 Found 40 hits Enz. Inhib. hit(s) with all data for entry = 50045307
Found 40 hits Enz. Inhib. hit(s) with all data for entry = 50045307 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056134
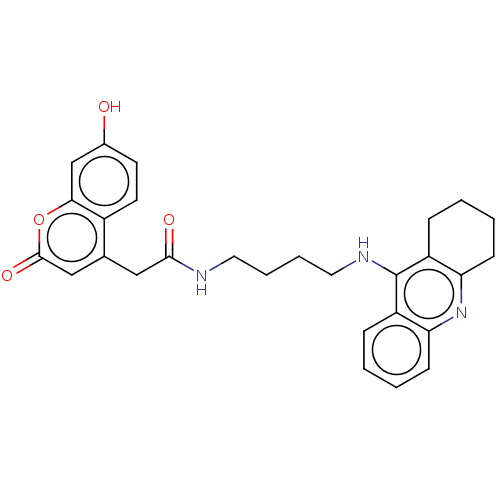
(CHEMBL3322160)Show SMILES Oc1ccc2c(CC(=O)NCCCCNc3c4CCCCc4nc4ccccc34)cc(=O)oc2c1 Show InChI InChI=1S/C28H29N3O4/c32-19-11-12-20-18(16-27(34)35-25(20)17-19)15-26(33)29-13-5-6-14-30-28-21-7-1-3-9-23(21)31-24-10-4-2-8-22(24)28/h1,3,7,9,11-12,16-17,32H,2,4-6,8,10,13-15H2,(H,29,33)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex by Lineweaver-Burk plot |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056134

(CHEMBL3322160)Show SMILES Oc1ccc2c(CC(=O)NCCCCNc3c4CCCCc4nc4ccccc34)cc(=O)oc2c1 Show InChI InChI=1S/C28H29N3O4/c32-19-11-12-20-18(16-27(34)35-25(20)17-19)15-26(33)29-13-5-6-14-30-28-21-7-1-3-9-23(21)31-24-10-4-2-8-22(24)28/h1,3,7,9,11-12,16-17,32H,2,4-6,8,10,13-15H2,(H,29,33)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex by Lineweaver-Burk plot |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056126
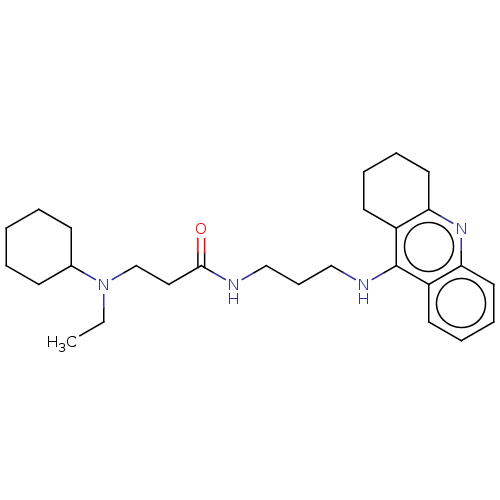
(CHEMBL3322141)Show SMILES CCN(CCC(=O)NCCCNc1c2CCCCc2nc2ccccc12)C1CCCCC1 Show InChI InChI=1S/C27H40N4O/c1-2-31(21-11-4-3-5-12-21)20-17-26(32)28-18-10-19-29-27-22-13-6-8-15-24(22)30-25-16-9-7-14-23(25)27/h6,8,13,15,21H,2-5,7,9-12,14,16-20H2,1H3,(H,28,32)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex by Lineweaver-Burk plot |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056126

