

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13988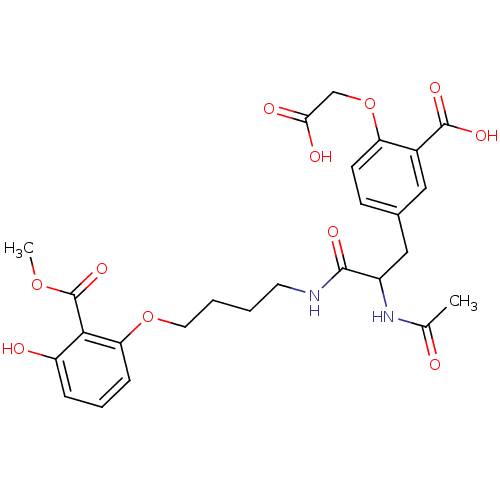 (1:1 racemic mixture | 2-(carboxymethoxy)-5-[2-acet...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.20E+3 | -32.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 3947-50 (2003) Article DOI: 10.1016/j.bmcl.2003.08.064 BindingDB Entry DOI: 10.7270/Q22R3PXB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13978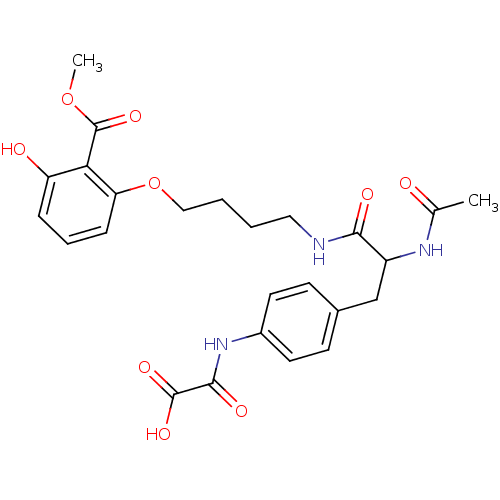 (({4-[2-acetamido-2-({4-[3-hydroxy-2-(methoxycarbon...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.10E+3 | -29.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 3947-50 (2003) Article DOI: 10.1016/j.bmcl.2003.08.064 BindingDB Entry DOI: 10.7270/Q22R3PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13977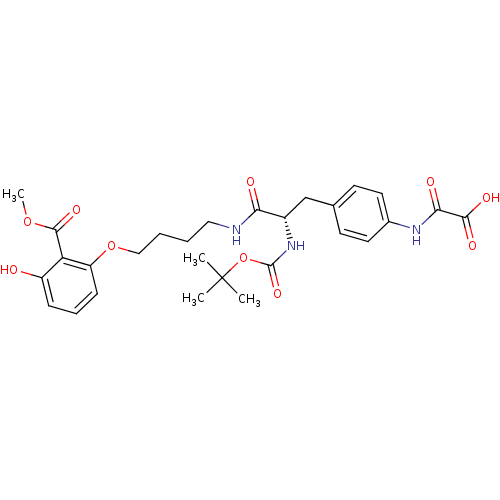 (({4-[(2S)-2-{[(tert-butoxy)carbonyl]amino}-2-({4-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.80E+3 | -28.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 3947-50 (2003) Article DOI: 10.1016/j.bmcl.2003.08.064 BindingDB Entry DOI: 10.7270/Q22R3PXB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13987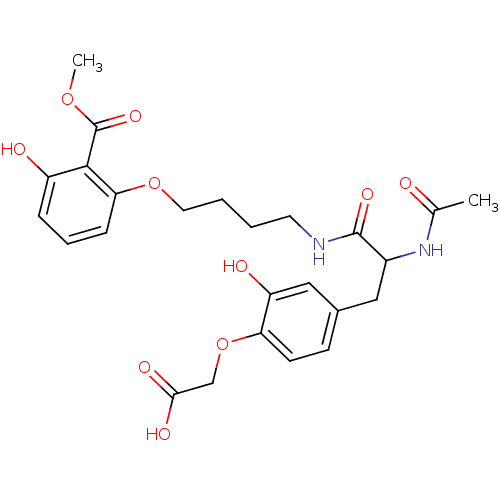 (1:1 racemic mixture | 2-{4-[2-acetamido-2-({4-[3-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 9.00E+3 | -28.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 3947-50 (2003) Article DOI: 10.1016/j.bmcl.2003.08.064 BindingDB Entry DOI: 10.7270/Q22R3PXB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13976 (Aminobenzoic acid analog 5 | CHEMBL116605) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80E+4 | -26.