

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50006842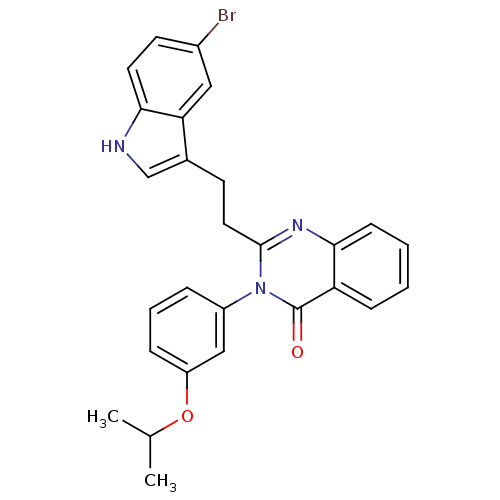 (2-[2-(5-Bromo-1H-indol-3-yl)-ethyl]-3-(3-isopropox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Evaluated for inhibition of [125I]-CCK-8S binding to cholecystokinin CCK-B receptor from mouse brain membranes at a concentration of 10 microM (in vi... | J Med Chem 35: 2534-42 (1992) BindingDB Entry DOI: 10.7270/Q2JM28MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50006838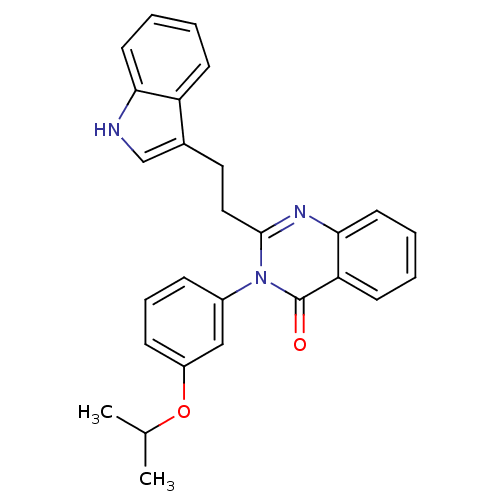 (2-[2-(1H-Indol-3-yl)-ethyl]-3-(3-isopropoxy-phenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Evaluated for inhibition of [125I]-CCK-8S binding to cholecystokinin CCK-B receptor from mouse brain membranes at a concentration of 10 microM (in vi... | J Med Chem 35: 2534-42 (1992) BindingDB Entry DOI: 10.7270/Q2JM28MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50006839 (2-[2-(1H-Indol-3-yl)-propyl]-3-(3-isopropoxy-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Evaluated for inhibition of [125I]-CCK-8S binding to cholecystokinin CCK-B receptor from mouse brain membranes at a concentration of 10 microM (in vi... | J Med Chem 35: 2534-42 (1992) BindingDB Entry DOI: 10.7270/Q2JM28MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50006840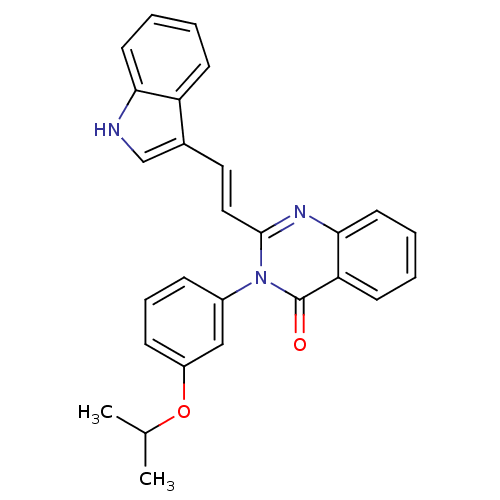 (2-[2-(1H-Indol-3-yl)-vinyl]-3-(3-isopropoxy-phenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Evaluated for inhibition of [125I]-CCK-8S binding to cholecystokinin CCK-B receptor from mouse brain membranes at a concentration of 10 microM (in vi... | J Med Chem 35: 2534-42 (1992) BindingDB Entry DOI: 10.7270/Q2JM28MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50006837 (2-[2-(1H-Indol-3-yl)-ethyl]-3-phenyl-3H-quinazolin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Evaluated for inhibition of [125I]-CCK-8S binding to cholecystokinin CCK-B receptor from mouse brain membranes at a concentration of 10 microM (in vi... | J Med Chem 35: 2534-42 (1992) BindingDB Entry DOI: 10.7270/Q2JM28MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50006836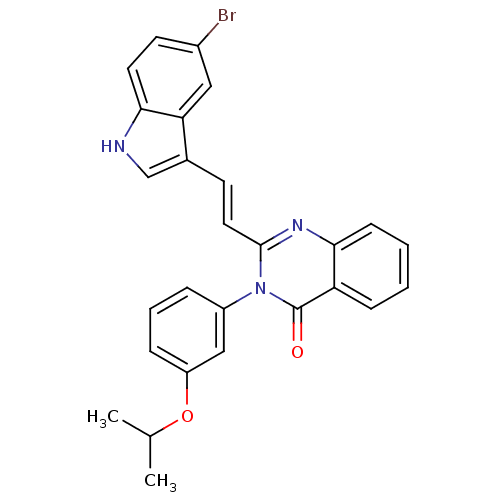 (2-[2-(5-Bromo-1H-indol-3-yl)-vinyl]-3-(3-isopropox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Evaluated for inhibition of [125I]-CCK-8S binding to cholecystokinin CCK-B receptor from mouse brain membranes at a concentration of 10 microM (in vi... | J Med Chem 35: 2534-42 (1992) BindingDB Entry DOI: 10.7270/Q2JM28MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50006841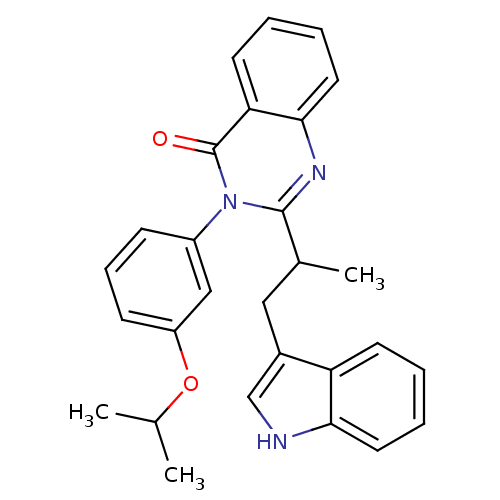 (2-[2-(1H-Indol-3-yl)-1-methyl-ethyl]-3-(3-isopropo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Evaluated for inhibition of [125I]-CCK-8S binding to cholecystokinin CCK-B receptor from mouse brain membranes at a concentration of 10 microM (in vi... | J Med Chem 35: 2534-42 (1992) BindingDB Entry DOI: 10.7270/Q2JM28MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||