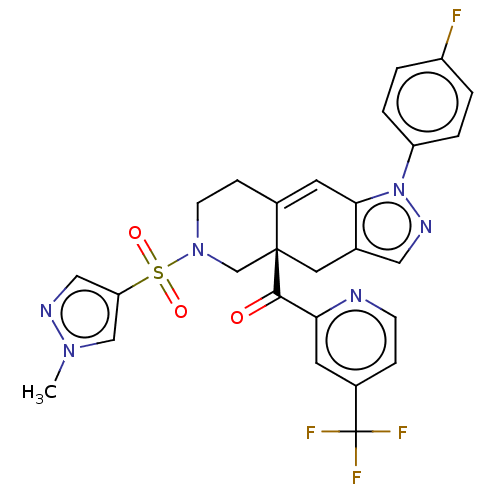 (CHEMBL4068611) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Inhibition of CYP3A5 (unknown origin) | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50584760 (CHEMBL2068968) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Time dependent inhibition of recombinant human CYP3A5 incubated for 30 mins in presence of NADPH generating system by LC-MS/MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01875 BindingDB Entry DOI: 10.7270/Q2PZ5DQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50364645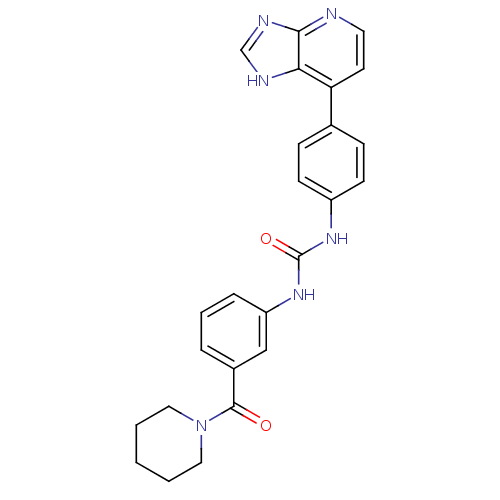 (CHEMBL1951342) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP3A5 in supersomes using midazolam as substrate after 5 mins by LC-MS/MS analysis | Bioorg Med Chem Lett 22: 1611-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.125 BindingDB Entry DOI: 10.7270/Q2T72HXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50364627 (CHEMBL1951322) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP3A5 in supersomes using midazolam as substrate after 5 mins by LC-MS/MS analysis | Bioorg Med Chem Lett 22: 1611-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.125 BindingDB Entry DOI: 10.7270/Q2T72HXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50364657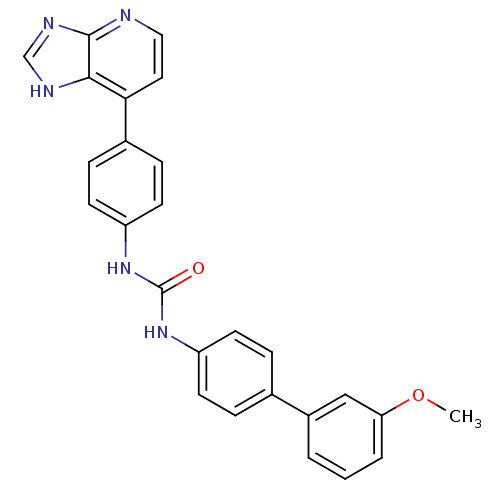 (CHEMBL1951447) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP3A5 in supersomes using midazolam as substrate after 5 mins by LC-MS/MS analysis | Bioorg Med Chem Lett 22: 1611-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.125 BindingDB Entry DOI: 10.7270/Q2T72HXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50364644 (CHEMBL1951341) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP3A5 in supersomes using midazolam as substrate after 5 mins by LC-MS/MS analysis | Bioorg Med Chem Lett 22: 