 Found 46 hits Enz. Inhib. hit(s) with all data for entry = 50008746
Found 46 hits Enz. Inhib. hit(s) with all data for entry = 50008746 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50067563

((S)-2-{[2'-Methyl-5-(pyridin-3-yloxymethyl)-biphen...)Show SMILES CSCC[C@H](NC(=O)c1ccc(COc2cccnc2)cc1-c1ccccc1C)C(O)=O Show InChI InChI=1S/C25H26N2O4S/c1-17-6-3-4-8-20(17)22-14-18(16-31-19-7-5-12-26-15-19)9-10-21(22)24(28)27-23(25(29)30)11-13-32-2/h3-10,12,14-15,23H,11,13,16H2,1-2H3,(H,27,28)(H,29,30)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound was evaluated against farnesyltransferase (FTase) |
Bioorg Med Chem Lett 9: 1069-74 (1999)
BindingDB Entry DOI: 10.7270/Q2X34WNT |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50067584

((S)-2-{[5-((R)-2-Amino-3-mercapto-propylamino)-bip...)Show SMILES CSCC[C@H](NC(=O)c1ccc(NC[C@@H](N)CS)cc1-c1ccccc1)C(O)=O Show InChI InChI=1S/C21H27N3O3S2/c1-29-10-9-19(21(26)27)24-20(25)17-8-7-16(23-12-15(22)13-28)11-18(17)14-5-3-2-4-6-14/h2-8,11,15,19,23,28H,9-10,12-13,22H2,1H3,(H,24,25)(H,26,27)/t15-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound was evaluated against farnesyltransferase (FTase) |
Bioorg Med Chem Lett 9: 1069-74 (1999)
BindingDB Entry DOI: 10.7270/Q2X34WNT |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50076811
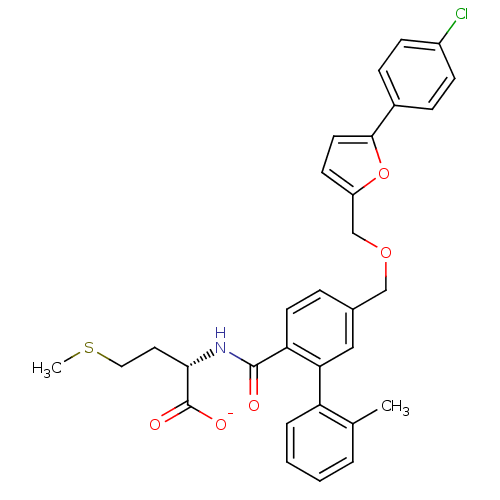
(CHEMBL10877 | Lithium; (S)-2-({5-[5-(4-chloro-phen...)Show SMILES CSCC[C@H](NC(=O)c1ccc(COCc2ccc(o2)-c2ccc(Cl)cc2)cc1-c1ccccc1C)C([O-])=O Show InChI InChI=1S/C31H30ClNO5S/c1-20-5-3-4-6-25(20)27-17-21(7-13-26(27)30(34)33-28(31(35)36)15-16-39-2)18-37-19-24-12-14-29(38-24)22-8-10-23(32)11-9-22/h3-14,17,28H,15-16,18-19H2,1-2H3,(H,33,34)(H,35,36)/p-1/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound was evaluated against farnesyltransferase (FTase) |
Bioorg Med Chem Lett 9: 1069-74 (1999)
BindingDB Entry DOI: 10.7270/Q2X34WNT |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50076809
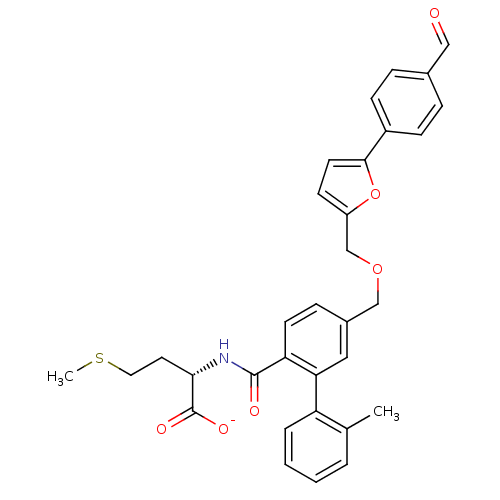
(CHEMBL417606 | Lithium; (S)-2-({5-[5-(4-formyl-phe...)Show SMILES CSCC[C@H](NC(=O)c1ccc(COCc2ccc(o2)-c2ccc(C=O)cc2)cc1-c1ccccc1C)C([O-])=O Show InChI InChI=1S/C32H31NO6S/c1-21-5-3-4-6-26(21)28-17-23(9-13-27(28)31(35)33-29(32(36)37)15-16-40-2)19-38-20-25-12-14-30(39-25)24-10-7-22(18-34)8-11-24/h3-14,17-18,29H,15-16,19-20H2,1-2H3,(H,33,35)(H,36,37)/p-1/t29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound was evaluated against farnesyltransferase (FTase) |
Bioorg Med Chem Lett 9: 1069-74 (1999)
BindingDB Entry DOI: 10.7270/Q2X34WNT |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50076798
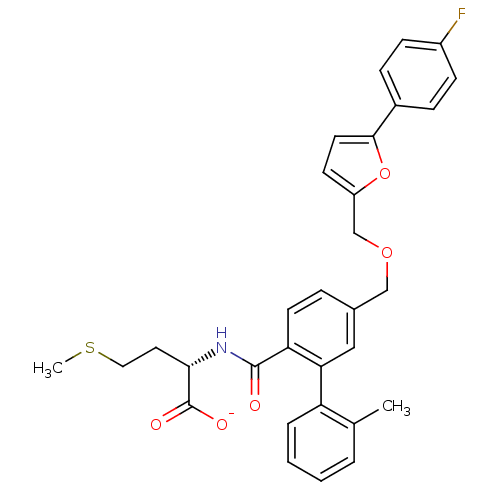
(CHEMBL273697 | Lithium; (S)-2-({5-[5-(4-fluoro-phe...)Show SMILES CSCC[C@H](NC(=O)c1ccc(COCc2ccc(o2)-c2ccc(F)cc2)cc1-c1ccccc1C)C([O-])=O Show InChI InChI=1S/C31H30FNO5S/c1-20-5-3-4-6-25(20)27-17-21(7-13-26(27)30(34)33-28(31(35)36)15-16-39-2)18-37-19-24-12-14-29(38-24)22-8-10-23(32)11-9-22/h3-14,17,28H,15-16,18-19H2,1-2H3,(H,33,34)(H,35,36)/p-1/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound was evaluated against farnesyltransferase (FTase) |
Bioorg Med Chem Lett 9: 1069-74 (1999)
BindingDB Entry DOI: 10.7270/Q2X34WNT |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50076802
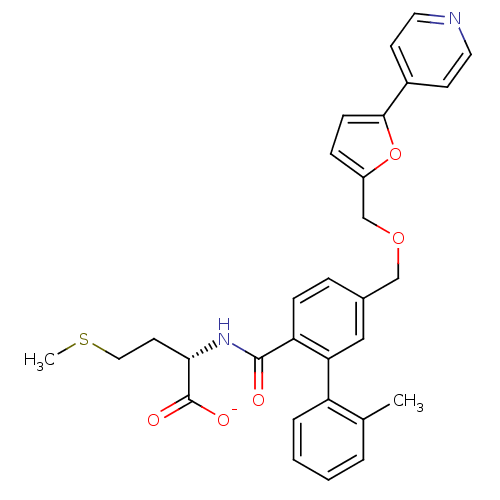
(CHEMBL10912 | Lithium; (S)-2-{[2'-methyl-5-(5-pyri...)Show SMILES CSCC[C@H](NC(=O)c1ccc(COCc2ccc(o2)-c2ccncc2)cc1-c1ccccc1C)C([O-])=O Show InChI InChI=1S/C30H30N2O5S/c1-20-5-3-4-6-24(20)26-17-21(7-9-25(26)29(33)32-27(30(34)35)13-16-38-2)18-36-19-23-8-10-28(37-23)22-11-14-31-15-12-22/h3-12,14-15,17,27H,13,16,18-19H2,1-2H3,(H,32,33)(H,34,35)/p-1/t27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound was evaluated against farnesyltransferase (FTase) |
Bioorg Med Chem Lett 9: 1069-74 (1999)
BindingDB Entry DOI: 10.7270/Q2X34WNT |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50076795
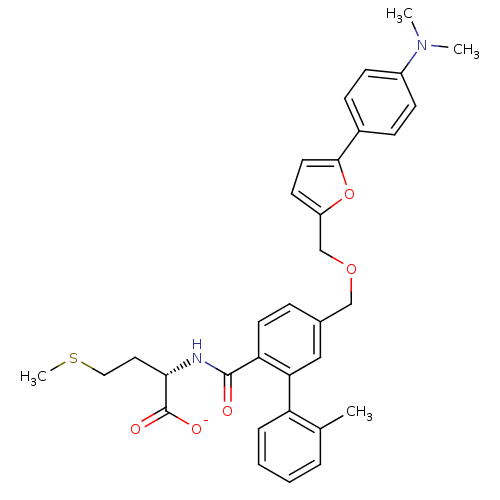
(CHEMBL11210 | Lithium; (S)-2-({5-[5-(4-dimethylami...)Show SMILES CSCC[C@H](NC(=O)c1ccc(COCc2ccc(o2)-c2ccc(cc2)N(C)C)cc1-c1ccccc1C)C([O-])=O Show InChI InChI=1S/C33H36N2O5S/c1-22-7-5-6-8-27(22)29-19-23(9-15-28(29)32(36)34-30(33(37)38)17-18-41-4)20-39-21-26-14-16-31(40-26)24-10-12-25(13-11-24)35(2)3/h5-16,19,30H,17-18,20-21H2,1-4H3,(H,34,36)(H,37,38)/p-1/t30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound was evaluated against farnesyltransferase (FTase) |
