

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM98538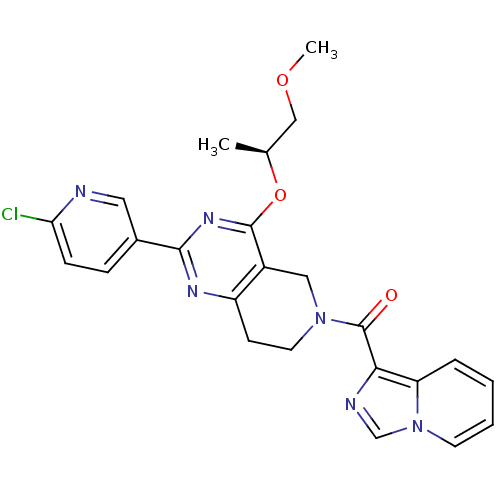 (US8492392, T-1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.430 | -53.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Fluorescence polarization assay: The compounds of the following examples had activity in reference assays by exhibiting the ability to inhibit the hy... | US Patent US8492392 (2013) BindingDB Entry DOI: 10.7270/Q2SN07M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM98540 (US8492392, 1-11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.600 | -52.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Fluorescence polarization assay: The compounds of the following examples had activity in reference assays by exhibiting the ability to inhibit the hy... | US Patent US8492392 (2013) BindingDB Entry DOI: 10.7270/Q2SN07M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50398012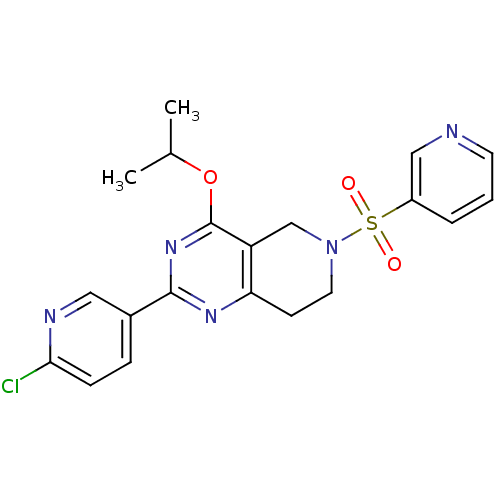 (CHEMBL2180422 | US8492392, 1-17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | 0.630 | -52.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Fluorescence polarization assay: The compounds of the following examples had activity in reference assays by exhibiting the ability to inhibit the hy... | US Patent US8492392 (2013) BindingDB Entry DOI: 10.7270/Q2SN07M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM98545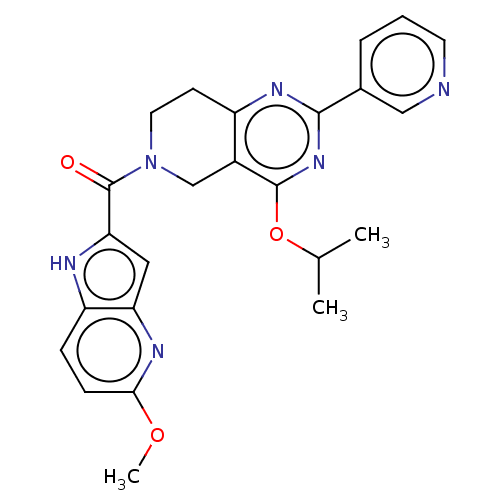 (US8492392, 1-20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.800 | -51.9 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Fluorescence polarization assay: The compounds of the following examples had activity in reference assays by exhibiting the ability to inhibit the hy... | US Patent US8492392 (2013) BindingDB Entry DOI: 10.7270/Q2SN07M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM98541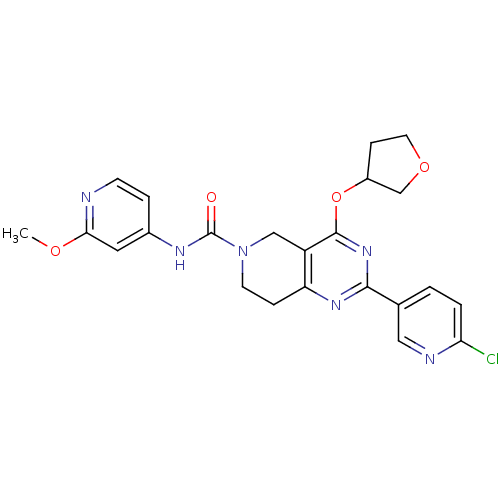 (US8492392, 1-13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.840 | -51.8 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Fluorescence polarization assay: The compounds of the following examples had activity in reference assays by exhibiting the ability to inhibit the hy... | US Patent US8492392 (2013) BindingDB Entry DOI: 10.7270/Q2SN07M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50398013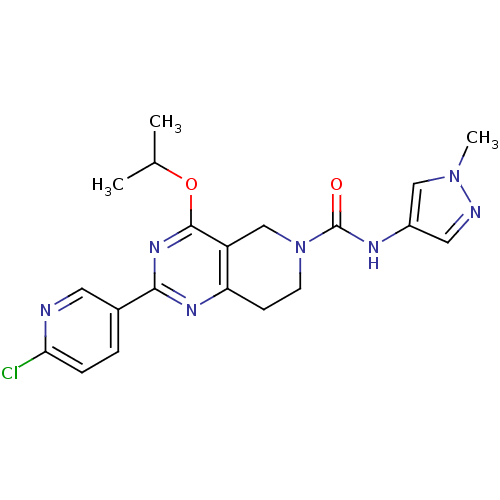 (CHEMBL2180421 | US8492392, K-2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.900 | -51.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Fluorescence polarization assay: The compounds of the following examples had activity in reference assays by exhibiting the ability to inhibit the hy... | US Patent US8492392 (2013) BindingDB Entry DOI: 10.7270/Q2SN07M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM98535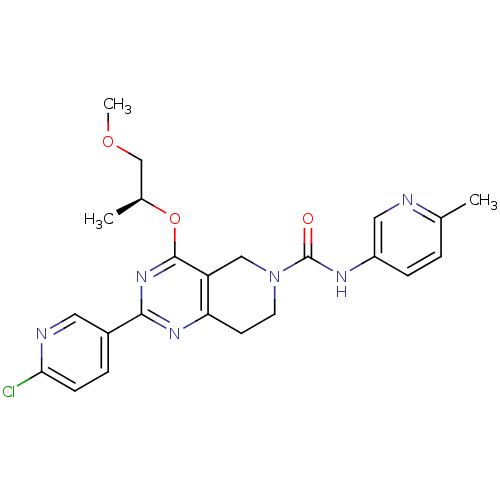 (US8492392, P-5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.920 | -51.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Fluorescence polarization assay: The compounds of the following examples had activity in reference assays by exhibiting the ability to inhibit the hy... | US Patent US8492392 (2013) BindingDB Entry DOI: 10.7270/Q2SN07M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50398011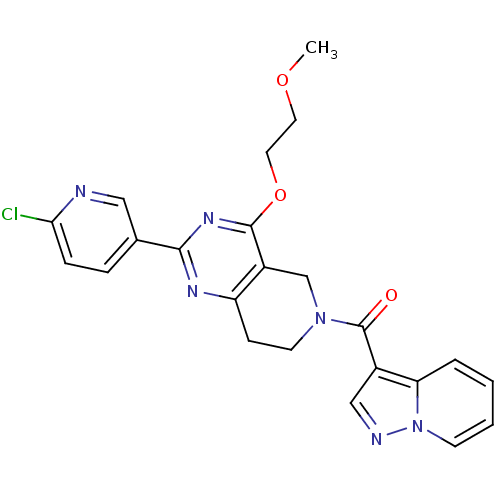 (CHEMBL2180423 | US8492392, O-2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.930 | -51.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Fluorescence polarization assay: The compounds of the following examples had activity in reference assays by exhibiting the ability to inhibit the hy... | US Patent US8492392 (2013) BindingDB Entry DOI: 10.7270/Q2SN07M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50401294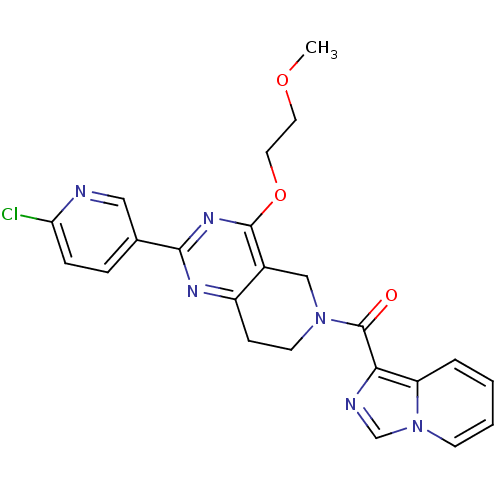 (CHEMBL2204538 | US8492392, Q-2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | 0.990 | -51.