

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1105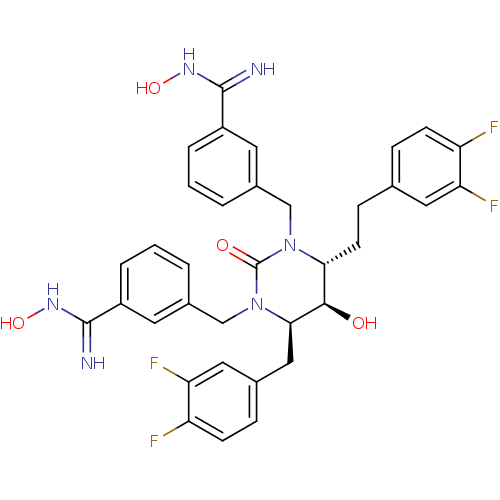 ((4R,5R,6R)-Tetrahydro-1,3-bis[(3-(N-hydroxycarboxi...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | -65.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Pharmaceuticals Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50404011 (CHEMBL36900) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company | Assay Description Inhibition constant of HIV protease inhibitors | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1150 ((4R,5R,6R)-Tetrahydro-1-(3-aminophenyl-methyl)-3-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company | Assay Description Inhibition constant of HIV protease inhibitors | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1104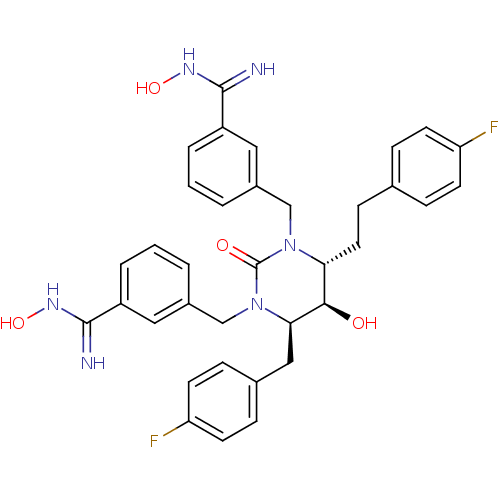 ((4R,5R,6R)-Tetrahydro-1,3-bis[(3-(N-hydroxycarboxi...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | -63.5 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Pharmaceuticals Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50404007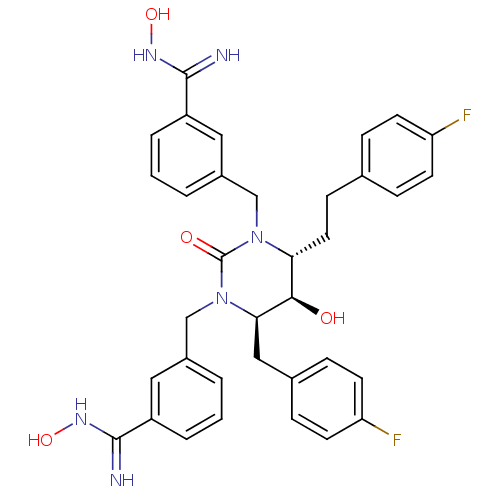 (CHEMBL116517) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company | Assay Description Inhibition constant of HIV protease inhibitors | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM164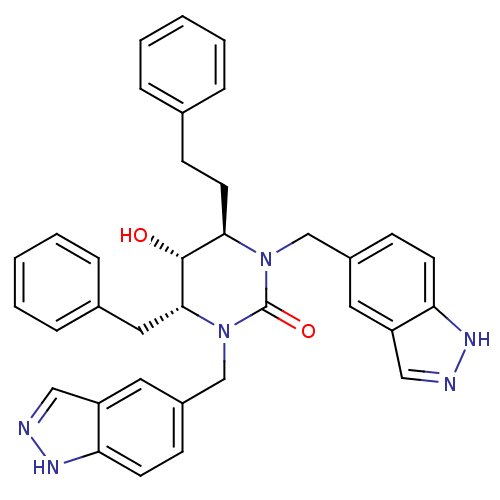 ((4R,5R,6R)-4-benzyl-5-hydroxy-1,3-bis(1H-indazol-5...