

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-lactamase (Enterobacter cloacae) | BDBM50290040 (CHEMBL67925 | Potassium; (5R,6S)-3-tert-butyl-6-((...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for beta-lactamase inhibition activity of Enterobacter cloacae after 15 min of preincubation with the enzyme at 37 degree C | Bioorg Med Chem Lett 7: 2217-2222 (1997) Article DOI: 10.1016/S0960-894X(97)00401-0 BindingDB Entry DOI: 10.7270/Q2M32VR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50021959 (CHEMBL777 | MM 14151 | US9120808, Clavulanic acid ...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for beta-lactamase inhibition activity of Escherichia coli after 15 min of preincubation with the enzyme at 37 degree C | Bioorg Med Chem Lett 7: 2217-2222 (1997) Article DOI: 10.1016/S0960-894X(97)00401-0 BindingDB Entry DOI: 10.7270/Q2M32VR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50290038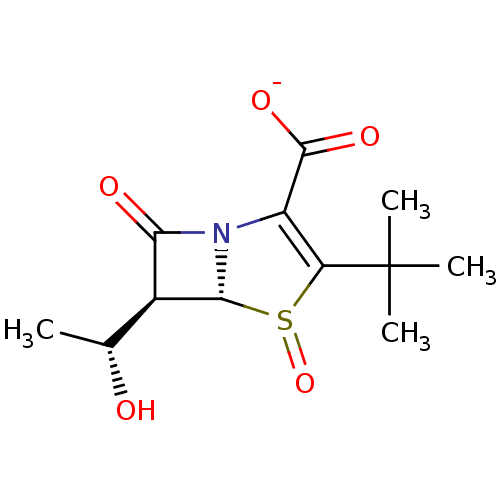 (CHEMBL419441 | Potassium; (5R,6S)-3-tert-butyl-6-(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for beta-lactamase inhibition activity of Enterobacter cloacae after 15 min of preincubation with the enzyme at 37 degree C | Bioorg Med Chem Lett 7: 2217-2222 (1997) Article DOI: 10.1016/S0960-894X(97)00401-0 BindingDB Entry DOI: 10.7270/Q2M32VR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50290039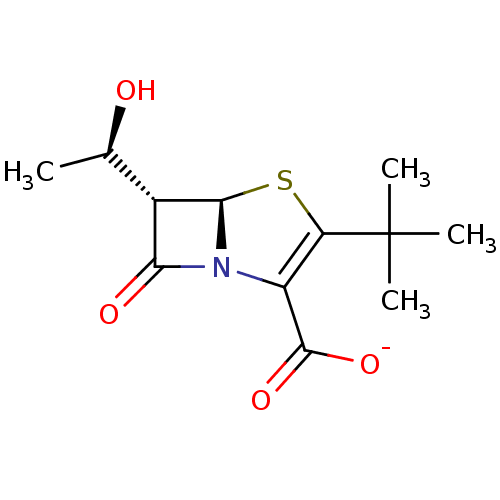 (CHEMBL69141 | Potassium; (5R,6S)-3-tert-butyl-6-((...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for beta-lactamase inhibition activity of Enterobacter cloacae after 15 min of preincubation with the enzyme at 37 degree C | Bioorg Med Chem Lett 7: 2217-2222 (1997) Article DOI: 10.1016/S0960-894X(97)00401-0 BindingDB Entry DOI: 10.7270/Q2M32VR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50021954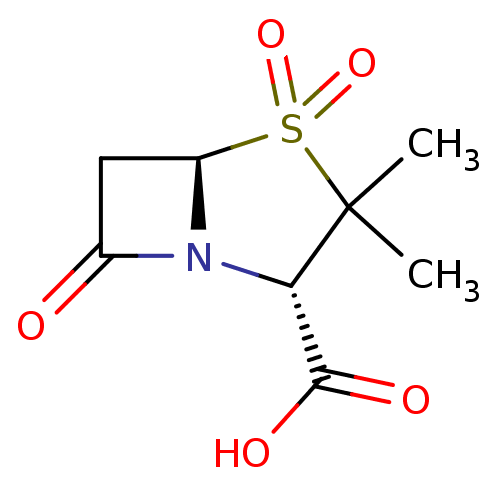 ((2S,5R)-3,3-Dimethyl-4,4,7-trioxo-4lambda*6*-thia-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for beta-lactamase inhibition activity of Escherichia coli after 15 min of preincubation with the enzyme at 37 degree C | Bioorg Med Chem Lett 7: 2217-2222 (1997) Article DOI: 10.1016/S0960-894X(97)00401-0 BindingDB Entry DOI: 10.7270/Q2M32VR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50021954 ((2S,5R)-3,3-Dimethyl-4,4,7-trioxo-4lambda*6*-thia-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for beta-lactamase inhibition activity of Escherichia cloacae after 15 min of preincubation with the enzyme at 37 degree C | Bioorg Med Chem Lett 7: 2217-2222 (1997) Article DOI: 10.1016/S0960-894X(97)00401-0 BindingDB Entry DOI: 10.7270/Q2M32VR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50290040 (CHEMBL67925 | Potassium; (5R,6S)-3-tert-butyl-6-((...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for beta-lactamase inhibition activity of Escherichia coli after 15 min of preincubation with the enzyme at 37 degree C | Bioorg Med Chem Lett 7: 2217-2222 (1997) Article DOI: 10.1016/S0960-894X(97)00401-0 BindingDB Entry DOI: 10.7270/Q2M32VR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50290039 (CHEMBL69141 | Potassium; (5R,6S)-3-tert-butyl-6-((...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for beta-lactamase inhibition activity of Escherichia coli after 15 min of preincubation with the enzyme at 37 degree C | Bioorg Med Chem Lett 7: 2217-2222 (1997) Article DOI: 10.1016/S0960-894X(97)00401-0 BindingDB Entry DOI: 10.7270/Q2M32VR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50290038 (CHEMBL419441 | Potassium; (5R,6S)-3-tert-butyl-6-(...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for beta-lactamase inhibition activity of Escherichia coli after 15 min of preincubation with the enzyme at 37 degree C | Bioorg Med Chem Lett 7: 2217-2222 (1997) Article DOI: 10.1016/S0960-894X(97)00401-0 BindingDB Entry DOI: 10.7270/Q2M32VR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50021959 (CHEMBL777 | MM 14151 | US9120808, Clavulanic acid ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for beta-lactamase inhibition activity of Enterobacter cloacae after 15 min of preincubation with the enzyme at 37 degree C | Bioorg Med Chem Lett 7: 2217-2222 (1997) Article DOI: 10.1016/S0960-894X(97)00401-0 BindingDB Entry DOI: 10.7270/Q2M32VR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||