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Fluorescence polarization assay: The compounds of the following examples had activity in reference assays by exhibiting the ability to inhibit the hy... | US Patent US8492392 (2013) BindingDB Entry DOI: 10.7270/Q2SN07M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM98544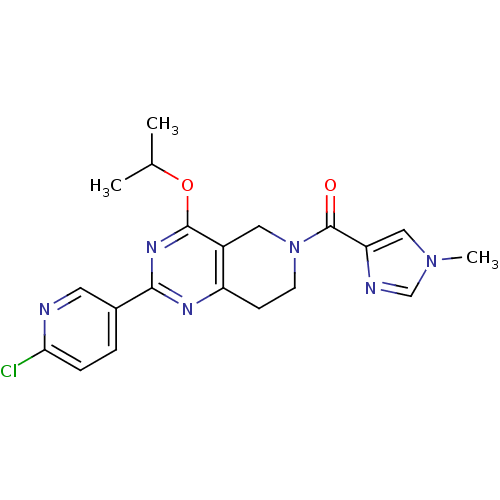 (CHEMBL2204537 | US8492392, 1-19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 4.55 | -47.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Fluorescence polarization assay: The compounds of the following examples had activity in reference assays by exhibiting the ability to inhibit the hy... | US Patent US8492392 (2013) BindingDB Entry DOI: 10.7270/Q2SN07M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM98539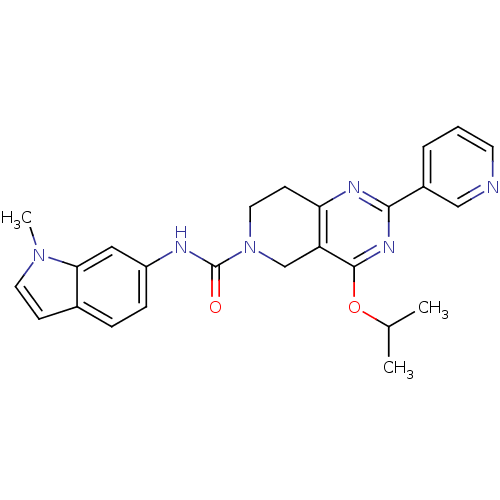 (US8492392, 1-8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 9.20 | -45.9 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Fluorescence polarization assay: The compounds of the following examples had activity in reference assays by exhibiting the ability to inhibit the hy... | US Patent US8492392 (2013) BindingDB Entry DOI: 10.7270/Q2SN07M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM98542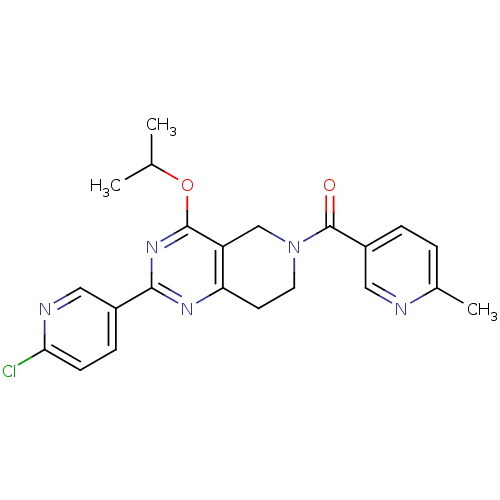 (US8492392, 1-14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 9.90 | -45.7 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Fluorescence polarization assay: The compounds of the following examples had activity in reference assays by exhibiting the ability to inhibit the hy... | US Patent US8492392 (2013) BindingDB Entry DOI: 10.7270/Q2SN07M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM98536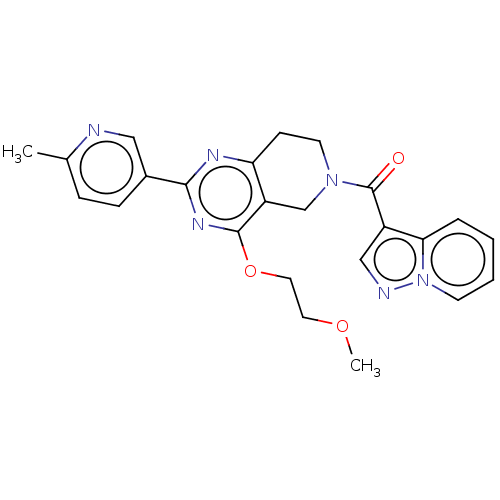 (US8492392, R-1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 12.5 | -45.1 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Fluorescence polarization assay: The compounds of the following examples had activity in reference assays by exhibiting the ability to inhibit the