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 3947-50 (2003) Article DOI: 10.1016/j.bmcl.2003.08.064 BindingDB Entry DOI: 10.7270/Q22R3PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM13976 (Aminobenzoic acid analog 5 | CHEMBL116605) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.50E+4 | -23.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 3947-50 (2003) Article DOI: 10.1016/j.bmcl.2003.08.064 BindingDB Entry DOI: 10.7270/Q22R3PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM13977 (({4-[(2S)-2-{[(tert-butoxy)carbonyl]amino}-2-({4-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.41E+5 | -21.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 3947-50 (2003) Article DOI: 10.1016/j.bmcl.2003.08.064 BindingDB Entry DOI: 10.7270/Q22R3PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM13987 (1:1 racemic mixture | 2-{4-[2-acetamido-2-({4-[3-h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 1.82E+5 | -21.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 3947-50 (2003) Article DOI: 10.1016/j.bmcl.2003.08.064 BindingDB Entry DOI: 10.7270/Q22R3PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13989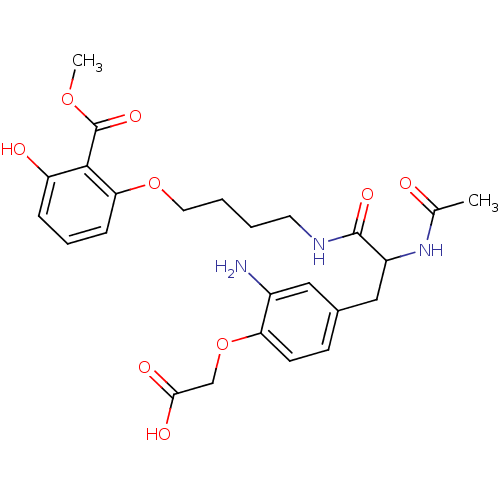 (1:1 racemic mixture | 2-{2-amino-4-[2-acetamido-2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >2.00E+5 | >-20.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 3947-50 (2003) Article DOI: 10.1016/j.bmcl.2003.08.064 BindingDB Entry DOI: 10.7270/Q22R3PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13985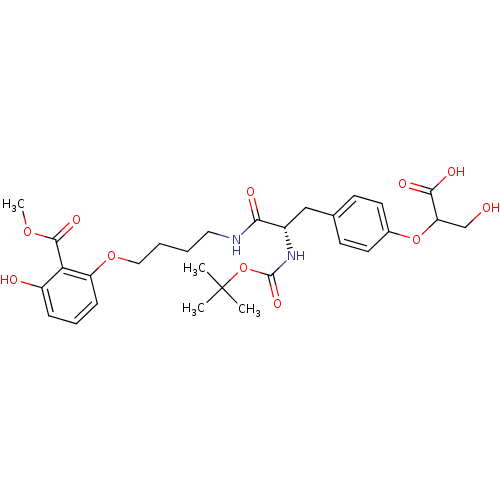 (2-{4-[(2S)-2-{[(tert-butoxy)carbonyl]amino}-2-({4-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >2.00E+5 | >-20.