Bioorg Med Chem Lett 9: 1069-74 (1999)
BindingDB Entry DOI: 10.7270/Q2X34WNT |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50076794
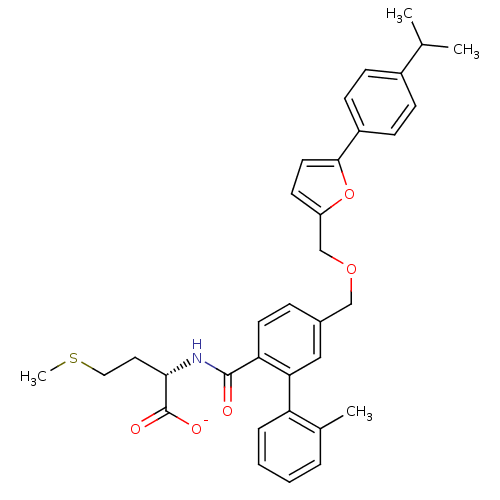
(CHEMBL276602 | Lithium; (S)-2-({5-[5-(4-isopropyl-...)Show SMILES CSCC[C@H](NC(=O)c1ccc(COCc2ccc(o2)-c2ccc(cc2)C(C)C)cc1-c1ccccc1C)C([O-])=O Show InChI InChI=1S/C34H37NO5S/c1-22(2)25-10-12-26(13-11-25)32-16-14-27(40-32)21-39-20-24-9-15-29(30(19-24)28-8-6-5-7-23(28)3)33(36)35-31(34(37)38)17-18-41-4/h5-16,19,22,31H,17-18,20-21H2,1-4H3,(H,35,36)(H,37,38)/p-1/t31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound was evaluated against farnesyltransferase (FTase) |
Bioorg Med Chem Lett 9: 1069-74 (1999)
BindingDB Entry DOI: 10.7270/Q2X34WNT |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50076791
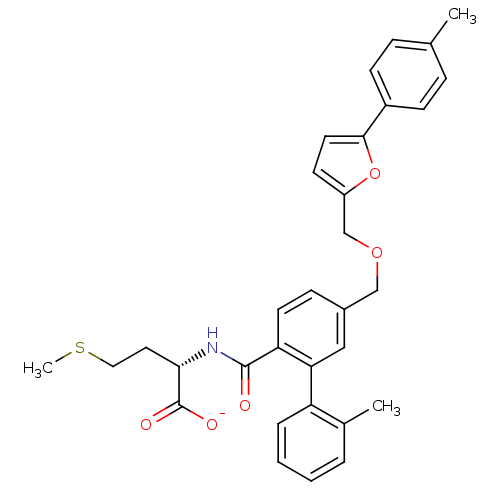
(CHEMBL10990 | Lithium; (S)-4-methylsulfanyl-2-{[2'...)Show SMILES CSCC[C@H](NC(=O)c1ccc(COCc2ccc(o2)-c2ccc(C)cc2)cc1-c1ccccc1C)C([O-])=O Show InChI InChI=1S/C32H33NO5S/c1-21-8-11-24(12-9-21)30-15-13-25(38-30)20-37-19-23-10-14-27(28(18-23)26-7-5-4-6-22(26)2)31(34)33-29(32(35)36)16-17-39-3/h4-15,18,29H,16-17,19-20H2,1-3H3,(H,33,34)(H,35,36)/p-1/t29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound was evaluated against farnesyltransferase (FTase) |
Bioorg Med Chem Lett 9: 1069-74 (1999)
BindingDB Entry DOI: 10.7270/Q2X34WNT |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50076810
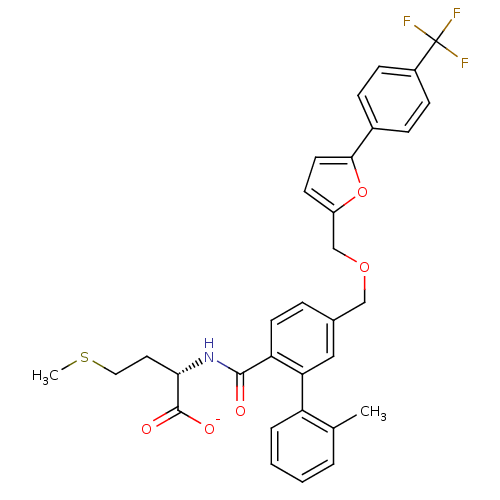
(CHEMBL428001 | Lithium; (S)-4-methylsulfanyl-2-({2...)Show SMILES CSCC[C@H](NC(=O)c1ccc(COCc2ccc(o2)-c2ccc(cc2)C(F)(F)F)cc1-c1ccccc1C)C([O-])=O Show InChI InChI=1S/C32H30F3NO5S/c1-20-5-3-4-6-25(20)27-17-21(7-13-26(27)30(37)36-28(31(38)39)15-16-42-2)18-40-19-24-12-14-29(41-24)22-8-10-23(11-9-22)32(33,34)35/h3-14,17,28H,15-16,18-19H2,1-2H3,(H,36,37)(H,38,39)/p-1/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound was evaluated against farnesyltransferase (FTase) |
Bioorg Med Chem Lett 9: 1069-74 (1999)
BindingDB Entry DOI: 10.7270/Q2X34WNT |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50076799
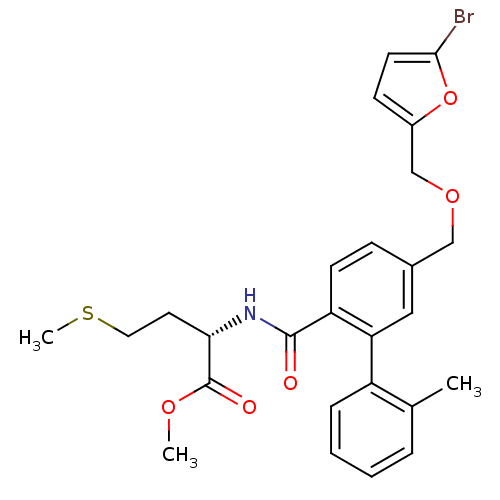
((S)-2-{[5-(5-Bromo-furan-2-ylmethoxymethyl)-2'-met...)Show SMILES COC(=O)[C@H](CCSC)NC(=O)c1ccc(COCc2ccc(Br)o2)cc1-c1ccccc1C Show InChI InChI=1S/C26H28BrNO5S/c1-17-6-4-5-7-20(17)22-14-18(15-32-16-19-9-11-24(27)33-19)8-10-21(22)25(29)28-23(12-13-34-3)26(30)31-2/h4-11,14,23H,12-13,15-16H2,1-3H3,(H,28,29)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound was evaluated against farnesyltransferase (FTase) |
Bioorg Med Chem Lett 9: 1069-74 (1999)
BindingDB Entry DOI: 10.7270/Q2X34WNT |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50076792
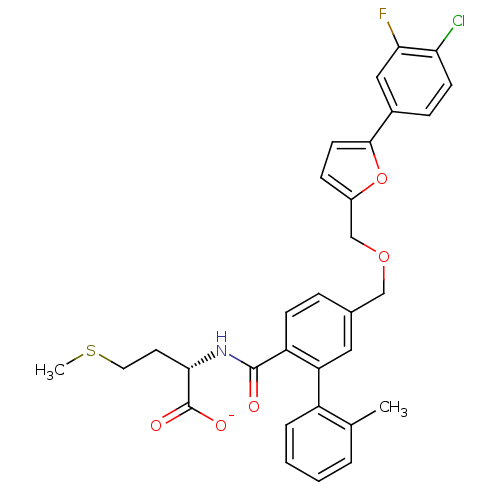
(CHEMBL10812 | Lithium; (S)-2-({5-[5-(4-chloro-3-fl...)Show SMILES CSCC[C@H](NC(=O)c1ccc(COCc2ccc(o2)-c2ccc(Cl)c(F)c2)cc1-c1ccccc1C)C([O-])=O Show InChI InChI=1S/C31H29ClFNO5S/c1-19-5-3-4-6-23(19)25-15-20(7-10-24(25)30(35)34-28(31(36)37)13-14-40-2)17-38-18-22-9-12-29(39-22)21-8-11-26(32)27(33)16-21/h3-12,15-16,28H,13-14,17-18H2,1-2H3,(H,34,35)(H,36,37)/p-1/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound was evaluated against farnesyltransferase (FTase) |
Bioorg Med Chem Lett 9: 1069-74 (1999)
BindingDB Entry DOI: 10.7270/Q2X34WNT |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50076797
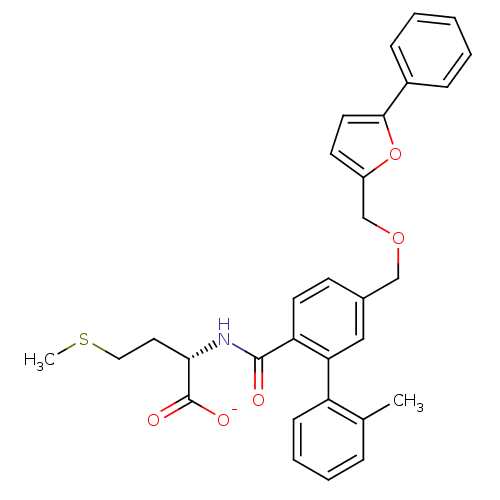
(CHEMBL10955 | Lithium; (S)-2-{[2'-methyl-5-(5-phen...)Show SMILES CSCC[C@H](NC(=O)c1ccc(COCc2ccc(o2)-c2ccccc2)cc1-c1ccccc1C)C([O-])=O Show InChI InChI=1S/C31H31NO5S/c1-21-8-6-7-11-25(21)27-18-22(12-14-26(27)30(33)32-28(31(34)35)16-17-38-2)19-36-20-24-13-15-29(37-24)23-9-4-3-5-10-23/h3-15,18,28H,16-17,19-20H2,1-2H3,(H,32,33)(H,34,35)/p-1/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound was evaluated against farnesyltransferase (FTase) |
Bioorg Med Chem Lett 9: 1069-74 (1999)
BindingDB Entry DOI: 10.7270/Q2X34WNT |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50076801
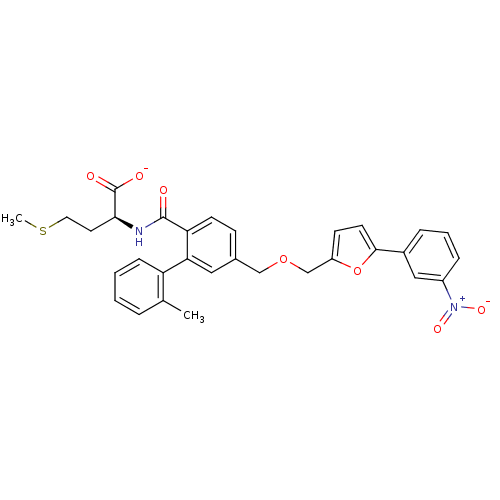
(CHEMBL430096 | Lithium; (S)-2-({2'-methyl-5-[5-(3-...)Show SMILES CSCC[C@H](NC(=O)c1ccc(COCc2ccc(o2)-c2cccc(c2)[N+]([O-])=O)cc1-c1ccccc1C)C([O-])=O Show InChI InChI=1S/C31H30N2O7S/c1-20-6-3-4-9-25(20)27-16-21(10-12-26(27)30(34)32-28(31(35)36)14-15-41-2)18-39-19-24-11-13-29(40-24)22-7-5-8-23(17-22)33(37)38/h3-13,16-17,28H,14-15,18-19H2,1-2H3,(H,32,34)(H,35,36)/p-1/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound was evaluated against farnesyltransferase (FTase) |
