

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1F (Homo sapiens (Human)) | BDBM50091438 ((E)-3-(3,4-Dichloro-phenyl)-N-{5-[4-(5-hydroxy-1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for 5-hydroxytryptamine 1F receptor was determined | Bioorg Med Chem Lett 11: 2177-80 (2001) BindingDB Entry DOI: 10.7270/Q2BP023S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM50091438 ((E)-3-(3,4-Dichloro-phenyl)-N-{5-[4-(5-hydroxy-1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for 5-hydroxytryptamine 1E receptor was determined | Bioorg Med Chem Lett 11: 2177-80 (2001) BindingDB Entry DOI: 10.7270/Q2BP023S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50091438 ((E)-3-(3,4-Dichloro-phenyl)-N-{5-[4-(5-hydroxy-1H-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for Dopamine receptor D3 was determined | Bioorg Med Chem Lett 11: 2177-80 (2001) BindingDB Entry DOI: 10.7270/Q2BP023S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50091438 ((E)-3-(3,4-Dichloro-phenyl)-N-{5-[4-(5-hydroxy-1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for 5-hydroxytryptamine 1B receptor was determined | Bioorg Med Chem Lett 11: 2177-80 (2001) BindingDB Entry DOI: 10.7270/Q2BP023S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50091438 ((E)-3-(3,4-Dichloro-phenyl)-N-{5-[4-(5-hydroxy-1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for Dopamine receptor D2 was determined | Bioorg Med Chem Lett 11: 2177-80 (2001) BindingDB Entry DOI: 10.7270/Q2BP023S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50091438 ((E)-3-(3,4-Dichloro-phenyl)-N-{5-[4-(5-hydroxy-1H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for 5-hydroxytryptamine 2A receptor was determined | Bioorg Med Chem Lett 11: 2177-80 (2001) BindingDB Entry DOI: 10.7270/Q2BP023S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50091438 ((E)-3-(3,4-Dichloro-phenyl)-N-{5-[4-(5-hydroxy-1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for 5-hydroxytryptamine 2B receptor was determined | Bioorg Med Chem Lett 11: 2177-80 (2001) BindingDB Entry DOI: 10.7270/Q2BP023S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50103142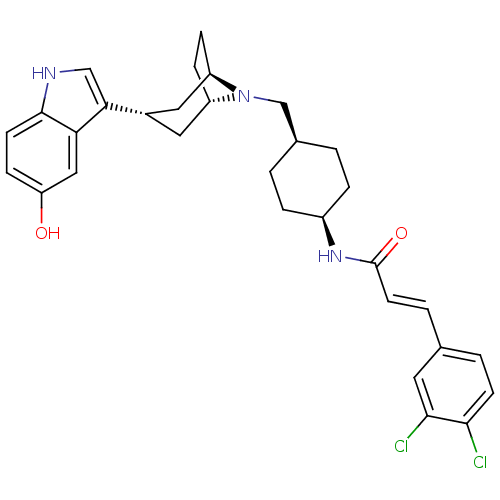 ((E)-3-(3,4-Dichloro-phenyl)-N-{4-[(R)-3-((2S,7aS)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity of the compound for C-C chemokine receptor type 2B was determined | Bioorg Med Chem Lett 11: 2177-80 (2001) BindingDB Entry DOI: 10.7270/Q2BP023S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50103142 ((E)-3-(3,4-Dichloro-phenyl)-N-{4-[(R)-3-((2S,7aS)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [125I]-MCP-1 binding to the cloned C-C chemokine receptor type 2B expressed in CHO cells | Bioorg Med Chem Lett 11: 2177-80 (2001) BindingDB Entry DOI: 10.7270/Q2BP023S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50091438 ((E)-3-(3,4-Dichloro-phenyl)-N-{5-[4-(5-hydroxy-1H-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [125I]MCP-1 binding to the cloned C-C chemokine receptor type 2B expressed in CHO cells | Bioorg Med Chem Lett 11: 2177-80 (2001) BindingDB Entry DOI: 10.7270/Q2BP023S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50091438 ((E)-3-(3,4-Dichloro-phenyl)-N-{5-[4-(5-hydroxy-1H-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [125I]-MCP-1 binding to the cloned C-C chemokine receptor type 2B expressed in CHO cells | Bioorg Med Chem Lett 11: 2177-80 (2001) BindingDB Entry DOI: 10.7270/Q2BP023S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50091438 ((E)-3-(3,4-Dichloro-phenyl)-N-{5-[4-(5-hydroxy-1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for 5-hydroxytryptamine 7 receptor was determined | Bioorg Med Chem Lett 11: 2177-80 (2001) BindingDB Entry DOI: 10.7270/Q2BP023S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50091438 ((E)-3-(3,4-Dichloro-phenyl)-N-{5-[4-(5-hydroxy-1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for 