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 3947-50 (2003) Article DOI: 10.1016/j.bmcl.2003.08.064 BindingDB Entry DOI: 10.7270/Q22R3PXB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13983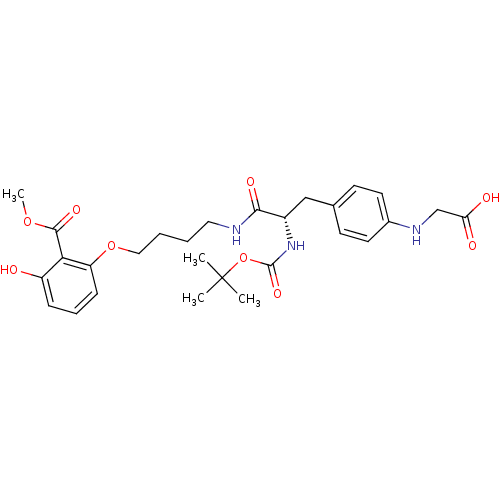 (2-({4-[(2S)-2-{[(tert-butoxy)carbonyl]amino}-2-({4...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >2.00E+5 | >-20.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 3947-50 (2003) Article DOI: 10.1016/j.bmcl.2003.08.064 BindingDB Entry DOI: 10.7270/Q22R3PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13982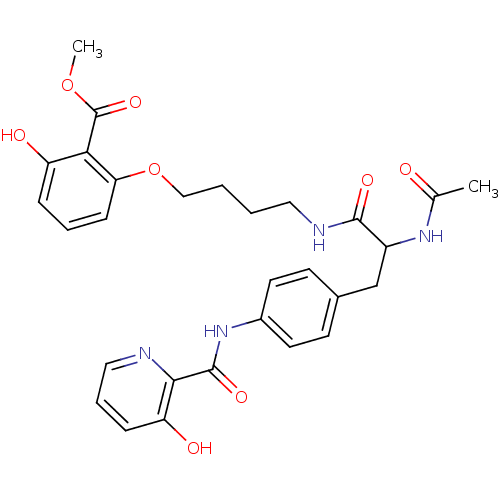 (1:1 racemic mixture | methyl 2-[4-(2-acetamido-3-{...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >2.00E+5 | >-20.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 3947-50 (2003) Article DOI: 10.1016/j.bmcl.2003.08.064 BindingDB Entry DOI: 10.7270/Q22R3PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13981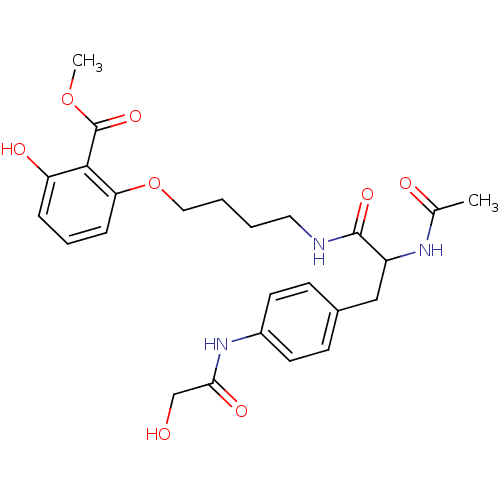 (1:1 racemic mixture | methyl 2-(4-{2-acetamido-3-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >2.00E+5 | >-20.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 3947-50 (2003) Article DOI: 10.1016/j.bmcl.2003.08.064 BindingDB Entry DOI: 10.7270/Q22R3PXB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13980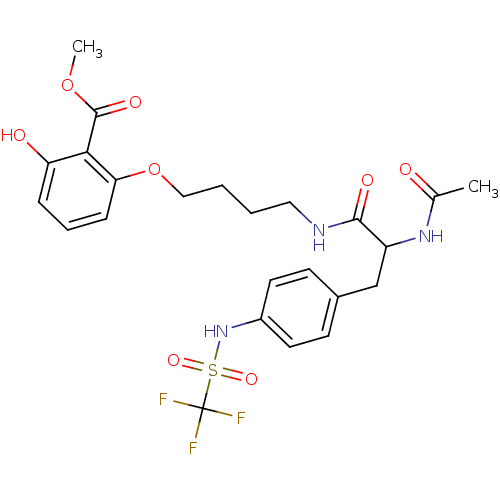 (1:1 racemic mixture | methyl 2-(4-{2-acetamido-3-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >2.00E+5 | >-20.