Bioorg Med Chem Lett 9: 1069-74 (1999)
BindingDB Entry DOI: 10.7270/Q2X34WNT |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50076793
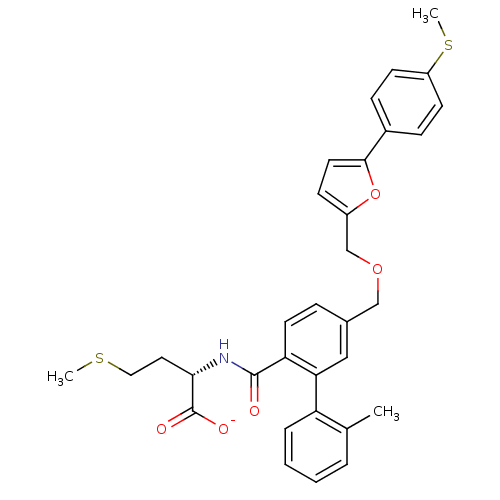
(CHEMBL11003 | Lithium; (S)-2-({2'-methyl-5-[5-(4-m...)Show SMILES CSCC[C@H](NC(=O)c1ccc(COCc2ccc(o2)-c2ccc(SC)cc2)cc1-c1ccccc1C)C([O-])=O Show InChI InChI=1S/C32H33NO5S2/c1-21-6-4-5-7-26(21)28-18-22(8-14-27(28)31(34)33-29(32(35)36)16-17-39-2)19-37-20-24-11-15-30(38-24)23-9-12-25(40-3)13-10-23/h4-15,18,29H,16-17,19-20H2,1-3H3,(H,33,34)(H,35,36)/p-1/t29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound was evaluated against farnesyltransferase (FTase) |
Bioorg Med Chem Lett 9: 1069-74 (1999)
BindingDB Entry DOI: 10.7270/Q2X34WNT |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50076803
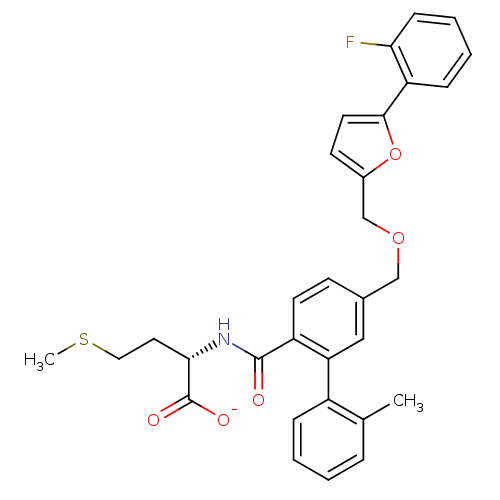
(CHEMBL10970 | Lithium; (S)-2-({5-[5-(2-fluoro-phen...)Show SMILES CSCC[C@H](NC(=O)c1ccc(COCc2ccc(o2)-c2ccccc2F)cc1-c1ccccc1C)C([O-])=O Show InChI InChI=1S/C31H30FNO5S/c1-20-7-3-4-8-23(20)26-17-21(11-13-24(26)30(34)33-28(31(35)36)15-16-39-2)18-37-19-22-12-14-29(38-22)25-9-5-6-10-27(25)32/h3-14,17,28H,15-16,18-19H2,1-2H3,(H,33,34)(H,35,36)/p-1/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound was evaluated against farnesyltransferase (FTase) |
Bioorg Med Chem Lett 9: 1069-74 (1999)
BindingDB Entry DOI: 10.7270/Q2X34WNT |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50076790
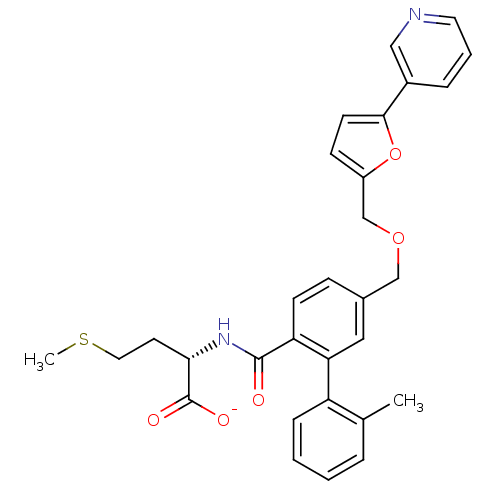
(CHEMBL10902 | Lithium; (S)-2-{[2'-methyl-5-(5-pyri...)Show SMILES CSCC[C@H](NC(=O)c1ccc(COCc2ccc(o2)-c2cccnc2)cc1-c1ccccc1C)C([O-])=O Show InChI InChI=1S/C30H30N2O5S/c1-20-6-3-4-8-24(20)26-16-21(9-11-25(26)29(33)32-27(30(34)35)13-15-38-2)18-36-19-23-10-12-28(37-23)22-7-5-14-31-17-22/h3-12,14,16-17,27H,13,15,18-19H2,1-2H3,(H,32,33)(H,34,35)/p-1/t27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound was evaluated against farnesyltransferase (FTase) |
Bioorg Med Chem Lett 9: 1069-74 (1999)
BindingDB Entry DOI: 10.7270/Q2X34WNT |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50076800
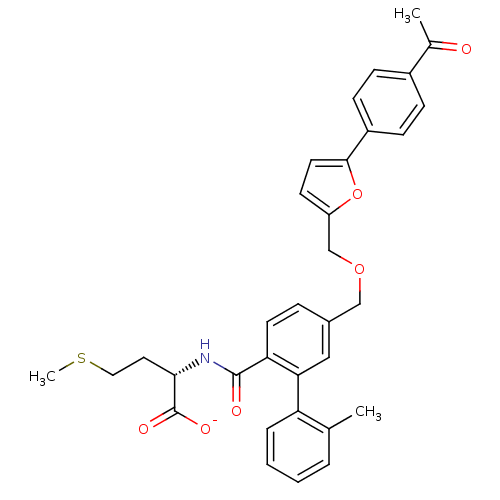
(CHEMBL430307 | Lithium; (S)-2-({5-[5-(4-acetyl-phe...)Show SMILES CSCC[C@H](NC(=O)c1ccc(COCc2ccc(o2)-c2ccc(cc2)C(C)=O)cc1-c1ccccc1C)C([O-])=O Show InChI InChI=1S/C33H33NO6S/c1-21-6-4-5-7-27(21)29-18-23(8-14-28(29)32(36)34-30(33(37)38)16-17-41-3)19-39-20-26-13-15-31(40-26)25-11-9-24(10-12-25)22(2)35/h4-15,18,30H,16-17,19-20H2,1-3H3,(H,34,36)(H,37,38)/p-1/t30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound was evaluated against farnesyltransferase (FTase) |
Bioorg Med Chem Lett 9: 1069-74 (1999)
BindingDB Entry DOI: 10.7270/Q2X34WNT |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50076788
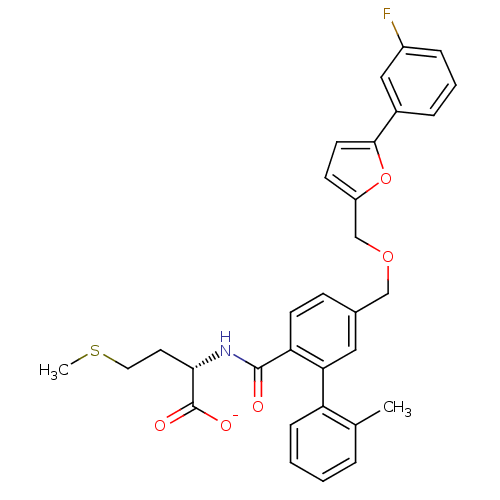
(CHEMBL11195 | Lithium; (S)-2-({5-[5-(3-fluoro-phen...)Show SMILES CSCC[C@H](NC(=O)c1ccc(COCc2ccc(o2)-c2cccc(F)c2)cc1-c1ccccc1C)C([O-])=O Show InChI InChI=1S/C31H30FNO5S/c1-20-6-3-4-9-25(20)27-16-21(10-12-26(27)30(34)33-28(31(35)36)14-15-39-2)18-37-19-24-11-13-29(38-24)22-7-5-8-23(32)17-22/h3-13,16-17,28H,14-15,18-19H2,1-2H3,(H,33,34)(H,35,36)/p-1/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound was evaluated against farnesyltransferase (FTase) |
Bioorg Med Chem Lett 9: 1069-74 (1999)
BindingDB Entry DOI: 10.7270/Q2X34WNT |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50076806
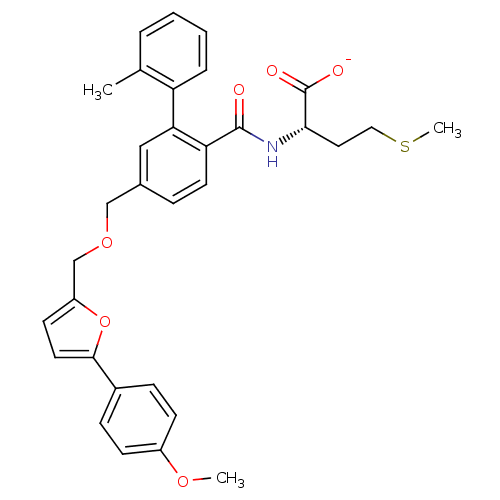
(CHEMBL273265 | Lithium; (S)-2-({5-[5-(4-methoxy-ph...)Show SMILES COc1ccc(cc1)-c1ccc(COCc2ccc(C(=O)N[C@@H](CCSC)C([O-])=O)c(c2)-c2ccccc2C)o1 Show InChI InChI=1S/C32H33NO6S/c1-21-6-4-5-7-26(21)28-18-22(8-14-27(28)31(34)33-29(32(35)36)16-17-40-3)19-38-20-25-13-15-30(39-25)23-9-11-24(37-2)12-10-23/h4-15,18,29H,16-17,19-20H2,1-3H3,(H,33,34)(H,35,36)/p-1/t29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound was evaluated against farnesyltransferase (FTase) |
Bioorg Med Chem Lett 9: 1069-74 (1999)
BindingDB Entry DOI: 10.7270/Q2X34WNT |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50076804
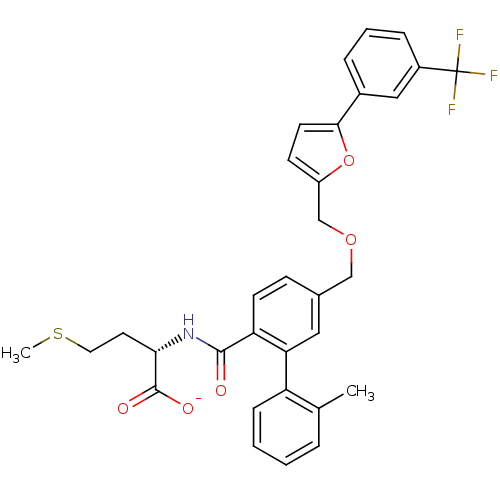