5-hydroxytryptamine 2C receptor was determined | Bioorg Med Chem Lett 11: 2177-80 (2001) BindingDB Entry DOI: 10.7270/Q2BP023S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50233075 (3-(3,4-dichlorophenyl)-N-((1s,4s)-4-((4-(5-hydroxy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [125I]-MCP-1 binding to the cloned C-C chemokine receptor type 2B expressed in CHO cells | Bioorg Med Chem Lett 11: 2177-80 (2001) BindingDB Entry DOI: 10.7270/Q2BP023S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50233075 (3-(3,4-dichlorophenyl)-N-((1s,4s)-4-((4-(5-hydroxy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [125I]-MCP-1 binding to the cloned C-C chemokine receptor type 2B expressed in CHO cells | Bioorg Med Chem Lett 11: 2177-80 (2001) BindingDB Entry DOI: 10.7270/Q2BP023S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50091438 ((E)-3-(3,4-Dichloro-phenyl)-N-{5-[4-(5-hydroxy-1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for 5-hydroxytryptamine 6 receptor was determined | Bioorg Med Chem Lett 11: 2177-80 (2001) BindingDB Entry DOI: 10.7270/Q2BP023S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50091438 ((E)-3-(3,4-Dichloro-phenyl)-N-{5-[4-(5-hydroxy-1H-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for 5-hydroxytryptamine 1A receptor was determined | Bioorg Med Chem Lett 11: 2177-80 (2001) BindingDB Entry DOI: 10.7270/Q2BP023S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50233073 (CHEMBL399472 | N-((1s,4s)-4-((4-(1H-indol-3-yl)pip...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [125I]-MCP-1 binding to the cloned C-C chemokine receptor type 2B expressed in CHO cells | Bioorg Med Chem Lett 11: 2177-80 (2001) BindingDB Entry DOI: 10.7270/Q2BP023S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50233075 (3-(3,4-dichlorophenyl)-N-((1s,4s)-4-((4-(5-hydroxy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [125I]-MCP-1 binding to the cloned C-C chemokine receptor type 2B expressed in CHO cells | Bioorg Med Chem Lett 11: 2177-80 (2001) BindingDB Entry DOI: 10.7270/Q2BP023S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50103141 ((E)-3-(3,4-Dichloro-phenyl)-N-{5-[(1R,5S)-3-(5-hyd...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [125I]-MCP-1 binding to the cloned C-C chemokine receptor type 2B expressed in CHO cells | Bioorg Med Chem Lett 11: 2177-80 (2001) BindingDB Entry DOI: 10.7270/Q2BP023S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50103141 ((E)-3-(3,4-Dichloro-phenyl)-N-{5-[(1R,5S)-3-(5-hyd...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [125I]MCP-1 binding to the cloned C-C chemokine receptor type 2B expressed in CHO cells | Bioorg Med Chem Lett 11: 2177-80 (2001) BindingDB Entry DOI: 10.7270/Q2BP023S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50233071 (3-(3,4-dichlorophenyl)-N-((1r,4r)-4-((4-(5-hydroxy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [125I]MCP-1 binding to the cloned C-C chemokine receptor type 2B expressed in CHO cells | Bioorg Med Chem Lett 11: 2177-80 (2001) BindingDB Entry DOI: 10.7270/Q2BP023S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50103142 ((E)-3-(3,4-Dichloro-phenyl)-N-{4-[(R)-3-((2S,7aS)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for 5-hydroxytryptamine 2B receptor was determined | Bioorg Med Chem Lett 11: 2177-80 (2001) BindingDB Entry DOI: 10.7270/Q2BP023S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50103141 ((E)-3-(3,4-Dichloro-phenyl)-N-{5-[(1R,5S)-3-(5-hyd...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [125I]-MCP-1 binding to the cloned C-C chemokine receptor type 2B expressed in CHO cells | Bioorg Med Chem Lett 11: 2177-80 (2001) BindingDB Entry DOI: 10.7270/Q2BP023S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50103141 ((E)-3-(3,4-Dichloro-phenyl)-N-{5-[(1R,5S)-3-(5-hyd...