hy... | US Patent US8492392 (2013) BindingDB Entry DOI: 10.7270/Q2SN07M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50401302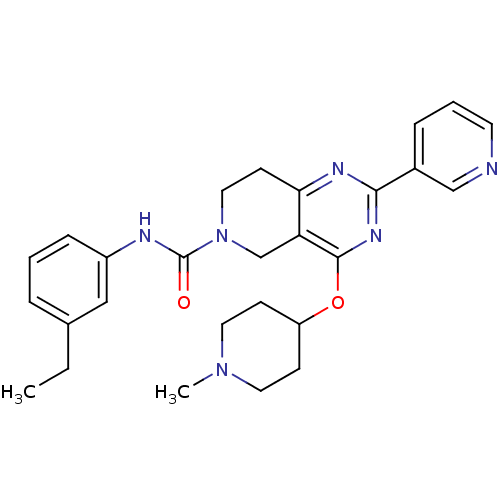 (CHEMBL2205203 | US8492392, 1-7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 14 | -44.8 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Fluorescence polarization assay: The compounds of the following examples had activity in reference assays by exhibiting the ability to inhibit the hy... | US Patent US8492392 (2013) BindingDB Entry DOI: 10.7270/Q2SN07M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM98543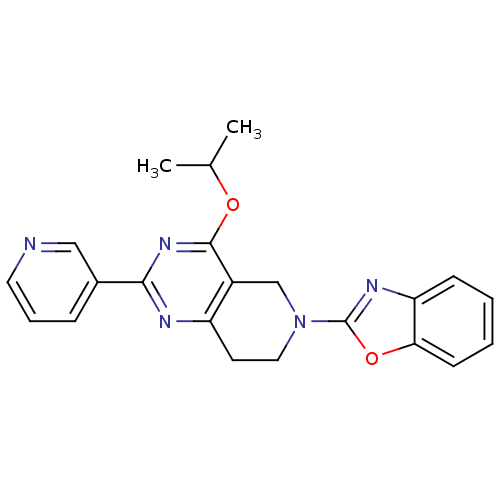 (US8492392, 1-18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 15.9 | -44.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Fluorescence polarization assay: The compounds of the following examples had activity in reference assays by exhibiting the ability to inhibit the hy... | US Patent US8492392 (2013) BindingDB Entry DOI: 10.7270/Q2SN07M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM98537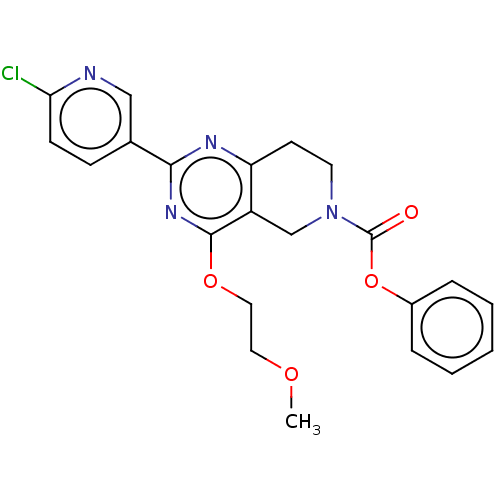 (US8492392, S-1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 16.8 | -44.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Fluorescence polarization assay: The compounds of the following examples had activity in reference assays by exhibiting the ability to inhibit the hy... | US Patent US8492392 (2013) BindingDB Entry DOI: 10.7270/Q2SN07M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM98534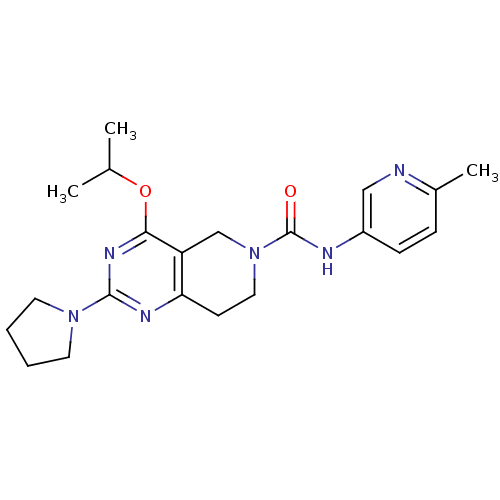 (US8492392, N-5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 31.4 | -42.8 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Fluorescence polarization assay: The compounds of the following examples had activity in reference assays by exhibiting the ability to inhibit the hy... | US Patent US8492392 (2013) BindingDB Entry DOI: 10.7270/Q2SN07M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||