(CHEMBL3322141)Show SMILES CCN(CCC(=O)NCCCNc1c2CCCCc2nc2ccccc12)C1CCCCC1 Show InChI InChI=1S/C27H40N4O/c1-2-31(21-11-4-3-5-12-21)20-17-26(32)28-18-10-19-29-27-22-13-6-8-15-24(22)30-25-16-9-7-14-23(25)27/h6,8,13,15,21H,2-5,7,9-12,14,16-20H2,1H3,(H,28,32)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex by Lineweaver-Burk plot |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex by Lineweaver-Burk plot |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056127
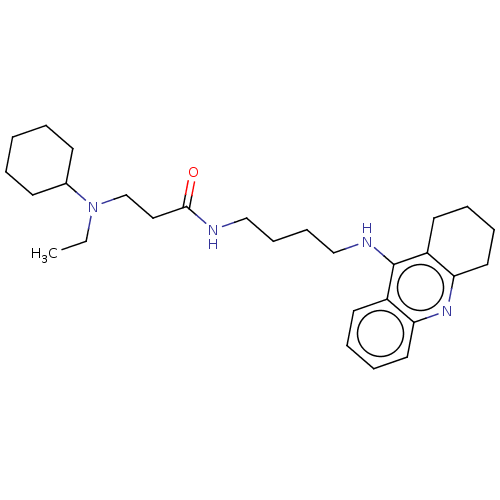
(CHEMBL3322142)Show SMILES CCN(CCC(=O)NCCCCNc1c2CCCCc2nc2ccccc12)C1CCCCC1 Show InChI InChI=1S/C28H42N4O/c1-2-32(22-12-4-3-5-13-22)21-18-27(33)29-19-10-11-20-30-28-23-14-6-8-16-25(23)31-26-17-9-7-15-24(26)28/h6,8,14,16,22H,2-5,7,9-13,15,17-21H2,1H3,(H,29,33)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 199 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex by Lineweaver-Burk plot |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 225 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex by Lineweaver-Burk plot |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056127

(CHEMBL3322142)Show SMILES CCN(CCC(=O)NCCCCNc1c2CCCCc2nc2ccccc12)C1CCCCC1 Show InChI InChI=1S/C28H42N4O/c1-2-32(22-12-4-3-5-13-22)21-18-27(33)29-19-10-11-20-30-28-23-14-6-8-16-25(23)31-26-17-9-7-15-24(26)28/h6,8,14,16,22H,2-5,7,9-13,15,17-21H2,1H3,(H,29,33)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 328 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex by Lineweaver-Burk plot |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056135
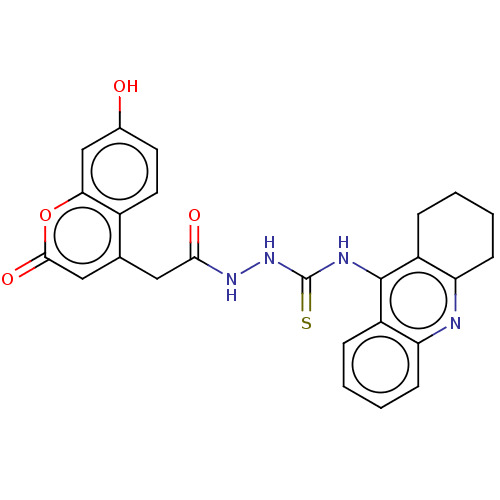
(CHEMBL3322161)Show SMILES Oc1ccc2c(CC(=O)NNC(=S)Nc3c4CCCCc4nc4ccccc34)cc(=O)oc2c1 Show InChI InChI=1S/C25H22N4O4S/c30-15-9-10-16-14(12-23(32)33-21(16)13-15)11-22(31)28-29-25(34)27-24-17-5-1-3-7-19(17)26-20-8-4-2-6-18(20)24/h1,3,5,7,9-10,12-13,30H,2,4,6,8,11H2,(H,28,31)(H2,26,27,29,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex by Lineweaver-Burk plot |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50327939

(7-methoxytacrine | CHEMBL1256415)Show InChI InChI=1S/C14H16N2O/c1-17-9-6-7-13-11(8-9)14(15)10-4-2-3-5-12(10)16-13/h6-8H,2-5H2,1H3,(H2,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex by Lineweaver-Burk plot |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056136