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company | Assay Description Inhibition constant of HIV protease inhibitors | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1150 ((4R,5R,6R)-Tetrahydro-1-(3-aminophenyl-methyl)-3-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065089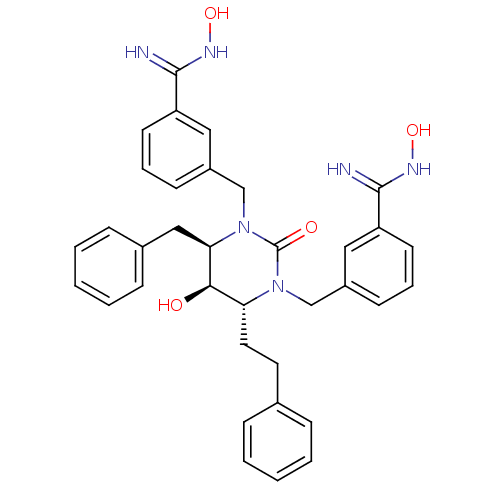 ((4R,5R,6R)-Tetrahydro-1,3-bis[(3-benzamide oxime)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company | Assay Description Inhibition constant of HIV protease inhibitors | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM182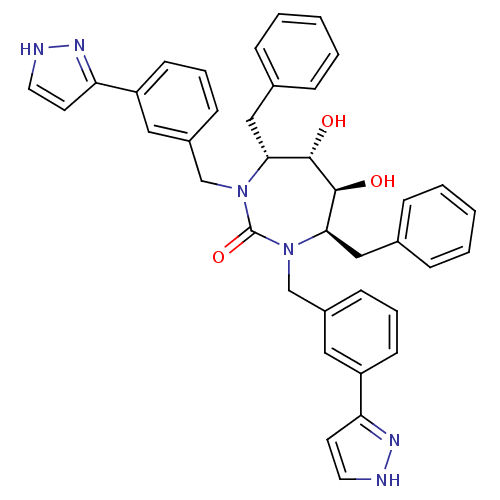 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis({...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company | Assay Description Inhibition constant of HIV protease inhibitors | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1110 ((4R,5R,6R)-1,3-bis[(3-amino-1H-indazol-5-yl)methyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | -62.5 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Pharmaceuticals Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1110 ((4R,5R,6R)-1,3-bis[(3-amino-1H-indazol-5-yl)methyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company | Assay Description Inhibition constant of HIV protease inhibitors | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1113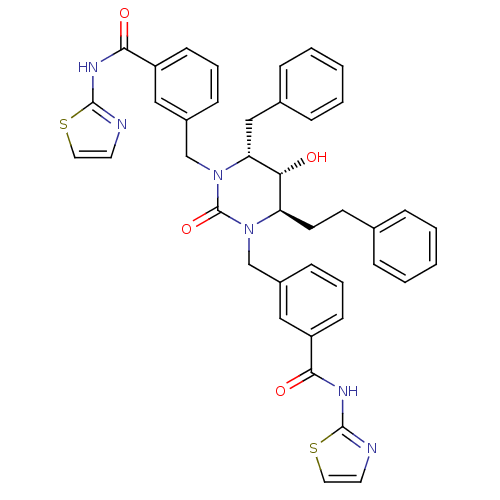 ((4R,5R,6R)-Tetrahydro-5-hydroxy-1,3-bis[(3-(thiazo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company | Assay Description Inhibition constant of HIV protease inhibitors | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1113 ((4R,5R,6R)-Tetrahydro-5-hydroxy-1,3-bis[(3-(thiazo...