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [125I]-MCP-1 binding to the cloned C-C chemokine receptor type 2B expressed in CHO cells | Bioorg Med Chem Lett 11: 2177-80 (2001) BindingDB Entry DOI: 10.7270/Q2BP023S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50233072 ((E)-3-(3,4-Dichloro-phenyl)-N-{4-[4-(1H-indol-3-yl...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [125I]MCP-1 binding to the cloned C-C chemokine receptor type 2B expressed in CHO cells | Bioorg Med Chem Lett 11: 2177-80 (2001) BindingDB Entry DOI: 10.7270/Q2BP023S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50103142 ((E)-3-(3,4-Dichloro-phenyl)-N-{4-[(R)-3-((2S,7aS)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | <794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity relative to indolopiperidine (compound 1) for 5-hydroxytryptamine 7 receptor was determined | Bioorg Med Chem Lett 11: 2177-80 (2001) BindingDB Entry DOI: 10.7270/Q2BP023S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50103142 ((E)-3-(3,4-Dichloro-phenyl)-N-{4-[(R)-3-((2S,7aS)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for 5-hydroxytryptamine 1B receptor was determined | Bioorg Med Chem Lett 11: 2177-80 (2001) BindingDB Entry DOI: 10.7270/Q2BP023S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50103142 ((E)-3-(3,4-Dichloro-phenyl)-N-{4-[(R)-3-((2S,7aS)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity relative to indolopiperidine (compound 1) for Dopamine receptor D2 was determined | Bioorg Med Chem Lett 11: 2177-80 (2001) BindingDB Entry DOI: 10.7270/Q2BP023S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50103142 ((E)-3-(3,4-Dichloro-phenyl)-N-{4-[(R)-3-((2S,7aS)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for 5-hydroxytryptamine 6 receptor was determined | Bioorg Med Chem Lett 11: 2177-80 (2001) BindingDB Entry DOI: 10.7270/Q2BP023S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50103142 ((E)-3-(3,4-Dichloro-phenyl)-N-{4-[(R)-3-((2S,7aS)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity relative to indolopiperidine (compound 1) for Dopamine receptor D3 was determined | Bioorg Med Chem Lett 11: 2177-80 (2001) BindingDB Entry DOI: 10.7270/Q2BP023S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1F (Homo sapiens (Human)) | BDBM50103142 ((E)-3-(3,4-Dichloro-phenyl)-N-{4-[(R)-3-((2S,7aS)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | <3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for 5-hydroxytryptamine 1F receptor was determined | Bioorg Med Chem Lett 11: 2177-80 (2001) BindingDB Entry DOI: 10.7270/Q2BP023S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50103142 ((E)-3-(3,4-Dichloro-phenyl)-N-{4-[(R)-3-((2S,7aS)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | <3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for 5-hydroxytryptamine 2C receptor was determined | Bioorg Med Chem Lett 11: 2177-80 (2001) BindingDB Entry DOI: 10.7270/Q2BP023S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM50103142 ((E)-3-(3,4-Dichloro-phenyl)-N-{4-[(R)-3-((2S,7aS)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | <6.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for 5-hydroxytryptamine 1E receptor was determined | Bioorg Med Chem Lett 11: 2177-80 (2001) BindingDB Entry DOI: 10.7270/Q2BP023S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50103142 ((E)-3-(3,4-Dichloro-phenyl)-N-{4-[(R)-3-((2S,7aS)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for 5-hydroxytryptamine 1A receptor was determined | Bioorg Med Chem Lett 11: 2177-80 (2001) BindingDB Entry DOI: 10.7270/Q2BP023S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50103142 ((E)-3-(3,4-Dichloro-phenyl)-N-{4-[(R)-3-((2S,7aS)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | <2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for 5-hydroxytryptamine 2A receptor was determined | Bioorg Med Chem Lett 11: 2177-80 (2001) BindingDB Entry DOI: 10.7270/Q2BP023S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||