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 3947-50 (2003) Article DOI: 10.1016/j.bmcl.2003.08.064 BindingDB Entry DOI: 10.7270/Q22R3PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13979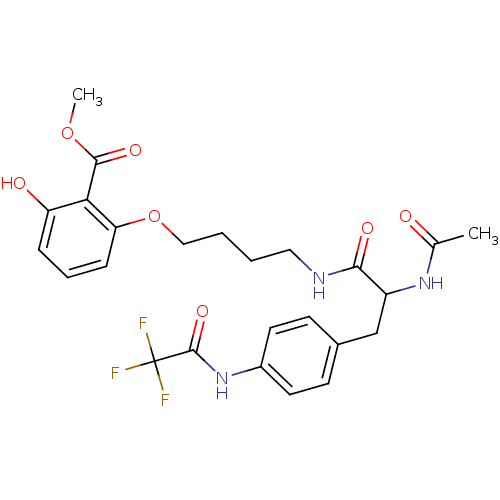 (1:1 racemic mixture | methyl 2-(4-{2-acetamido-3-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >2.00E+5 | >-20.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 3947-50 (2003) Article DOI: 10.1016/j.bmcl.2003.08.064 BindingDB Entry DOI: 10.7270/Q22R3PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13984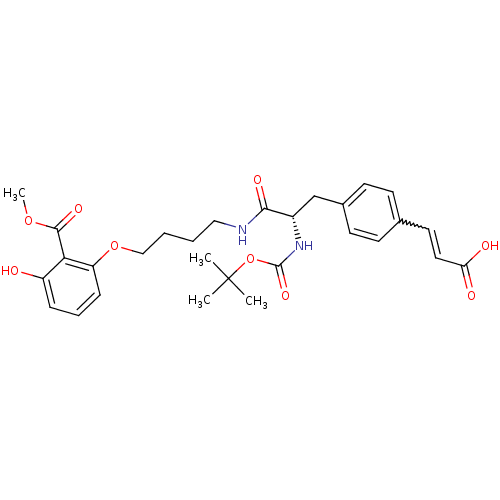 ((2Z)-3-{4-[(2S)-2-{[(tert-butoxy)carbonyl]amino}-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >2.00E+5 | >-20.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 3947-50 (2003) Article DOI: 10.1016/j.bmcl.2003.08.064 BindingDB Entry DOI: 10.7270/Q22R3PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM13986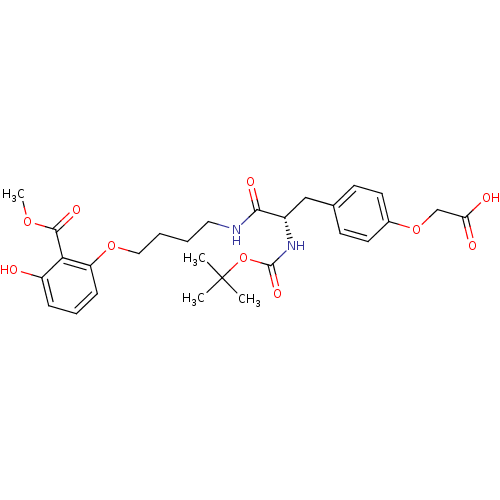 (2-{4-[(2S)-2-{[(tert-butoxy)carbonyl]amino}-2-({4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >2.00E+5 | >-20.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 3947-50 (2003) Article DOI: 10.1016/j.bmcl.2003.08.064 BindingDB Entry DOI: 10.7270/Q22R3PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13986 (2-{4-[(2S)-2-{[(tert-butoxy)carbonyl]amino}-2-({4-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20E+5 | -20.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 3947-50 (2003) Article DOI: 10.1016/j.bmcl.2003.08.064 BindingDB Entry DOI: 10.7270/Q22R3PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||