1611-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.125 BindingDB Entry DOI: 10.7270/Q2T72HXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50364650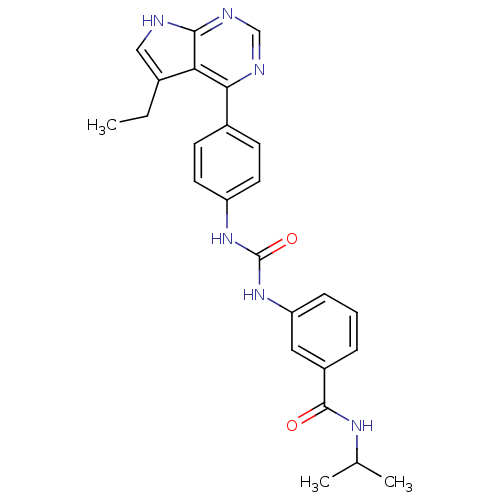 (CHEMBL1951347) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP3A5 in supersomes using midazolam as substrate after 5 mins by LC-MS/MS analysis | Bioorg Med Chem Lett 22: 1611-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.125 BindingDB Entry DOI: 10.7270/Q2T72HXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50364642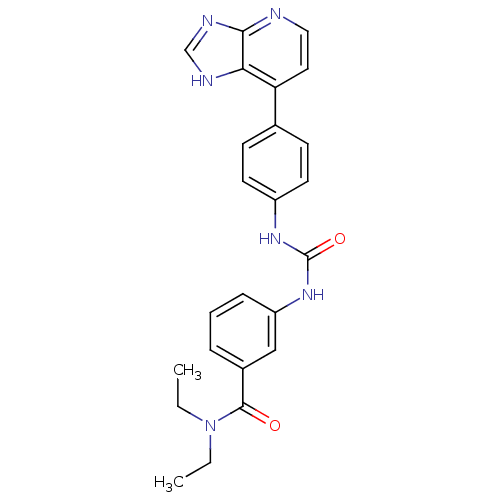 (CHEMBL1951338) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP3A5 in supersomes using midazolam as substrate after 5 mins by LC-MS/MS analysis | Bioorg Med Chem Lett 22: 1611-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.125 BindingDB Entry DOI: 10.7270/Q2T72HXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50457685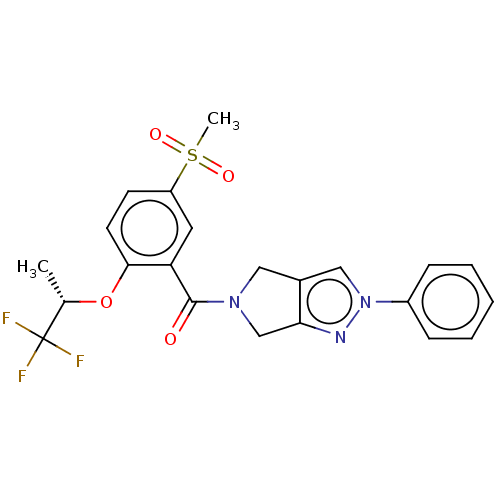 (CHEMBL4210484) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience LLC Curated by ChEMBL | Assay Description Inhibition of CYP3A5 in human liver microsomes using midazolam as substrate measured after 10 mins in presence of NADPH by LC-MS/MS analysis | J Med Chem 61: 6018-6033 (2018) Article DOI: 10.1021/acs.jmedchem.8b00372 BindingDB Entry DOI: 10.7270/Q2GB26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50457698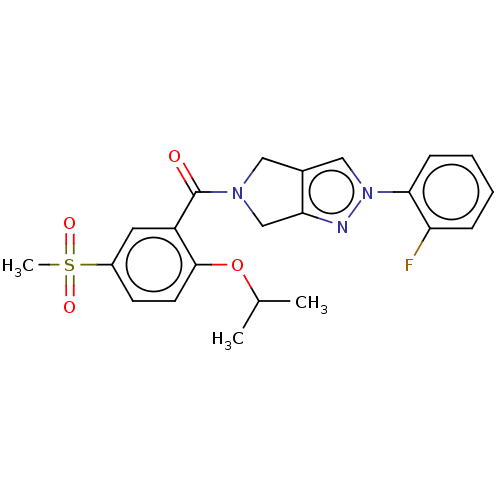 (CHEMBL4213968) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience LLC Curated by ChEMBL | Assay Description Inhibition of CYP3A5 in human liver microsomes using midazolam as substrate measured after 10 mins in presence of NADPH by LC-MS/MS analysis | J Med Chem 61: 6018-6033 (2018) Article DOI: 10.1021/acs.jmedchem.8b00372 BindingDB Entry DOI: 10.7270/Q2GB26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50457706 (CHEMBL4216391) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience LLC Curated by ChEMBL | Assay Description Inhibition of CYP3A5 in human liver microsomes using midazolam as substrate measured after 10 mins in presence of NADPH by LC-MS/MS analysis | J Med Chem 61: 6018-6033 (2018) Article DOI: 10.1021/acs.jmedchem.8b00372 BindingDB Entry DOI: 10.7270/Q2GB26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50457691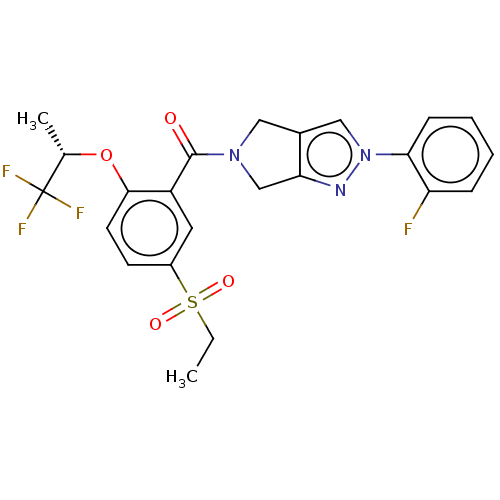 (CHEMBL4209849) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience LLC Curated by ChEMBL | Assay Description Inhibition of CYP3A5 in human liver microsomes using midazolam as substrate measured after 10 mins in presence of NADPH by LC-MS/MS analysis | J Med Chem 61: 6018-6033 (2018) Article DOI: 10.1021/acs.jmedchem.8b00372 BindingDB Entry DOI: 10.7270/Q2GB26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50457699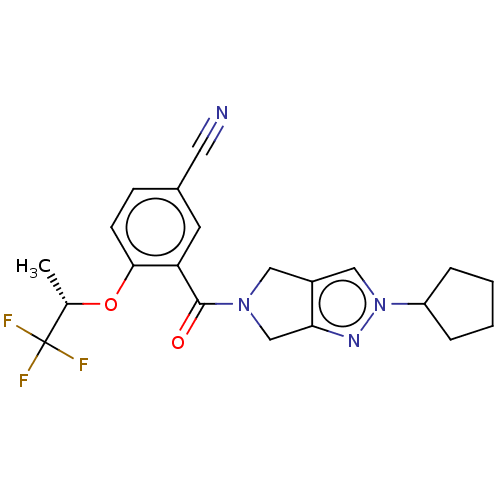 (CHEMBL4210095) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience LLC Curated by ChEMBL | Assay Description Inhibition of CYP3A5 in human liver microsomes using midazolam as substrate measured after 10 mins in presence of NADPH by LC-MS/MS analysis | J Med Chem 61: 6018-6033 (2018) Article DOI: 10.1021/acs.jmedchem.8b00372 BindingDB Entry DOI: 10.7270/Q2GB26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50457692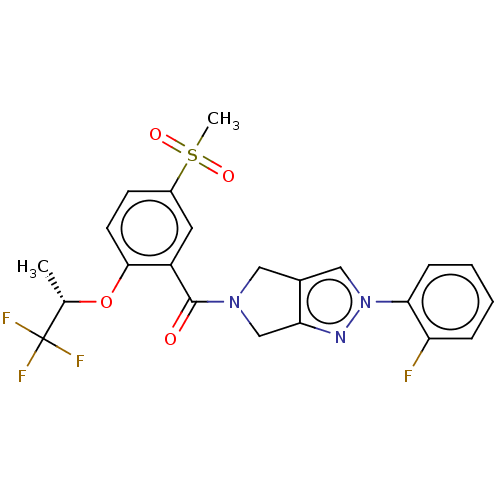 (CHEMBL4211012) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience LLC Curated by ChEMBL | Assay Description Inhibition of CYP3A5 in human liver microsomes using midazolam as substrate measured after 10 mins in presence of NADPH by LC-MS/MS analysis | J Med Chem 61: 6018-6033 (2018) Article DOI: 10.1021/acs.jmedchem.8b00372 BindingDB Entry DOI: 10.7270/Q2GB26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50457712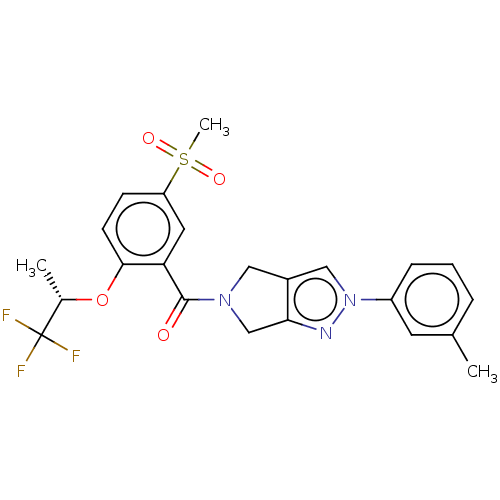 (CHEMBL4206122) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience LLC Curated by ChEMBL | Assay Description Inhibition of CYP3A5 in human liver microsomes using midazolam as substrate measured after 10 mins in presence of NADPH by LC-MS/MS analysis | J Med Chem 61: 6018-6033 (2018) Article DOI: 10.1021/acs.jmedchem.8b00372 BindingDB Entry DOI: 10.7270/Q2GB26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50457688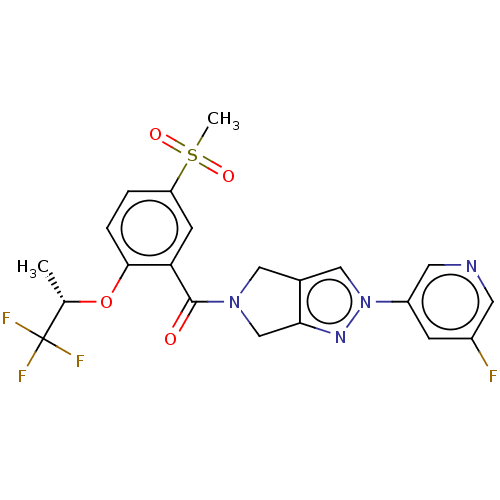 (CHEMBL4206639) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience LLC Curated by ChEMBL | Assay Description Inhibition of CYP3A5 in human liver microsomes using midazolam as substrate measured after 10 mins in presence of NADPH by LC-MS/MS analysis | J Med Chem 61: 6018-6033 (2018) Article DOI: 10.1021/acs.jmedchem.8b00372 BindingDB Entry DOI: 10.7270/Q2GB26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50457711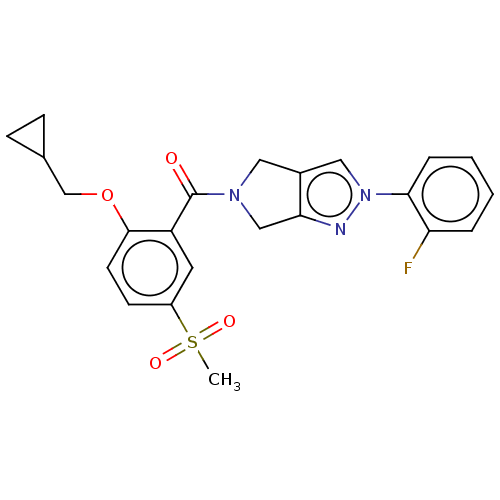 (CHEMBL4211426) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience LLC Curated by ChEMBL | Assay Description Inhibition of CYP3A5 in human liver microsomes using midazolam as substrate measured after 10 mins in presence of NADPH by LC-MS/MS analysis | J Med Chem 61: 6018-6033 (2018) Article DOI: 10.1021/acs.jmedchem.8b00372 BindingDB Entry DOI: 10.7270/Q2GB26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50457687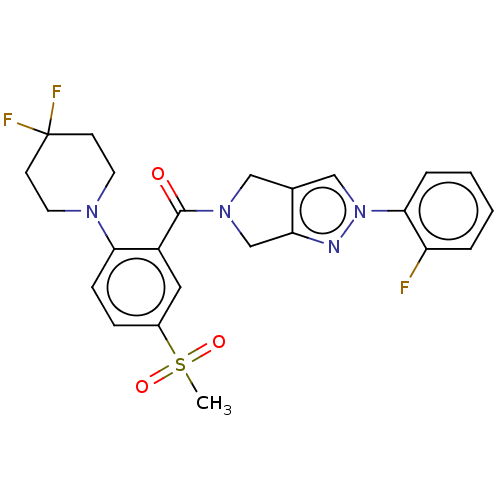 (CHEMBL4205471) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience LLC Curated by ChEMBL | Assay Description Inhibition of CYP3A5 in human liver microsomes using midazolam as substrate measured after 10 mins in presence of NADPH by LC-MS/MS analysis | J Med Chem 61: 6018-6033 (2018) Article DOI: 10.1021/acs.jmedchem.8b00372 BindingDB Entry DOI: 10.7270/Q2GB26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50457695 (CHEMBL4217712) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience LLC Curated by ChEMBL | Assay Description Inhibition of CYP3A5 in human liver microsomes using midazolam as substrate measured after 10 mins in presence of NADPH by LC-MS/MS analysis | J Med Chem 61: 6018-6033 (2018) Article DOI: 10.1021/acs.jmedchem.8b00372 BindingDB Entry DOI: 10.7270/Q2GB26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50457702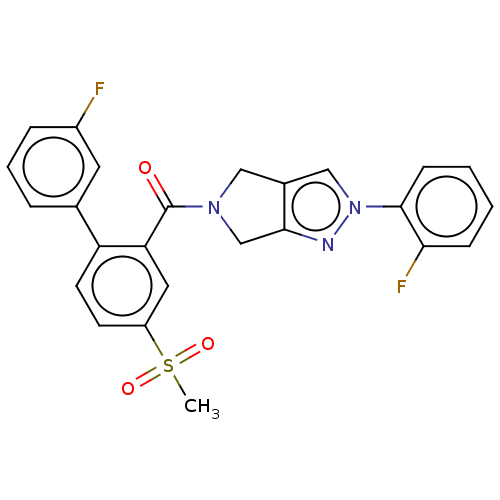 (CHEMBL4205995) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience LLC Curated by ChEMBL | Assay Description Inhibition of CYP3A5 in human liver microsomes using midazolam as substrate measured after 10 mins in presence of NADPH by LC-MS/MS analysis | J Med Chem 61: 6018-6033 (2018) Article DOI: 10.1021/acs.jmedchem.8b00372 BindingDB Entry DOI: 10.7270/Q2GB26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50457703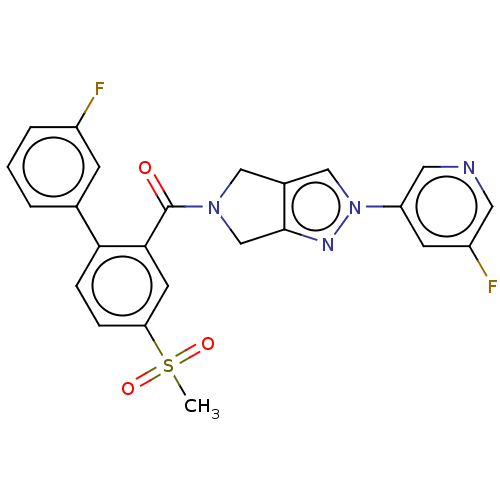 (CHEMBL4214250) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience LLC Curated by ChEMBL | Assay Description Inhibition of CYP3A5 in human liver microsomes using midazolam as substrate measured after 10 mins in presence of NADPH by LC-MS/MS analysis | J Med Chem 61: 6018-6033 (2018) Article DOI: 10.1021/acs.jmedchem.8b00372 BindingDB Entry DOI: 10.7270/Q2GB26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50457689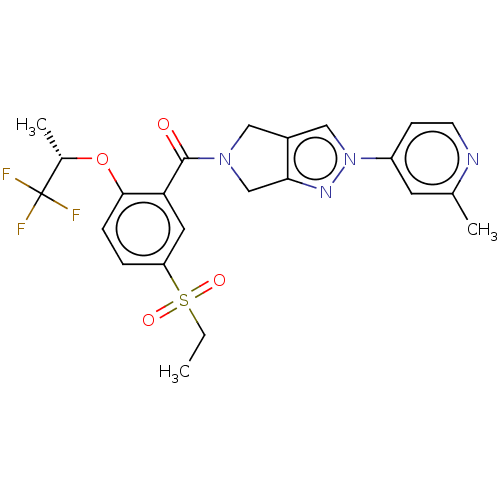 (CHEMBL4206403) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience LLC Curated by ChEMBL | Assay Description Inhibition of CYP3A5 in human liver microsomes using midazolam as substrate measured after 10 mins in presence of NADPH by LC-MS/MS analysis | J Med Chem 61: 6018-6033 (2018) Article DOI: 10.1021/acs.jmedchem.8b00372 BindingDB Entry DOI: 10.7270/Q2GB26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50457709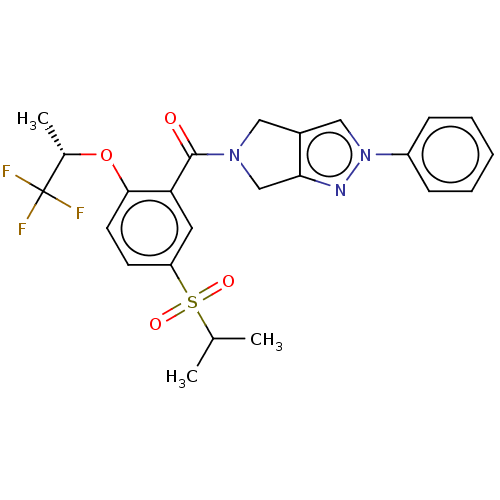 (CHEMBL4211293) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience LLC Curated by ChEMBL | Assay Description Inhibition of CYP3A5 in human liver microsomes using midazolam as substrate measured after 10 mins in presence of NADPH by LC-MS/MS analysis | J Med Chem 61: 6018-6033 (2018) Article DOI: 10.1021/acs.jmedchem.8b00372 BindingDB Entry DOI: 10.7270/Q2GB26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50457697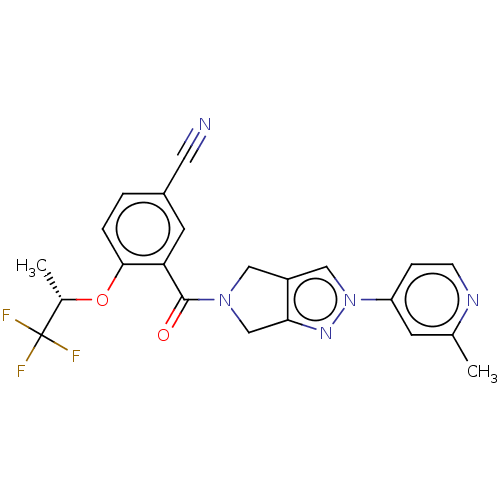 (CHEMBL4215015) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience LLC Curated by ChEMBL | Assay Description Inhibition of CYP3A5 in human liver microsomes using midazolam as substrate measured after 10 mins in presence of NADPH by LC-MS/MS analysis | J Med Chem 61: 6018-6033 (2018) Article DOI: 10.1021/acs.jmedchem.8b00372 BindingDB Entry DOI: 10.7270/Q2GB26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50457708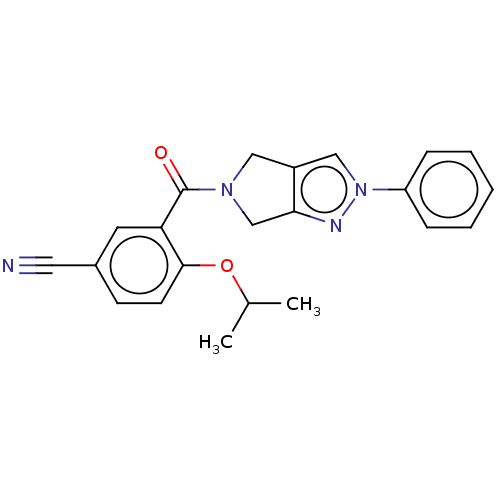 (CHEMBL4202824) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience LLC Curated by ChEMBL | Assay Description Inhibition of CYP3A5 in human liver microsomes using midazolam as substrate measured after 10 mins in presence of NADPH by LC-MS/MS analysis | J Med Chem 61: 6018-6033 (2018) Article DOI: 10.1021/acs.jmedchem.8b00372 BindingDB Entry DOI: 10.7270/Q2GB26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50457696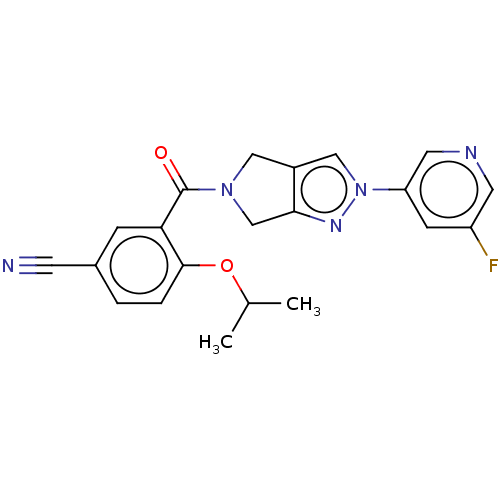 (CHEMBL4214486) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience LLC Curated by ChEMBL | Assay Description Inhibition of CYP3A5 in human liver microsomes using midazolam as substrate measured after 10 mins in presence of NADPH by LC-MS/MS analysis | J Med Chem 61: 6018-6033 (2018) Article DOI: 10.1021/acs.jmedchem.8b00372 BindingDB Entry DOI: 10.7270/Q2GB26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50457694 (CHEMBL4211535) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience LLC Curated by ChEMBL | Assay Description Inhibition of CYP3A5 in human liver microsomes using midazolam as substrate measured after 10 mins in presence of NADPH by LC-MS/MS analysis | J Med Chem 61: 6018-6033 (2018) Article DOI: 10.1021/acs.jmedchem.8b00372 BindingDB Entry DOI: 10.7270/Q2GB26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50457686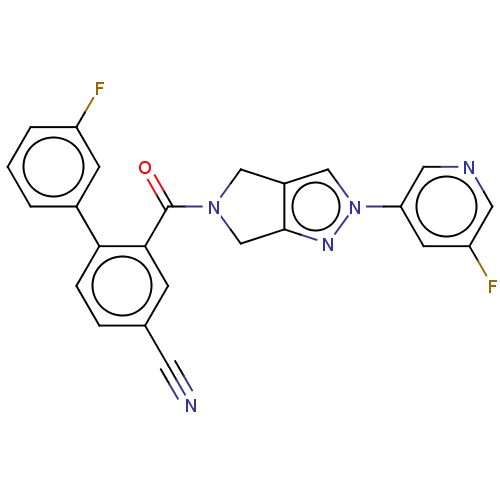 (CHEMBL4206650) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience LLC Curated by ChEMBL | Assay Description Inhibition of CYP3A5 in human liver microsomes using midazolam as substrate measured after 10 mins in presence of NADPH by LC-MS/MS analysis | J Med Chem 61: 6018-6033 (2018) Article DOI: 10.1021/acs.jmedchem.8b00372 BindingDB Entry DOI: 10.7270/Q2GB26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50457705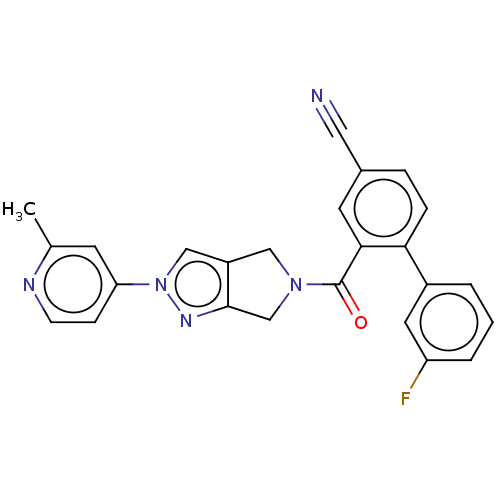 (CHEMBL4212425) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience LLC Curated by ChEMBL | Assay Description Inhibition of CYP3A5 in human liver microsomes using midazolam as substrate measured after 10 mins in presence of NADPH by LC-MS/MS analysis | J Med Chem 61: 6018-6033 (2018) Article DOI: 10.1021/acs.jmedchem.8b00372 BindingDB Entry DOI: 10.7270/Q2GB26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50457707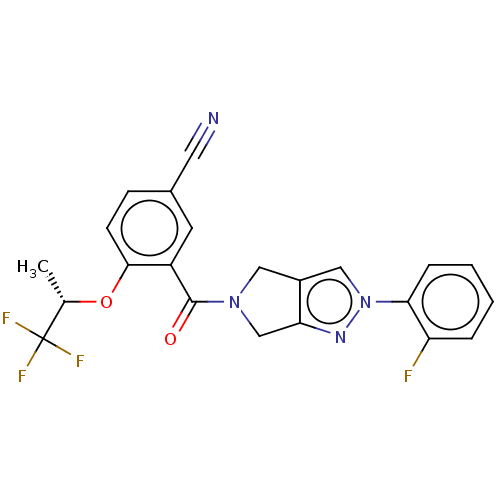 (CHEMBL4210589) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience LLC Curated by ChEMBL | Assay Description Inhibition of CYP3A5 in human liver microsomes using midazolam as substrate measured after 10 mins in presence of NADPH by LC-MS/MS analysis | J Med Chem 61: 6018-6033 (2018) Article DOI: 10.1021/acs.jmedchem.8b00372 BindingDB Entry DOI: 10.7270/Q2GB26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 110 total ) | Next | Last >> |