(CHEMBL273507 | Lithium; (S)-4-methylsulfanyl-2-({2...)Show SMILES CSCC[C@H](NC(=O)c1ccc(COCc2ccc(o2)-c2cccc(c2)C(F)(F)F)cc1-c1ccccc1C)C([O-])=O Show InChI InChI=1S/C32H30F3NO5S/c1-20-6-3-4-9-25(20)27-16-21(10-12-26(27)30(37)36-28(31(38)39)14-15-42-2)18-40-19-24-11-13-29(41-24)22-7-5-8-23(17-22)32(33,34)35/h3-13,16-17,28H,14-15,18-19H2,1-2H3,(H,36,37)(H,38,39)/p-1/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound was evaluated against farnesyltransferase (FTase) |
Bioorg Med Chem Lett 9: 1069-74 (1999)
BindingDB Entry DOI: 10.7270/Q2X34WNT |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50076789
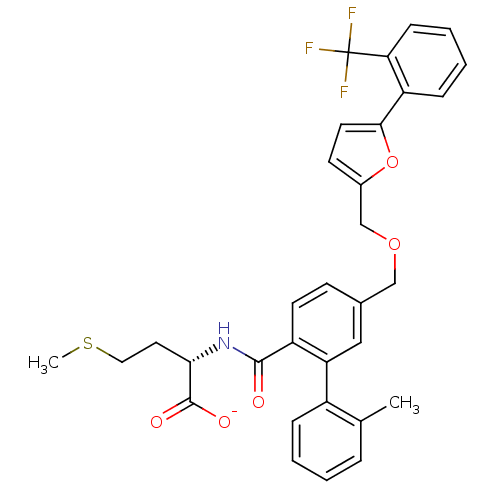
(CHEMBL274672 | Lithium; (S)-4-methylsulfanyl-2-({2...)Show SMILES CSCC[C@H](NC(=O)c1ccc(COCc2ccc(o2)-c2ccccc2C(F)(F)F)cc1-c1ccccc1C)C([O-])=O Show InChI InChI=1S/C32H30F3NO5S/c1-20-7-3-4-8-23(20)26-17-21(11-13-24(26)30(37)36-28(31(38)39)15-16-42-2)18-40-19-22-12-14-29(41-22)25-9-5-6-10-27(25)32(33,34)35/h3-14,17,28H,15-16,18-19H2,1-2H3,(H,36,37)(H,38,39)/p-1/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound was evaluated against farnesyltransferase (FTase) |
Bioorg Med Chem Lett 9: 1069-74 (1999)
BindingDB Entry DOI: 10.7270/Q2X34WNT |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50076807
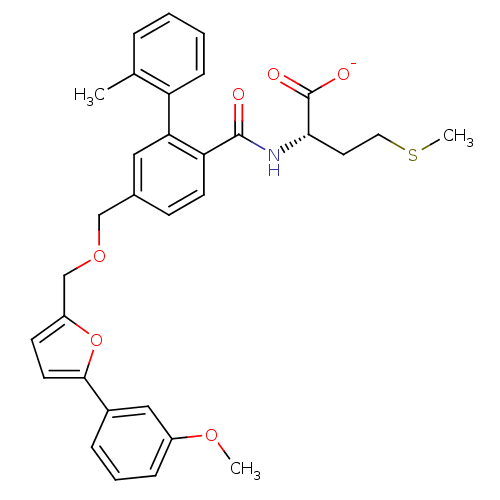
(CHEMBL273721 | Lithium; (S)-2-({5-[5-(3-methoxy-ph...)Show SMILES COc1cccc(c1)-c1ccc(COCc2ccc(C(=O)N[C@@H](CCSC)C([O-])=O)c(c2)-c2ccccc2C)o1 Show InChI InChI=1S/C32H33NO6S/c1-21-7-4-5-10-26(21)28-17-22(11-13-27(28)31(34)33-29(32(35)36)15-16-40-3)19-38-20-25-12-14-30(39-25)23-8-6-9-24(18-23)37-2/h4-14,17-18,29H,15-16,19-20H2,1-3H3,(H,33,34)(H,35,36)/p-1/t29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound was evaluated against farnesyltransferase (FTase) |
Bioorg Med Chem Lett 9: 1069-74 (1999)
BindingDB Entry DOI: 10.7270/Q2X34WNT |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50076808
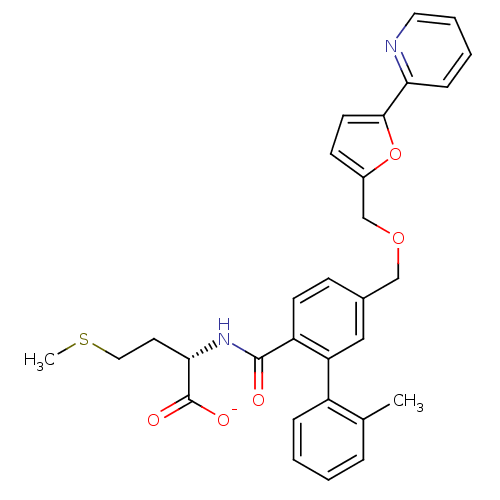
(CHEMBL10762 | Lithium; (S)-2-{[2'-methyl-5-(5-pyri...)Show SMILES CSCC[C@H](NC(=O)c1ccc(COCc2ccc(o2)-c2ccccn2)cc1-c1ccccc1C)C([O-])=O Show InChI InChI=1S/C30H30N2O5S/c1-20-7-3-4-8-23(20)25-17-21(10-12-24(25)29(33)32-27(30(34)35)14-16-38-2)18-36-19-22-11-13-28(37-22)26-9-5-6-15-31-26/h3-13,15,17,27H,14,16,18-19H2,1-2H3,(H,32,33)(H,34,35)/p-1/t27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound was evaluated against farnesyltransferase (FTase) |
Bioorg Med Chem Lett 9: 1069-74 (1999)
BindingDB Entry DOI: 10.7270/Q2X34WNT |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50076813
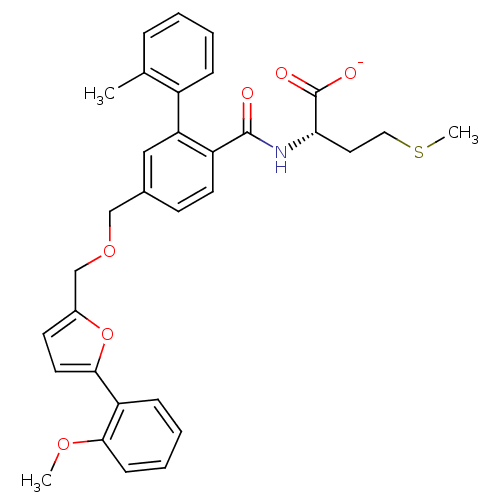
(CHEMBL10687 | Lithium; (S)-2-({5-[5-(2-methoxy-phe...)Show SMILES COc1ccccc1-c1ccc(COCc2ccc(C(=O)N[C@@H](CCSC)C([O-])=O)c(c2)-c2ccccc2C)o1 Show InChI InChI=1S/C32H33NO6S/c1-21-8-4-5-9-24(21)27-18-22(12-14-25(27)31(34)33-28(32(35)36)16-17-40-3)19-38-20-23-13-15-30(39-23)26-10-6-7-11-29(26)37-2/h4-15,18,28H,16-17,19-20H2,1-3H3,(H,33,34)(H,35,36)/p-1/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound was evaluated against farnesyltransferase (FTase) |
Bioorg Med Chem Lett 9: 1069-74 (1999)
BindingDB Entry DOI: 10.7270/Q2X34WNT |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50076812
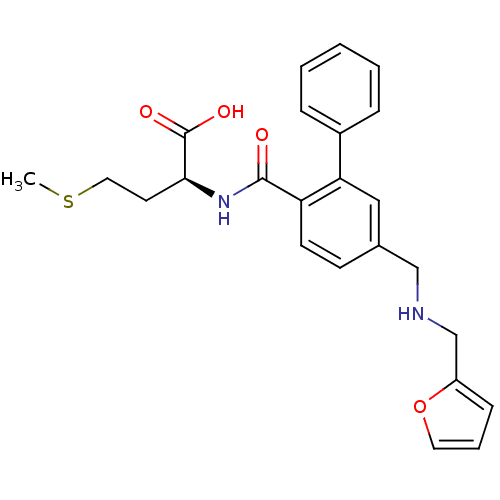
((S)-2-[(5-{[(Furan-2-ylmethyl)-amino]-methyl}-biph...)Show SMILES CSCC[C@H](NC(=O)c1ccc(CNCc2ccco2)cc1-c1ccccc1)C(O)=O Show InChI InChI=1S/C24H26N2O4S/c1-31-13-11-22(24(28)29)26-23(27)20-10-9-17(15-25-16-19-8-5-12-30-19)14-21(20)18-6-3-2-4-7-18/h2-10,12,14,22,25H,11,13,15-16H2,1H3,(H,26,27)(H,28,29)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound was evaluated against farnesyltransferase (FTase) |
Bioorg Med Chem Lett 9: 1069-74 (1999)
BindingDB Entry DOI: 10.7270/Q2X34WNT |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50076805
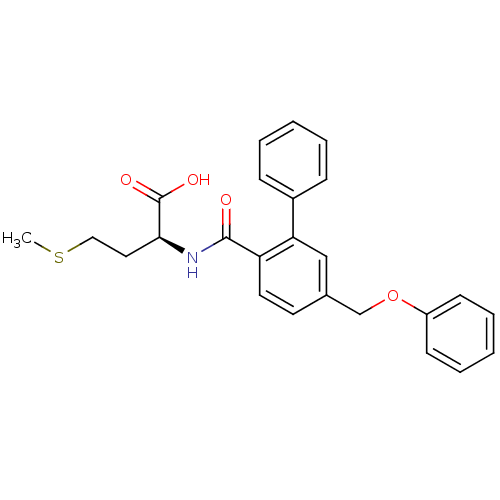
((S)-4-Methylsulfanyl-2-[(5-phenoxymethyl-biphenyl-...)Show SMILES CSCC[C@H](NC(=O)c1ccc(COc2ccccc2)cc1-c1ccccc1)C(O)=O Show InChI InChI=1S/C25H25NO4S/c1-31-15-14-23(25(28)29)26-24(27)21-13-12-18(17-30-20-10-6-3-7-11-20)16-22(21)19-8-4-2-5-9-19/h2-13,16,23H,14-15,17H2,1H3,(H,26,27)(H,28,29)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound was evaluated against farnesyltransferase (FTase) |
Bioorg Med Chem Lett 9: 1069-74 (1999)
BindingDB Entry DOI: 10.7270/Q2X34WNT |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50076795