(CHEMBL3322162)Show SMILES Oc1ccc2c(CC(=O)N\N=C3/SCC(=O)N3c3c4CCCCc4nc4ccccc34)cc(=O)oc2c1 Show InChI InChI=1S/C27H22N4O5S/c32-16-9-10-17-15(12-25(35)36-22(17)13-16)11-23(33)29-30-27-31(24(34)14-37-27)26-18-5-1-3-7-20(18)28-21-8-4-2-6-19(21)26/h1,3,5,7,9-10,12-13,32H,2,4,6,8,11,14H2,(H,29,33)/b30-27- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex by Lineweaver-Burk plot |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50327939

(7-methoxytacrine | CHEMBL1256415)Show InChI InChI=1S/C14H16N2O/c1-17-9-6-7-13-11(8-9)14(15)10-4-2-3-5-12(10)16-13/h6-8H,2-5H2,1H3,(H2,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex by Lineweaver-Burk plot |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056136

(CHEMBL3322162)Show SMILES Oc1ccc2c(CC(=O)N\N=C3/SCC(=O)N3c3c4CCCCc4nc4ccccc34)cc(=O)oc2c1 Show InChI InChI=1S/C27H22N4O5S/c32-16-9-10-17-15(12-25(35)36-22(17)13-16)11-23(33)29-30-27-31(24(34)14-37-27)26-18-5-1-3-7-20(18)28-21-8-4-2-6-19(21)26/h1,3,5,7,9-10,12-13,32H,2,4,6,8,11,14H2,(H,29,33)/b30-27- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex by Lineweaver-Burk plot |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056135

(CHEMBL3322161)Show SMILES Oc1ccc2c(CC(=O)NNC(=S)Nc3c4CCCCc4nc4ccccc34)cc(=O)oc2c1 Show InChI InChI=1S/C25H22N4O4S/c30-15-9-10-16-14(12-23(32)33-21(16)13-15)11-22(31)28-29-25(34)27-24-17-5-1-3-7-19(17)26-20-8-4-2-6-18(20)24/h1,3,5,7,9-10,12-13,30H,2,4,6,8,11H2,(H,28,31)(H2,26,27,29,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex by Lineweaver-Burk plot |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056134

(CHEMBL3322160)Show SMILES Oc1ccc2c(CC(=O)NCCCCNc3c4CCCCc4nc4ccccc34)cc(=O)oc2c1 Show InChI InChI=1S/C28H29N3O4/c32-19-11-12-20-18(16-27(34)35-25(20)17-19)15-26(33)29-13-5-6-14-30-28-21-7-1-3-9-23(21)31-24-10-4-2-8-22(24)28/h1,3,7,9,11-12,16-17,32H,2,4-6,8,10,13-15H2,(H,29,33)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE pre-incubated for 5 mins before acetylthiocholine substrate addition by Ellman's method |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human BuChE pre-incubated for 5 mins before butylthiocholine substrate addition by Ellman's method |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056126

(CHEMBL3322141)Show SMILES CCN(CCC(=O)NCCCNc1c2CCCCc2nc2ccccc12)C1CCCCC1 Show InChI InChI=1S/C27H40N4O/c1-2-31(21-11-4-3-5-12-21)20-17-26(32)28-18-10-19-29-27-22-13-6-8-15-24(22)30-25-16-9-7-14-23(25)27/h6,8,13,15,21H,2-5,7,9-12,14,16-20H2,1H3,(H,28,32)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE pre-incubated for 5 mins before acetylthiocholine substrate addition by Ellman's method |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056127

(CHEMBL3322142)Show SMILES CCN(CCC(=O)NCCCCNc1c2CCCCc2nc2ccccc12)C1CCCCC1 Show InChI InChI=1S/C28H42N4O/c1-2-32(22-12-4-3-5-13-22)21-18-27(33)29-19-10-11-20-30-28-23-14-6-8-16-25(23)31-26-17-9-7-15-24(26)28/h6,8,14,16,22H,2-5,7,9-13,15,17-21H2,1H3,(H,29,33)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE pre-incubated for 5 mins before acetylthiocholine substrate addition by Ellman's method |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056124

