

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymidylate synthase (Lactobacillus casei) | BDBM50028378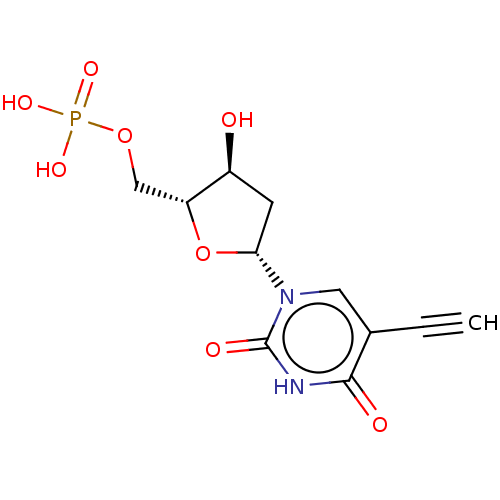 (CHEMBL3143871 | Phosphoric acid mono-[5-(5-ethynyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was evaluated for the inhibition of dTMP synthetase from Lactobacillus casei | J Med Chem 24: 1385-8 (1982) BindingDB Entry DOI: 10.7270/Q2MS3RSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50010239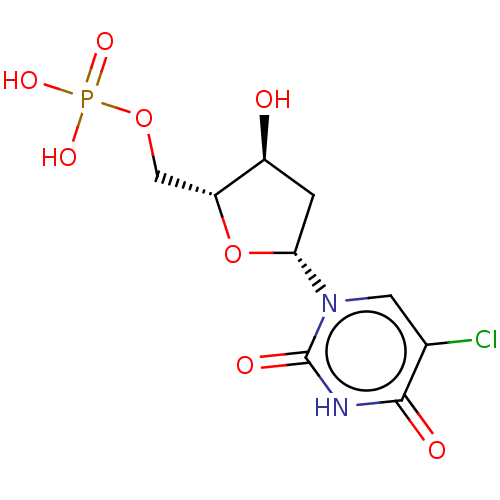 (CHEMBL1236538) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was evaluated for the inhibition of dTMP synthetase from Lactobacillus casei | J Med Chem 24: 1385-8 (1982) BindingDB Entry DOI: 10.7270/Q2MS3RSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50028376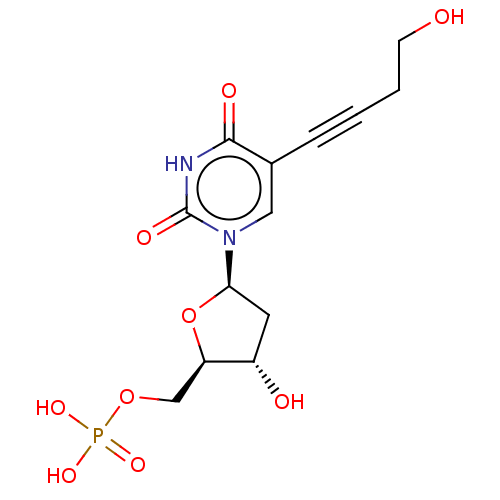 (CHEMBL3143870 | Phosphoric acid mono-{3-hydroxy-5-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was evaluated for the inhibition of dTMP synthetase from Lactobacillus casei | J Med Chem 24: 1385-8 (1982) BindingDB Entry DOI: 10.7270/Q2MS3RSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50028372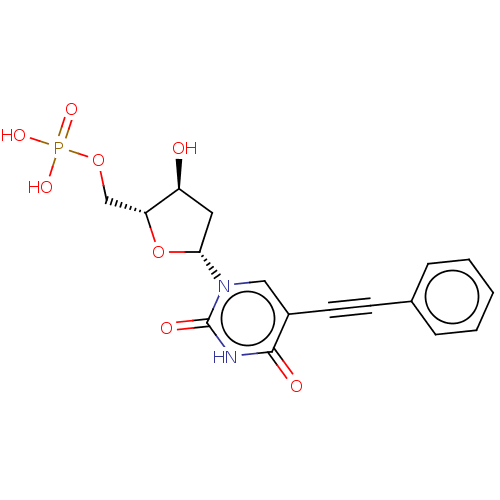 (CHEMBL3143873 | Phosphoric acid mono-[5-(2,4-dioxo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was evaluated for the inhibition of dTMP synthetase from Lactobacillus casei | J Med Chem 24: 1385-8 (1982) BindingDB Entry DOI: 10.7270/Q2MS3RSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50028375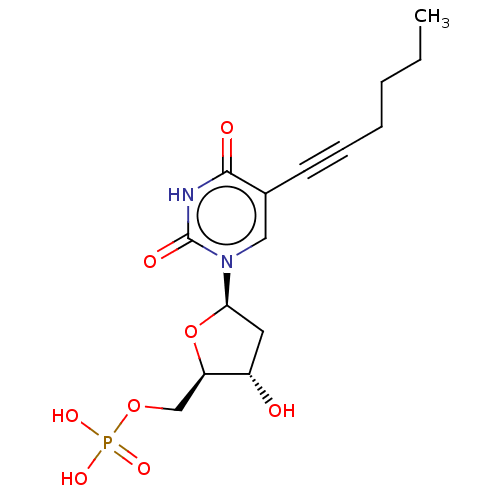 (CHEMBL3143872 | Phosphoric acid mono-[5-(5-hex-1-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was evaluated for the inhibition of dTMP synthetase from Lactobacillus casei | J Med Chem 24: 1385-8 (1982) BindingDB Entry DOI: 10.7270/Q2MS3RSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50028377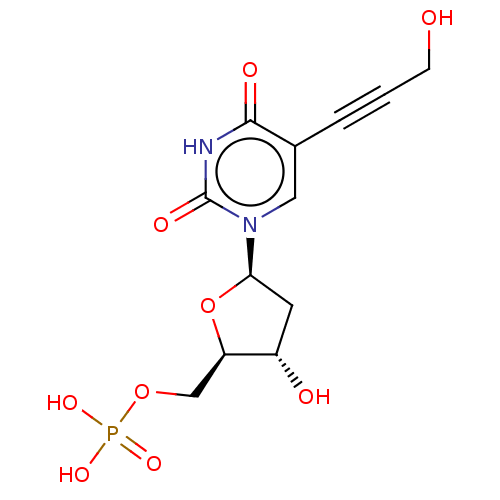 (CHEMBL3143869 | Phosphoric acid mono-{3-hydroxy-5-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was evaluated for the inhibition of dTMP synthetase from Lactobacillus casei | J Med Chem 24: 1385-8 (1982) BindingDB Entry DOI: 10.7270/Q2MS3RSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||