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | -62.5 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Pharmaceuticals Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1149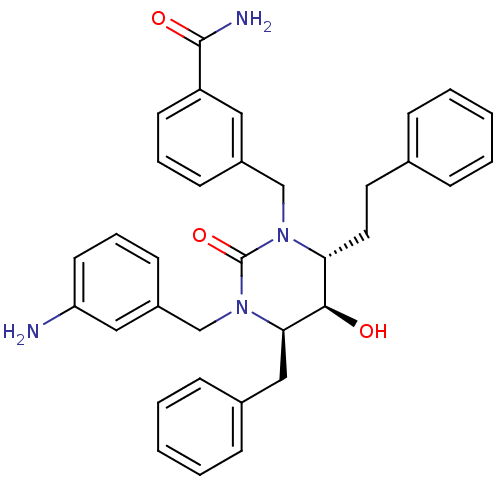 ((4R,5R,6R)-Tetrahydro-1-(3-aminophenyl-methyl)-3-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM172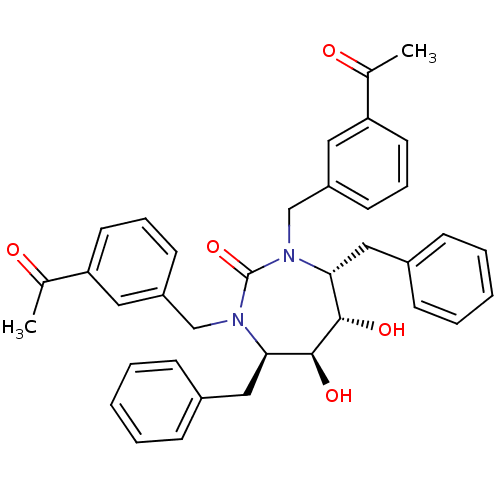 ((4R,5S,6S,7R)-4,7-dibenzyl-1,3-bis[(3-acetylphenyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company | Assay Description Inhibition constant of HIV protease inhibitors | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1106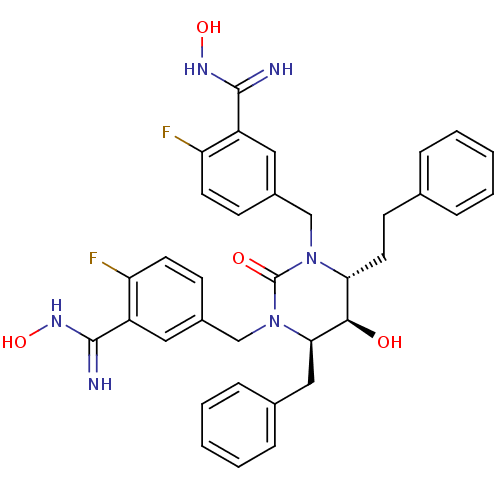 ((4R,5R,6R)-Tetrahydro-1,3-bis[(3-(N-hydroxycarboxi...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | -60.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Pharmaceuticals Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50404006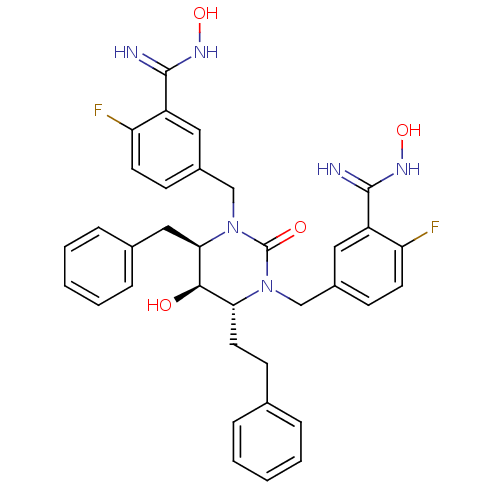 (CHEMBL366576) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company | Assay Description Inhibition constant of HIV protease inhibitors | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1149 ((4R,5R,6R)-Tetrahydro-1-(3-aminophenyl-methyl)-3-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company | Assay Description Inhibition constant of HIV protease inhibitors | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1108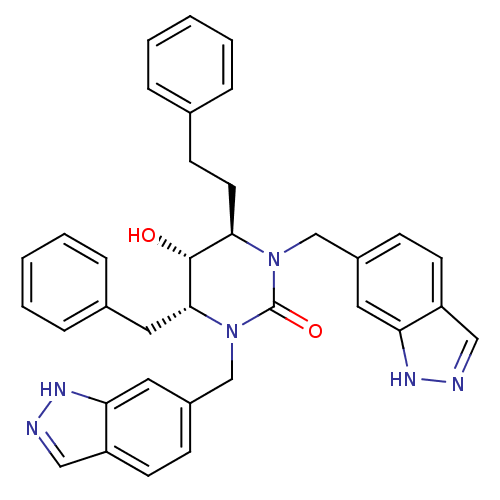 ((4R,5R,6R)-4-benzyl-5-hydroxy-1,3-bis(1H-indazol-6...