(CHEMBL11210 | Lithium; (S)-2-({5-[5-(4-dimethylami...)Show SMILES CSCC[C@H](NC(=O)c1ccc(COCc2ccc(o2)-c2ccc(cc2)N(C)C)cc1-c1ccccc1C)C([O-])=O Show InChI InChI=1S/C33H36N2O5S/c1-22-7-5-6-8-27(22)29-19-23(9-15-28(29)32(36)34-30(33(37)38)17-18-41-4)20-39-21-26-14-16-31(40-26)24-10-12-25(13-11-24)35(2)3/h5-16,19,30H,17-18,20-21H2,1-4H3,(H,34,36)(H,37,38)/p-1/t30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
EC50 is measured as inhibition of ras processing in a whole cell assay for farnesyltransferase (FTase) |
Bioorg Med Chem Lett 9: 1069-74 (1999)
BindingDB Entry DOI: 10.7270/Q2X34WNT |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50076791

(CHEMBL10990 | Lithium; (S)-4-methylsulfanyl-2-{[2'...)Show SMILES CSCC[C@H](NC(=O)c1ccc(COCc2ccc(o2)-c2ccc(C)cc2)cc1-c1ccccc1C)C([O-])=O Show InChI InChI=1S/C32H33NO5S/c1-21-8-11-24(12-9-21)30-15-13-25(38-30)20-37-19-23-10-14-27(28(18-23)26-7-5-4-6-22(26)2)31(34)33-29(32(35)36)16-17-39-3/h4-15,18,29H,16-17,19-20H2,1-3H3,(H,33,34)(H,35,36)/p-1/t29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
EC50 is measured as inhibition of ras processing in a whole cell assay for farnesyltransferase (FTase) |
Bioorg Med Chem Lett 9: 1069-74 (1999)
BindingDB Entry DOI: 10.7270/Q2X34WNT |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50076802