(CHEMBL3322157)Show SMILES CN(CCC(=O)NCCCCNc1c2CCCCc2nc2ccccc12)C1CCCCC1 Show InChI InChI=1S/C27H40N4O/c1-31(21-11-3-2-4-12-21)20-17-26(32)28-18-9-10-19-29-27-22-13-5-7-15-24(22)30-25-16-8-6-14-23(25)27/h5,7,13,15,21H,2-4,6,8-12,14,16-20H2,1H3,(H,28,32)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE pre-incubated for 5 mins before acetylthiocholine substrate addition by Ellman's method |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50056127

(CHEMBL3322142)Show SMILES CCN(CCC(=O)NCCCCNc1c2CCCCc2nc2ccccc12)C1CCCCC1 Show InChI InChI=1S/C28H42N4O/c1-2-32(22-12-4-3-5-13-22)21-18-27(33)29-19-10-11-20-30-28-23-14-6-8-16-25(23)31-26-17-9-7-15-24(26)28/h6,8,14,16,22H,2-5,7,9-13,15,17-21H2,1H3,(H,29,33)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 228 | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human BuChE pre-incubated for 5 mins before butylthiocholine substrate addition by Ellman's method |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056125

(CHEMBL3322140)Show SMILES CCN(CCC(=O)NCCNc1c2CCCCc2nc2ccccc12)C1CCCCC1 Show InChI InChI=1S/C26H38N4O/c1-2-30(20-10-4-3-5-11-20)19-16-25(31)27-17-18-28-26-21-12-6-8-14-23(21)29-24-15-9-7-13-22(24)26/h6,8,12,14,20H,2-5,7,9-11,13,15-19H2,1H3,(H,27,31)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 233 | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE pre-incubated for 5 mins before acetylthiocholine substrate addition by Ellman's method |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50056126

(CHEMBL3322141)Show SMILES CCN(CCC(=O)NCCCNc1c2CCCCc2nc2ccccc12)C1CCCCC1 Show InChI InChI=1S/C27H40N4O/c1-2-31(21-11-4-3-5-12-21)20-17-26(32)28-18-10-19-29-27-22-13-6-8-15-24(22)30-25-16-9-7-14-23(25)27/h6,8,13,15,21H,2-5,7,9-12,14,16-20H2,1H3,(H,28,32)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 267 | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human BuChE pre-incubated for 5 mins before butylthiocholine substrate addition by Ellman's method |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056131
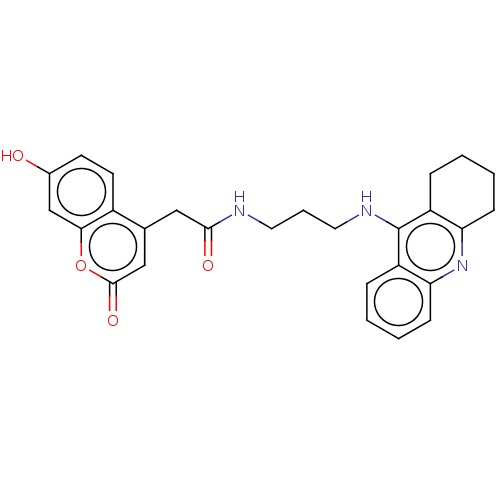
(CHEMBL3322159)Show SMILES Oc1ccc2c(CC(=O)NCCCNc3c4CCCCc4nc4ccccc34)cc(=O)oc2c1 Show InChI InChI=1S/C27H27N3O4/c31-18-10-11-19-17(15-26(33)34-24(19)16-18)14-25(32)28-12-5-13-29-27-20-6-1-3-8-22(20)30-23-9-4-2-7-21(23)27/h1,3,6,8,10-11,15-16,31H,2,4-5,7,9,12-14H2,(H,28,32)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 277 | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE pre-incubated for 5 mins before acetylthiocholine substrate addition by Ellman's method |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50056134