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company | Assay Description Inhibition constant of HIV protease inhibitors | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1108 ((4R,5R,6R)-4-benzyl-5-hydroxy-1,3-bis(1H-indazol-6...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | -60.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Pharmaceuticals Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1144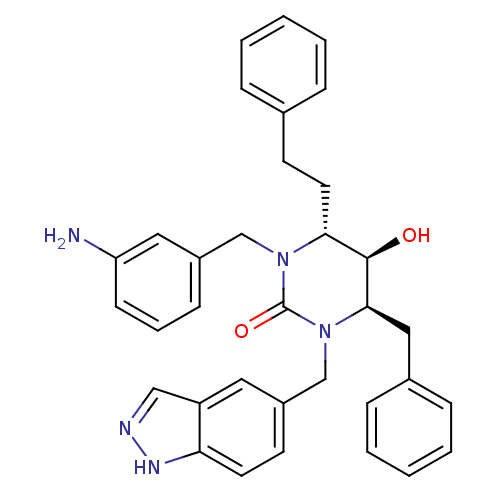 ((4R,5R,6R)-1-[(3-aminophenyl)methyl]-4-benzyl-5-hy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1112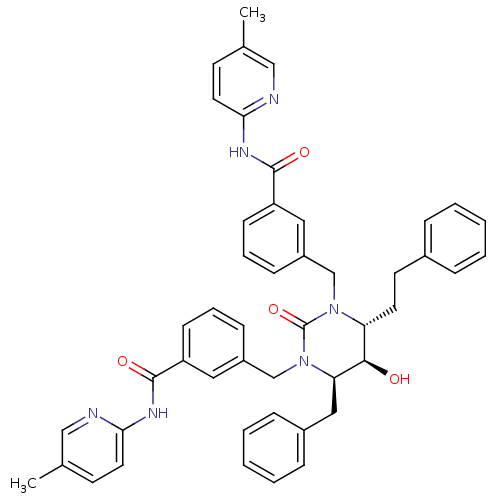 ((4R,5R,6R)-Tetrahydro-5-hydroxy-1,3-bis[(3-(5-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | -59.9 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Pharmaceuticals Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1112 ((4R,5R,6R)-Tetrahydro-5-hydroxy-1,3-bis[(3-(5-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company | Assay Description Inhibition constant of HIV protease inhibitors | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1144 ((4R,5R,6R)-1-[(3-aminophenyl)methyl]-4-benzyl-5-hy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company | Assay Description Inhibition constant of HIV protease inhibitors | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1080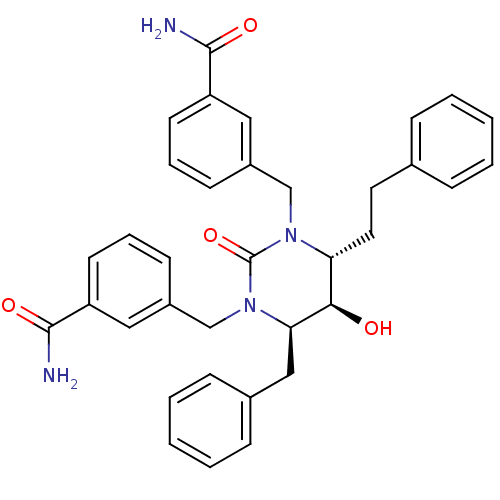 ((4R,5R,6R)-Tetrahydro-1,3-bis[(3-benzamido)methyl]...