(CHEMBL10912 | Lithium; (S)-2-{[2'-methyl-5-(5-pyri...)Show SMILES CSCC[C@H](NC(=O)c1ccc(COCc2ccc(o2)-c2ccncc2)cc1-c1ccccc1C)C([O-])=O Show InChI InChI=1S/C30H30N2O5S/c1-20-5-3-4-6-24(20)26-17-21(7-9-25(26)29(33)32-27(30(34)35)13-16-38-2)18-36-19-23-8-10-28(37-23)22-11-14-31-15-12-22/h3-12,14-15,17,27H,13,16,18-19H2,1-2H3,(H,32,33)(H,34,35)/p-1/t27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >0.300 | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
EC50 is measured as inhibition of ras processing in a whole cell assay for farnesyltransferase (FTase) |
Bioorg Med Chem Lett 9: 1069-74 (1999)
BindingDB Entry DOI: 10.7270/Q2X34WNT |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50076790

(CHEMBL10902 | Lithium; (S)-2-{[2'-methyl-5-(5-pyri...)Show SMILES CSCC[C@H](NC(=O)c1ccc(COCc2ccc(o2)-c2cccnc2)cc1-c1ccccc1C)C([O-])=O Show InChI InChI=1S/C30H30N2O5S/c1-20-6-3-4-8-24(20)26-16-21(9-11-25(26)29(33)32-27(30(34)35)13-15-38-2)18-36-19-23-10-12-28(37-23)22-7-5-14-31-17-22/h3-12,14,16-17,27H,13,15,18-19H2,1-2H3,(H,32,33)(H,34,35)/p-1/t27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
EC50 is measured as inhibition of ras processing in a whole cell assay for farnesyltransferase (FTase) |
Bioorg Med Chem Lett 9: 1069-74 (1999)
BindingDB Entry DOI: 10.7270/Q2X34WNT |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50076809