(CHEMBL3322160)Show SMILES Oc1ccc2c(CC(=O)NCCCCNc3c4CCCCc4nc4ccccc34)cc(=O)oc2c1 Show InChI InChI=1S/C28H29N3O4/c32-19-11-12-20-18(16-27(34)35-25(20)17-19)15-26(33)29-13-5-6-14-30-28-21-7-1-3-9-23(21)31-24-10-4-2-8-22(24)28/h1,3,7,9,11-12,16-17,32H,2,4-6,8,10,13-15H2,(H,29,33)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 328 | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human BuChE pre-incubated for 5 mins before butylthiocholine substrate addition by Ellman's method |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056129
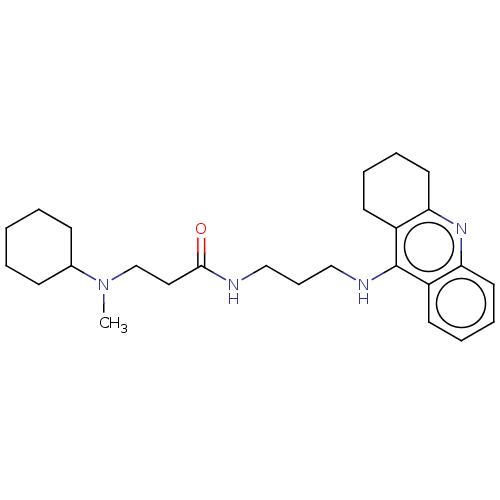
(CHEMBL3322156)Show SMILES CN(CCC(=O)NCCCNc1c2CCCCc2nc2ccccc12)C1CCCCC1 Show InChI InChI=1S/C26H38N4O/c1-30(20-10-3-2-4-11-20)19-16-25(31)27-17-9-18-28-26-21-12-5-7-14-23(21)29-24-15-8-6-13-22(24)26/h5,7,12,14,20H,2-4,6,8-11,13,15-19H2,1H3,(H,27,31)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 398 | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE pre-incubated for 5 mins before acetylthiocholine substrate addition by Ellman's method |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE pre-incubated for 5 mins before acetylthiocholine substrate addition by Ellman's method |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056128
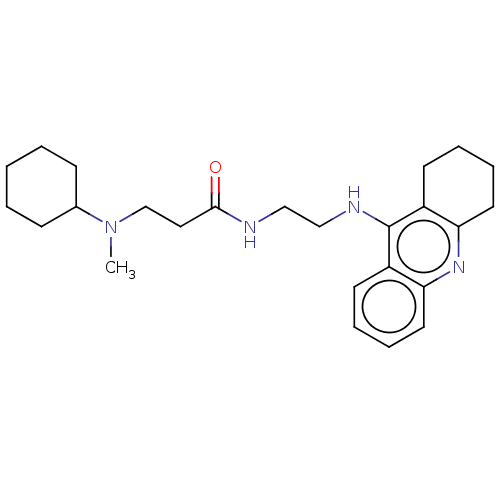
(CHEMBL3322155)Show SMILES CN(CCC(=O)NCCNc1c2CCCCc2nc2ccccc12)C1CCCCC1 Show InChI InChI=1S/C25H36N4O/c1-29(19-9-3-2-4-10-19)18-15-24(30)26-16-17-27-25-20-11-5-7-13-22(20)28-23-14-8-6-12-21(23)25/h5,7,11,13,19H,2-4,6,8-10,12,14-18H2,1H3,(H,26,30)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE pre-incubated for 5 mins before acetylthiocholine substrate addition by Ellman's method |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50056125

(CHEMBL3322140)Show SMILES CCN(CCC(=O)NCCNc1c2CCCCc2nc2ccccc12)C1CCCCC1 Show InChI InChI=1S/C26H38N4O/c1-2-30(20-10-4-3-5-11-20)19-16-25(31)27-17-18-28-26-21-12-6-8-14-23(21)29-24-15-9-7-13-22(24)26/h6,8,12,14,20H,2-5,7,9-11,13,15-19H2,1H3,(H,27,31)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 765 | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human BuChE pre-incubated for 5 mins before butylthiocholine substrate addition by Ellman's method |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50056131