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company | Assay Description Inhibition constant of HIV protease inhibitors | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1109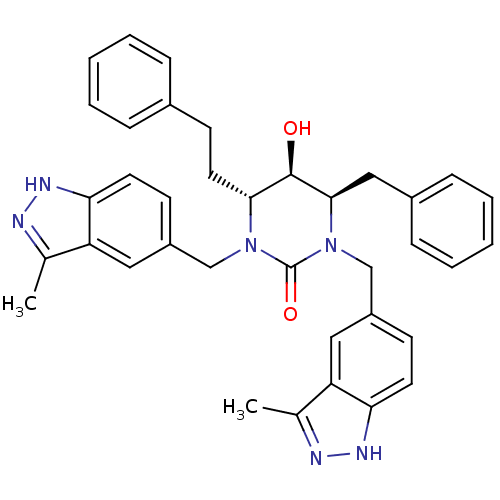 ((4R,5R,6R)-4-benzyl-5-hydroxy-1,3-bis[(3-methyl-1H...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | -59.4 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Pharmaceuticals Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1148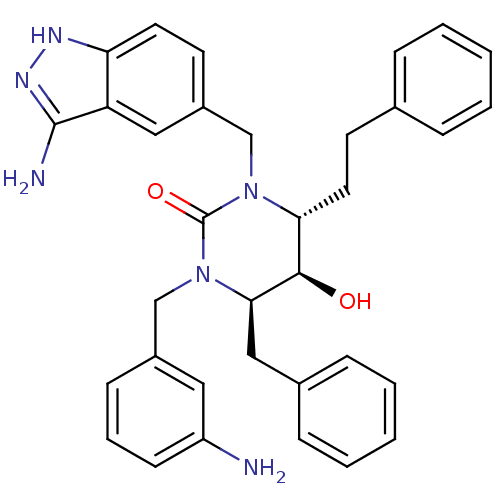 ((4R,5R,6R)-1-[(3-amino-1H-indazol-5-yl)methyl]-3-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1145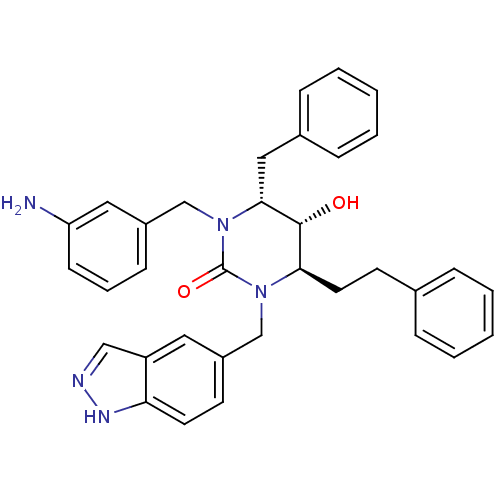 ((4R,5R,6R)-1-[(3-aminophenyl)methyl]-6-benzyl-5-hy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company | Assay Description Inhibition constant of HIV protease inhibitors | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1107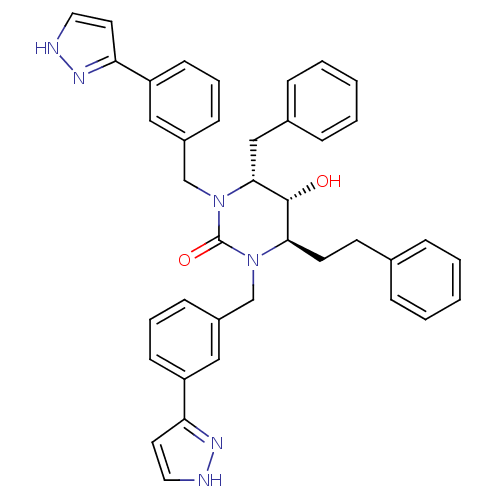 ((4R,5R,6R)-4-benzyl-5-hydroxy-6-(2-phenylethyl)-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | -59.4 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Pharmaceuticals Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1109 ((4R,5R,6R)-4-benzyl-5-hydroxy-1,3-bis[(3-methyl-1H...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company | Assay Description Inhibition constant of HIV protease inhibitors | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1107 ((4R,5R,6R)-4-benzyl-5-hydroxy-6-(2-phenylethyl)-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company | Assay Description Inhibition constant of HIV protease