(CHEMBL417606 | Lithium; (S)-2-({5-[5-(4-formyl-phe...)Show SMILES CSCC[C@H](NC(=O)c1ccc(COCc2ccc(o2)-c2ccc(C=O)cc2)cc1-c1ccccc1C)C([O-])=O Show InChI InChI=1S/C32H31NO6S/c1-21-5-3-4-6-26(21)28-17-23(9-13-27(28)31(35)33-29(32(36)37)15-16-40-2)19-38-20-25-12-14-30(39-25)24-10-7-22(18-34)8-11-24/h3-14,17-18,29H,15-16,19-20H2,1-2H3,(H,33,35)(H,36,37)/p-1/t29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >1 | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
EC50 is measured as inhibition of ras processing in a whole cell assay for farnesyltransferase (FTase) |
Bioorg Med Chem Lett 9: 1069-74 (1999)
BindingDB Entry DOI: 10.7270/Q2X34WNT |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50076798

(CHEMBL273697 | Lithium; (S)-2-({5-[5-(4-fluoro-phe...)Show SMILES CSCC[C@H](NC(=O)c1ccc(COCc2ccc(o2)-c2ccc(F)cc2)cc1-c1ccccc1C)C([O-])=O Show InChI InChI=1S/C31H30FNO5S/c1-20-5-3-4-6-25(20)27-17-21(7-13-26(27)30(34)33-28(31(35)36)15-16-39-2)18-37-19-24-12-14-29(38-24)22-8-10-23(32)11-9-22/h3-14,17,28H,15-16,18-19H2,1-2H3,(H,33,34)(H,35,36)/p-1/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
EC50 is measured as inhibition of ras processing in a whole cell assay for farnesyltransferase (FTase) |
Bioorg Med Chem Lett 9: 1069-74 (1999)
BindingDB Entry DOI: 10.7270/Q2X34WNT |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50076788

(CHEMBL11195 | Lithium; (S)-2-({5-[5-(3-fluoro-phen...)Show SMILES CSCC[C@H](NC(=O)c1ccc(COCc2ccc(o2)-c2cccc(F)c2)cc1-c1ccccc1C)C([O-])=O Show InChI InChI=1S/C31H30FNO5S/c1-20-6-3-4-9-25(20)27-16-21(10-12-26(27)30(34)33-28(31(35)36)14-15-39-2)18-37-19-24-11-13-29(38-24)22-7-5-8-23(32)17-22/h3-13,16-17,28H,14-15,18-19H2,1-2H3,(H,33,34)(H,35,36)/p-1/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
EC50 is measured as inhibition of ras processing in a whole cell assay for farnesyltransferase (FTase) |
Bioorg Med Chem Lett 9: 1069-74 (1999)
BindingDB Entry DOI: 10.7270/Q2X34WNT |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50076813

(CHEMBL10687 | Lithium; (S)-2-({5-[5-(2-methoxy-phe...)Show SMILES COc1ccccc1-c1ccc(COCc2ccc(C(=O)N[C@@H](CCSC)C([O-])=O)c(c2)-c2ccccc2C)o1 Show InChI InChI=1S/C32H33NO6S/c1-21-8-4-5-9-24(21)27-18-22(12-14-25(27)31(34)33-28(32(35)36)16-17-40-3)19-38-20-23-13-15-30(39-23)26-10-6-7-11-29(26)37-2/h4-15,18,28H,16-17,19-20H2,1-3H3,(H,33,34)(H,35,36)/p-1/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | >1 | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
EC50 is measured as inhibition of ras processing in a whole cell assay for farnesyltransferase (FTase) |
Bioorg Med Chem Lett 9: 1069-74 (1999)
BindingDB Entry DOI: 10.7270/Q2X34WNT |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50076794

(CHEMBL276602 | Lithium; (S)-2-({5-[5-(4-isopropyl-...)Show SMILES CSCC[C@H](NC(=O)c1ccc(COCc2ccc(o2)-c2ccc(cc2)C(C)C)cc1-c1ccccc1C)C([O-])=O Show InChI InChI=1S/C34H37NO5S/c1-22(2)25-10-12-26(13-11-25)32-16-14-27(40-32)21-39-20-24-9-15-29(30(19-24)28-8-6-5-7-23(28)3)33(36)35-31(34(37)38)17-18-41-4/h5-16,19,22,31H,17-18,20-21H2,1-4H3,(H,35,36)(H,37,38)/p-1/t31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
EC50 is measured as inhibition of ras processing in a whole cell assay for farnesyltransferase (FTase) |
Bioorg Med Chem Lett 9: 1069-74 (1999)
BindingDB Entry DOI: 10.7270/Q2X34WNT |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50076793

(CHEMBL11003 | Lithium; (S)-2-({2'-methyl-5-[5-(4-m...)Show SMILES CSCC[C@H](NC(=O)c1ccc(COCc2ccc(o2)-c2ccc(SC)cc2)cc1-c1ccccc1C)C([O-])=O Show InChI InChI=1S/C32H33NO5S2/c1-21-6-4-5-7-26(21)28-18-22(8-14-27(28)31(34)33-29(32(35)36)16-17-39-2)19-37-20-24-11-15-30(38-24)23-9-12-25(40-3)13-10-23/h4-15,18,29H,16-17,19-20H2,1-3H3,(H,33,34)(H,35,36)/p-1/t29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
EC50 is measured as inhibition of ras processing in a whole cell assay for farnesyltransferase (FTase) |
Bioorg Med Chem Lett 9: 1069-74 (1999)
BindingDB Entry DOI: 10.7270/Q2X34WNT |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50076811

(CHEMBL10877 | Lithium; (S)-2-({5-[5-(4-chloro-phen...)Show SMILES CSCC[C@H](NC(=O)c1ccc(COCc2ccc(o2)-c2ccc(Cl)cc2)cc1-c1ccccc1C)C([O-])=O Show InChI InChI=1S/C31H30ClNO5S/c1-20-5-3-4-6-25(20)27-17-21(7-13-26(27)30(34)33-28(31(35)36)15-16-39-2)18-37-19-24-12-14-29(38-24)22-8-10-23(32)11-9-22/h3-14,17,28H,15-16,18-19H2,1-2H3,(H,33,34)(H,35,36)/p-1/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
EC50 is measured as inhibition of ras processing in a whole cell assay for farnesyltransferase (FTase) |
Bioorg Med Chem Lett 9: 1069-74 (1999)
BindingDB Entry DOI: 10.7270/Q2X34WNT |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50076800