(CHEMBL3322159)Show SMILES Oc1ccc2c(CC(=O)NCCCNc3c4CCCCc4nc4ccccc34)cc(=O)oc2c1 Show InChI InChI=1S/C27H27N3O4/c31-18-10-11-19-17(15-26(33)34-24(19)16-18)14-25(32)28-12-5-13-29-27-20-6-1-3-8-22(20)30-23-9-4-2-7-21(23)27/h1,3,6,8,10-11,15-16,31H,2,4-5,7,9,12-14H2,(H,28,32)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 827 | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human BuChE pre-incubated for 5 mins before butylthiocholine substrate addition by Ellman's method |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50056135

(CHEMBL3322161)Show SMILES Oc1ccc2c(CC(=O)NNC(=S)Nc3c4CCCCc4nc4ccccc34)cc(=O)oc2c1 Show InChI InChI=1S/C25H22N4O4S/c30-15-9-10-16-14(12-23(32)33-21(16)13-15)11-22(31)28-29-25(34)27-24-17-5-1-3-7-19(17)26-20-8-4-2-6-18(20)24/h1,3,5,7,9-10,12-13,30H,2,4,6,8,11H2,(H,28,31)(H2,26,27,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human BuChE pre-incubated for 5 mins before butylthiocholine substrate addition by Ellman's method |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50056128

(CHEMBL3322155)Show SMILES CN(CCC(=O)NCCNc1c2CCCCc2nc2ccccc12)C1CCCCC1 Show InChI InChI=1S/C25H36N4O/c1-29(19-9-3-2-4-10-19)18-15-24(30)26-16-17-27-25-20-11-5-7-13-22(20)28-23-14-8-6-12-21(23)25/h5,7,11,13,19H,2-4,6,8-10,12,14-18H2,1H3,(H,26,30)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human BuChE pre-incubated for 5 mins before butylthiocholine substrate addition by Ellman's method |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50056124

(CHEMBL3322157)Show SMILES CN(CCC(=O)NCCCCNc1c2CCCCc2nc2ccccc12)C1CCCCC1 Show InChI InChI=1S/C27H40N4O/c1-31(21-11-3-2-4-12-21)20-17-26(32)28-18-9-10-19-29-27-22-13-5-7-15-24(22)30-25-16-8-6-14-23(25)27/h5,7,13,15,21H,2-4,6,8-12,14,16-20H2,1H3,(H,28,32)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human BuChE pre-incubated for 5 mins before butylthiocholine substrate addition by Ellman's method |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50056129

(CHEMBL3322156)Show SMILES CN(CCC(=O)NCCCNc1c2CCCCc2nc2ccccc12)C1CCCCC1 Show InChI InChI=1S/C26H38N4O/c1-30(20-10-3-2-4-11-20)19-16-25(31)27-17-9-18-28-26-21-12-5-7-14-23(21)29-24-15-8-6-13-22(24)26/h5,7,12,14,20H,2-4,6,8-11,13,15-19H2,1H3,(H,27,31)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human BuChE pre-incubated for 5 mins before butylthiocholine substrate addition by Ellman's method |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056130
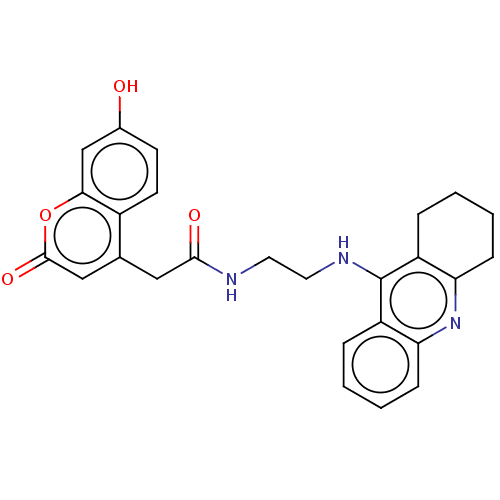
(CHEMBL3322158)Show SMILES Oc1ccc2c(CC(=O)NCCNc3c4CCCCc4nc4ccccc34)cc(=O)oc2c1 Show InChI InChI=1S/C26H25N3O4/c30-17-9-10-18-16(14-25(32)33-23(18)15-17)13-24(31)27-11-12-28-26-19-5-1-3-7-21(19)29-22-8-4-2-6-20(22)26/h1,3,5,7,9-10,14-15,30H,2,4,6,8,11-13H2,(H,27,31)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE pre-incubated for 5 mins before acetylthiocholine substrate addition by Ellman's method |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50056136