inhibitors | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1145 ((4R,5R,6R)-1-[(3-aminophenyl)methyl]-6-benzyl-5-hy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1148 ((4R,5R,6R)-1-[(3-amino-1H-indazol-5-yl)methyl]-3-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company | Assay Description Inhibition constant of HIV protease inhibitors | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1726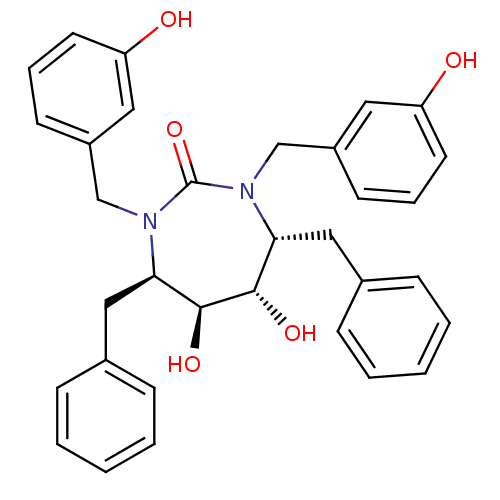 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis[(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company | Assay Description Inhibition constant of HIV protease inhibitors | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1146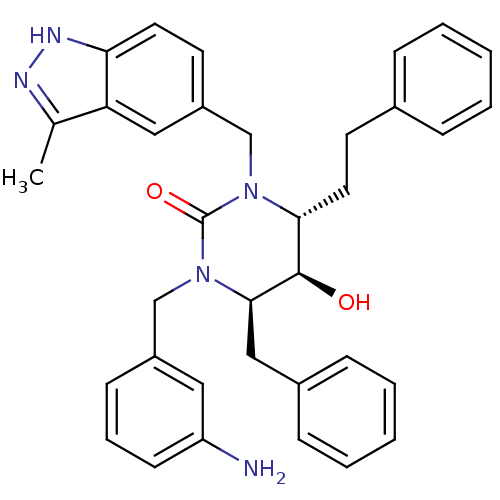 ((4R,5R,6R)-1-[(3-aminophenyl)methyl]-6-benzyl-5-hy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company | Assay Description Inhibition constant of HIV protease inhibitors | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1146 ((4R,5R,6R)-1-[(3-aminophenyl)methyl]-6-benzyl-5-hy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50408711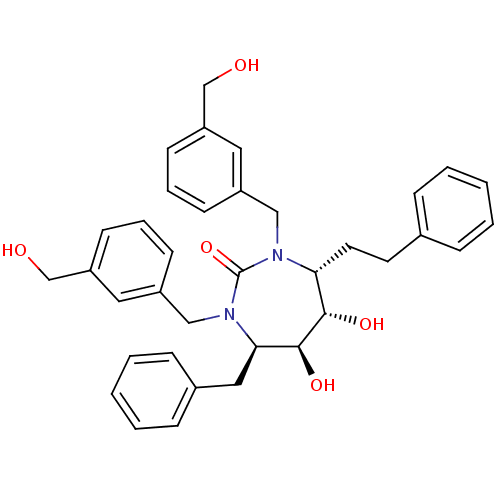 (CHEMBL277389) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company | Assay Description Inhibition constant of HIV protease inhibitors | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1730 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis({...