(CHEMBL430307 | Lithium; (S)-2-({5-[5-(4-acetyl-phe...)Show SMILES CSCC[C@H](NC(=O)c1ccc(COCc2ccc(o2)-c2ccc(cc2)C(C)=O)cc1-c1ccccc1C)C([O-])=O Show InChI InChI=1S/C33H33NO6S/c1-21-6-4-5-7-27(21)29-18-23(8-14-28(29)32(36)34-30(33(37)38)16-17-41-3)19-39-20-26-13-15-31(40-26)25-11-9-24(10-12-25)22(2)35/h4-15,18,30H,16-17,19-20H2,1-3H3,(H,34,36)(H,37,38)/p-1/t30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | >1 | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
EC50 is measured as inhibition of ras processing in a whole cell assay for farnesyltransferase (FTase) |
Bioorg Med Chem Lett 9: 1069-74 (1999)
BindingDB Entry DOI: 10.7270/Q2X34WNT |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50076807

(CHEMBL273721 | Lithium; (S)-2-({5-[5-(3-methoxy-ph...)Show SMILES COc1cccc(c1)-c1ccc(COCc2ccc(C(=O)N[C@@H](CCSC)C([O-])=O)c(c2)-c2ccccc2C)o1 Show InChI InChI=1S/C32H33NO6S/c1-21-7-4-5-10-26(21)28-17-22(11-13-27(28)31(34)33-29(32(35)36)15-16-40-3)19-38-20-25-12-14-30(39-25)23-8-6-9-24(18-23)37-2/h4-14,17-18,29H,15-16,19-20H2,1-3H3,(H,33,34)(H,35,36)/p-1/t29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | >1 | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
EC50 is measured as inhibition of ras processing in a whole cell assay for farnesyltransferase (FTase) |
Bioorg Med Chem Lett 9: 1069-74 (1999)
BindingDB Entry DOI: 10.7270/Q2X34WNT |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50076808

(CHEMBL10762 | Lithium; (S)-2-{[2'-methyl-5-(5-pyri...)Show SMILES CSCC[C@H](NC(=O)c1ccc(COCc2ccc(o2)-c2ccccn2)cc1-c1ccccc1C)C([O-])=O Show InChI InChI=1S/C30H30N2O5S/c1-20-7-3-4-8-23(20)25-17-21(10-12-24(25)29(33)32-27(30(34)35)14-16-38-2)18-36-19-22-11-13-28(37-22)26-9-5-6-15-31-26/h3-13,15,17,27H,14,16,18-19H2,1-2H3,(H,32,33)(H,34,35)/p-1/t27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >0.300 | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
EC50 is measured as inhibition of ras processing in a whole cell assay for farnesyltransferase (FTase) |
Bioorg Med Chem Lett 9: 1069-74 (1999)
BindingDB Entry DOI: 10.7270/Q2X34WNT |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50076810

(CHEMBL428001 | Lithium; (S)-4-methylsulfanyl-2-({2...)Show SMILES CSCC[C@H](NC(=O)c1ccc(COCc2ccc(o2)-c2ccc(cc2)C(F)(F)F)cc1-c1ccccc1C)C([O-])=O Show InChI InChI=1S/C32H30F3NO5S/c1-20-5-3-4-6-25(20)27-17-21(7-13-26(27)30(37)36-28(31(38)39)15-16-42-2)18-40-19-24-12-14-29(41-24)22-8-10-23(11-9-22)32(33,34)35/h3-14,17,28H,15-16,18-19H2,1-2H3,(H,36,37)(H,38,39)/p-1/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
EC50 is measured as inhibition of ras processing in a whole cell assay for farnesyltransferase (FTase) |
Bioorg Med Chem Lett 9: 1069-74 (1999)
BindingDB Entry DOI: 10.7270/Q2X34WNT |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50076797

(CHEMBL10955 | Lithium; (S)-2-{[2'-methyl-5-(5-phen...)Show SMILES CSCC[C@H](NC(=O)c1ccc(COCc2ccc(o2)-c2ccccc2)cc1-c1ccccc1C)C([O-])=O Show InChI InChI=1S/C31H31NO5S/c1-21-8-6-7-11-25(21)27-18-22(12-14-26(27)30(33)32-28(31(34)35)16-17-38-2)19-36-20-24-13-15-29(37-24)23-9-4-3-5-10-23/h3-15,18,28H,16-17,19-20H2,1-2H3,(H,32,33)(H,34,35)/p-1/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >1 | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
EC50 is measured as inhibition of ras processing in a whole cell assay for farnesyltransferase (FTase) |
Bioorg Med Chem Lett 9: 1069-74 (1999)
BindingDB Entry DOI: 10.7270/Q2X34WNT |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50076801

(CHEMBL430096 | Lithium; (S)-2-({2'-methyl-5-[5-(3-...)Show SMILES CSCC[C@H](NC(=O)c1ccc(COCc2ccc(o2)-c2cccc(c2)[N+]([O-])=O)cc1-c1ccccc1C)C([O-])=O Show InChI InChI=1S/C31H30N2O7S/c1-20-6-3-4-9-25(20)27-16-21(10-12-26(27)30(34)32-28(31(35)36)14-15-41-2)18-39-19-24-11-13-29(40-24)22-7-5-8-23(17-22)33(37)38/h3-13,16-17,28H,14-15,18-19H2,1-2H3,(H,32,34)(H,35,36)/p-1/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
EC50 is measured as inhibition of ras processing in a whole cell assay for farnesyltransferase (FTase) |
Bioorg Med Chem Lett 9: 1069-74 (1999)
BindingDB Entry DOI: 10.7270/Q2X34WNT |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50076806

(CHEMBL273265 | Lithium; (S)-2-({5-[5-(4-methoxy-ph...)Show SMILES COc1ccc(cc1)-c1ccc(COCc2ccc(C(=O)N[C@@H](CCSC)C([O-])=O)c(c2)-c2ccccc2C)o1 Show InChI InChI=1S/C32H33NO6S/c1-21-6-4-5-7-26(21)28-18-22(8-14-27(28)31(34)33-29(32(35)36)16-17-40-3)19-38-20-25-13-15-30(39-25)23-9-11-24(37-2)12-10-23/h4-15,18,29H,16-17,19-20H2,1-3H3,(H,33,34)(H,35,36)/p-1/t29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >1 | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
EC50 is measured as inhibition of ras processing in a whole cell assay for farnesyltransferase (FTase) |
Bioorg Med Chem Lett 9: 1069-74 (1999)
BindingDB Entry DOI: 10.7270/Q2X34WNT |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50076803

(CHEMBL10970 | Lithium; (S)-2-({5-[5-(2-fluoro-phen...)Show SMILES CSCC[C@H](NC(=O)c1ccc(COCc2ccc(o2)-c2ccccc2F)cc1-c1ccccc1C)C([O-])=O Show InChI InChI=1S/C31H30FNO5S/c1-20-7-3-4-8-23(20)26-17-21(11-13-24(26)30(34)33-28(31(35)36)15-16-39-2)18-37-19-22-12-14-29(38-22)25-9-5-6-10-27(25)32/h3-14,17,28H,15-16,18-19H2,1-2H3,(H,33,34)(H,35,36)/p-1/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | >1 | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
EC50 is measured as inhibition of ras processing in a whole cell assay for farnesyltransferase (FTase) |
Bioorg Med Chem Lett 9: 1069-74 (1999)
BindingDB Entry DOI: 10.7270/Q2X34WNT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data