(CHEMBL3322162)Show SMILES Oc1ccc2c(CC(=O)N\N=C3/SCC(=O)N3c3c4CCCCc4nc4ccccc34)cc(=O)oc2c1 Show InChI InChI=1S/C27H22N4O5S/c32-16-9-10-17-15(12-25(35)36-22(17)13-16)11-23(33)29-30-27-31(24(34)14-37-27)26-18-5-1-3-7-20(18)28-21-8-4-2-6-19(21)26/h1,3,5,7,9-10,12-13,32H,2,4,6,8,11,14H2,(H,29,33)/b30-27- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human BuChE pre-incubated for 5 mins before butylthiocholine substrate addition by Ellman's method |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056135

(CHEMBL3322161)Show SMILES Oc1ccc2c(CC(=O)NNC(=S)Nc3c4CCCCc4nc4ccccc34)cc(=O)oc2c1 Show InChI InChI=1S/C25H22N4O4S/c30-15-9-10-16-14(12-23(32)33-21(16)13-15)11-22(31)28-29-25(34)27-24-17-5-1-3-7-19(17)26-20-8-4-2-6-18(20)24/h1,3,5,7,9-10,12-13,30H,2,4,6,8,11H2,(H,28,31)(H2,26,27,29,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE pre-incubated for 5 mins before acetylthiocholine substrate addition by Ellman's method |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50056130

(CHEMBL3322158)Show SMILES Oc1ccc2c(CC(=O)NCCNc3c4CCCCc4nc4ccccc34)cc(=O)oc2c1 Show InChI InChI=1S/C26H25N3O4/c30-17-9-10-18-16(14-25(32)33-23(18)15-17)13-24(31)27-11-12-28-26-19-5-1-3-7-21(19)29-22-8-4-2-6-20(22)26/h1,3,5,7,9-10,14-15,30H,2,4,6,8,11-13H2,(H,27,31)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human BuChE pre-incubated for 5 mins before butylthiocholine substrate addition by Ellman's method |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50327939

(7-methoxytacrine | CHEMBL1256415)Show InChI InChI=1S/C14H16N2O/c1-17-9-6-7-13-11(8-9)14(15)10-4-2-3-5-12(10)16-13/h6-8H,2-5H2,1H3,(H2,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE pre-incubated for 5 mins before acetylthiocholine substrate addition by Ellman's method |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056136

(CHEMBL3322162)Show SMILES Oc1ccc2c(CC(=O)N\N=C3/SCC(=O)N3c3c4CCCCc4nc4ccccc34)cc(=O)oc2c1 Show InChI InChI=1S/C27H22N4O5S/c32-16-9-10-17-15(12-25(35)36-22(17)13-16)11-23(33)29-30-27-31(24(34)14-37-27)26-18-5-1-3-7-20(18)28-21-8-4-2-6-19(21)26/h1,3,5,7,9-10,12-13,32H,2,4,6,8,11,14H2,(H,29,33)/b30-27- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE pre-incubated for 5 mins before acetylthiocholine substrate addition by Ellman's method |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50327939

(7-methoxytacrine | CHEMBL1256415)Show InChI InChI=1S/C14H16N2O/c1-17-9-6-7-13-11(8-9)14(15)10-4-2-3-5-12(10)16-13/h6-8H,2-5H2,1H3,(H2,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human BuChE pre-incubated for 5 mins before butylthiocholine substrate addition by Ellman's method |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data