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company | Assay Description Inhibition constant of HIV protease inhibitors | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1102 ((4R,5R,6R)-Tetrahydro-1,3-bis[(3-carboxamidophenyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company | Assay Description Inhibition constant of HIV protease inhibitors | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1102 ((4R,5R,6R)-Tetrahydro-1,3-bis[(3-carboxamidophenyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.150 | -58.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Pharmaceuticals Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1131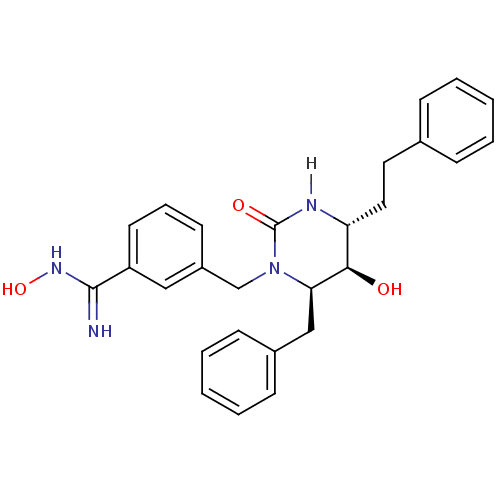 ((4R,5R,6R)-Tetrahydro-1-(3-(N-hydroxycarboximidami...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company | Assay Description Inhibition constant of HIV protease inhibitors | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1131 ((4R,5R,6R)-Tetrahydro-1-(3-(N-hydroxycarboximidami...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1090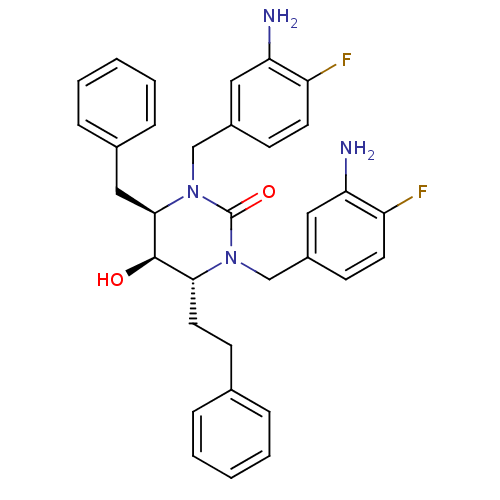 ((4R,5R,6R)-1,3-bis[(3-amino-4-fluorophenyl)methyl]...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company | Assay Description Inhibition constant of HIV protease inhibitors | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1143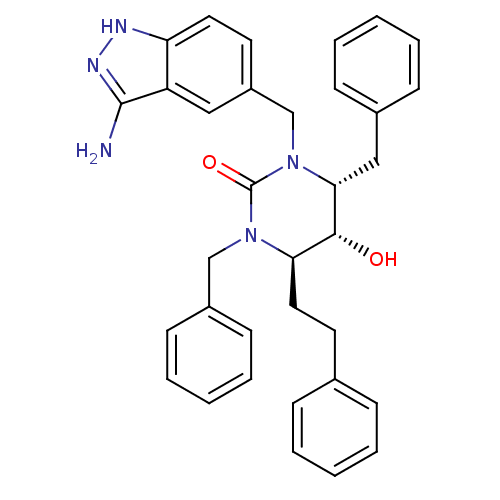 ((4R,5R,6R)-1-[(3-amino-1H-indazol-5-yl)methyl]-3,6...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company | Assay Description Inhibition constant of HIV protease inhibitors | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1143 ((4R,5R,6R)-1-[(3-amino-1H-indazol-5-yl)methyl]-3,6...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM151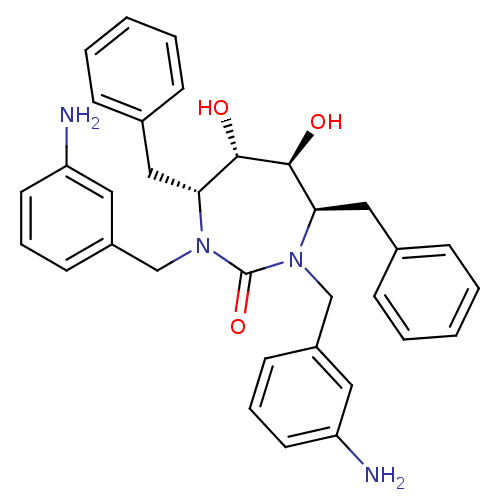 ((4R,5S,6S,7R)-1,3-bis[(3-aminophenyl)methyl]-4,7-d...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company | Assay Description Inhibition constant of HIV protease inhibitors | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1089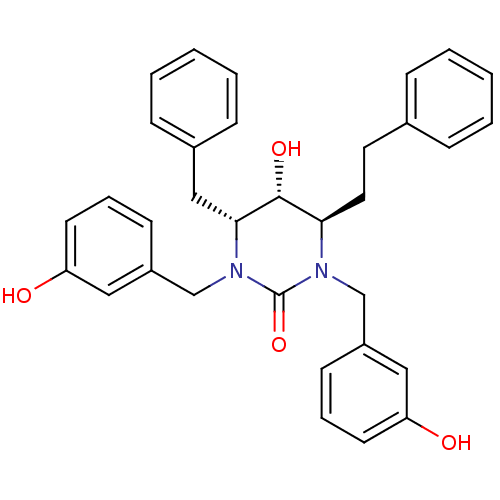 ((4R,5R,6R)-4-benzyl-5-hydroxy-1,3-bis[(3-hydroxyph...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company | Assay Description Inhibition constant of HIV protease inhibitors | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1101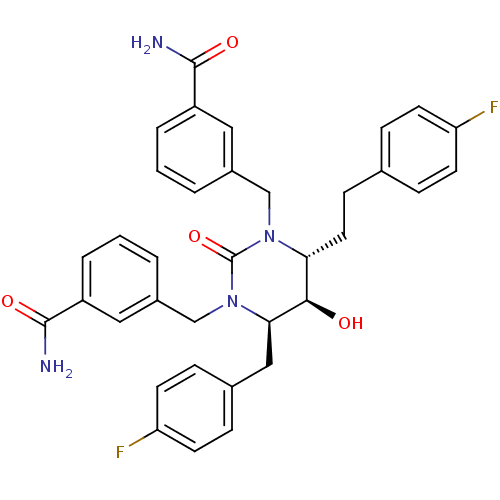 ((4R,5R,6R)-Tetrahydro-1,3-bis[(3-carboxamidophenyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company | Assay Description Inhibition constant of HIV protease inhibitors | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1101 ((4R,5R,6R)-Tetrahydro-1,3-bis[(3-carboxamidophenyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.310 | -56.5 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Pharmaceuticals Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1133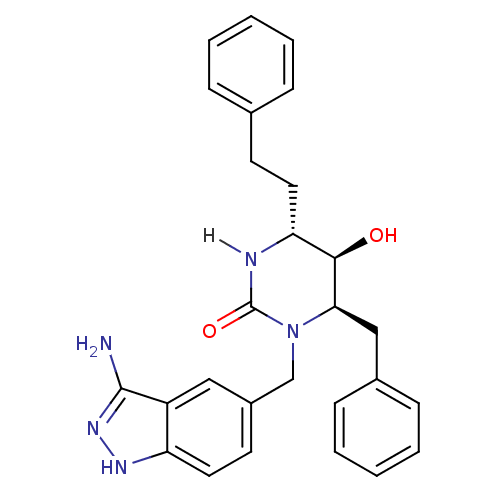 ((4R,5R,6R)-1-[(3-amino-1H-indazol-5-yl)methyl]-6-b...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 42: 135-52 (1999) Article DOI: 10.1021/jm9803626 BindingDB Entry DOI: 10.7270/Q28050S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 140 total ) | Next | Last >> |