 Found 134 hits Enz. Inhib. hit(s) with all data for entry = 50015967
Found 134 hits Enz. Inhib. hit(s) with all data for entry = 50015967 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50162995
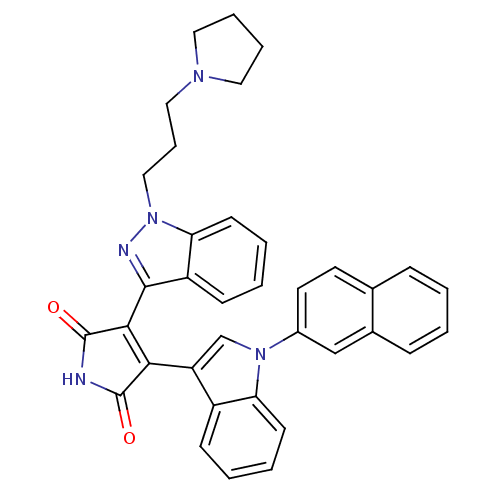
(3-[1-(1,2-Dihydro-pyridin-2-yl)-1H-indazol-3-yl]-4...)Show SMILES O=C1NC(=O)C(=C1c1cn(-c2ccc3ccccc3c2)c2ccccc12)c1nn(CCCN2CCCC2)c2ccccc12 |c:5| Show InChI InChI=1S/C36H31N5O2/c42-35-32(29-23-40(30-14-5-3-12-27(29)30)26-17-16-24-10-1-2-11-25(24)22-26)33(36(43)37-35)34-28-13-4-6-15-31(28)41(38-34)21-9-20-39-18-7-8-19-39/h1-6,10-17,22-23H,7-9,18-21H2,(H,37,42,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Potassium channel HERG |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50162996
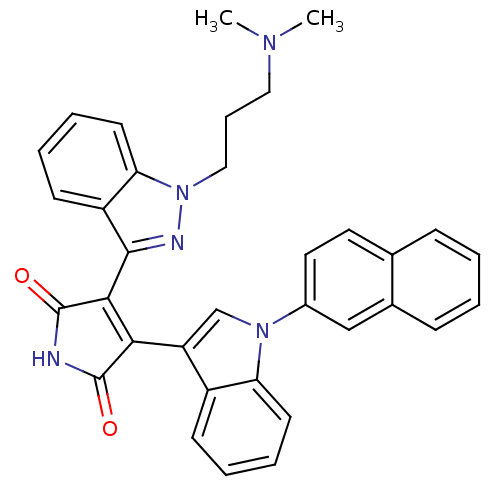
(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-[...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3ccc4ccccc4c3)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C34H29N5O2/c1-37(2)18-9-19-39-29-15-8-6-13-26(29)32(36-39)31-30(33(40)35-34(31)41)27-21-38(28-14-7-5-12-25(27)28)24-17-16-22-10-3-4-11-23(22)20-24/h3-8,10-17,20-21H,9,18-19H2,1-2H3,(H,35,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Potassium channel HERG |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50162990
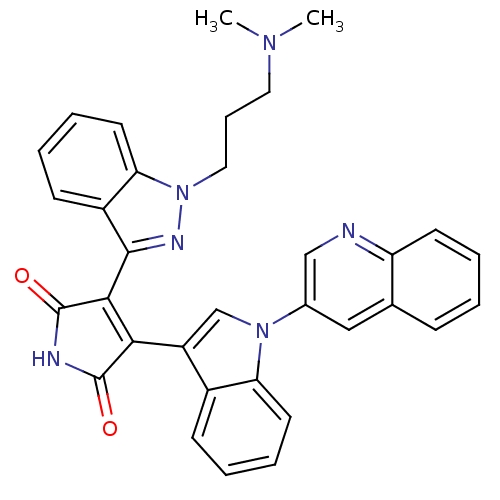
(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-(...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3cnc4ccccc4c3)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C33H28N6O2/c1-37(2)16-9-17-39-28-15-8-5-12-24(28)31(36-39)30-29(32(40)35-33(30)41)25-20-38(27-14-7-4-11-23(25)27)22-18-21-10-3-6-13-26(21)34-19-22/h3-8,10-15,18-20H,9,16-17H2,1-2H3,(H,35,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Potassium channel HERG |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50163000
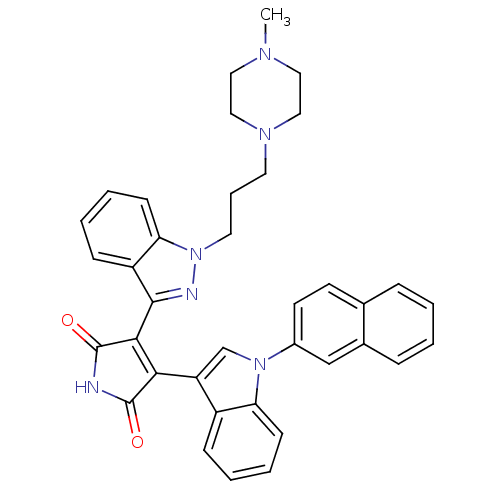
(3-(1-Piperazin-1-yl-1H-indazol-3-yl)-4-[1-(1,4,4a,...)Show SMILES CN1CCN(CCCn2nc(C3=C(C(=O)NC3=O)c3cn(-c4ccc5ccccc5c4)c4ccccc34)c3ccccc23)CC1 |t:11| Show InChI InChI=1S/C37H34N6O2/c1-40-19-21-41(22-20-40)17-8-18-43-32-14-7-5-12-29(32)35(39-43)34-33(36(44)38-37(34)45)30-24-42(31-13-6-4-11-28(30)31)27-16-15-25-9-2-3-10-26(25)23-27/h2-7,9-16,23-24H,8,17-22H2,1H3,(H,38,44,45) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Potassium channel HERG |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50017376

((+/-)1-(4-tert-butylphenyl)-4-(4-(hydroxydiphenylm...)Show SMILES CC(C)(C)c1ccc(cc1)C(O)CCCN1CCC(CC1)C(O)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C32H41NO2/c1-31(2,3)26-18-16-25(17-19-26)30(34)15-10-22-33-23-20-29(21-24-33)32(35,27-11-6-4-7-12-27)28-13-8-5-9-14-28/h4-9,11-14,16-19,29-30,34-35H,10,15,20-24H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Potassium channel HERG |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50162992
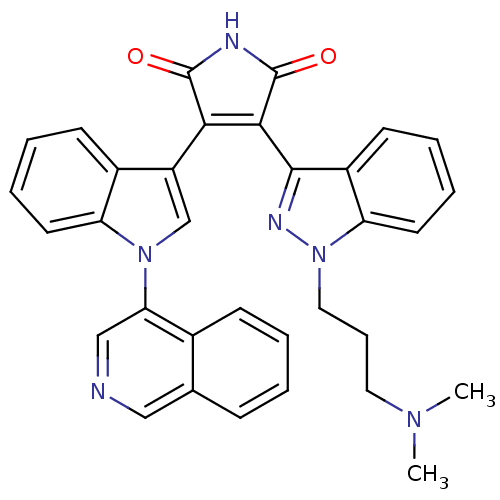
(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-(...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3cncc4ccccc34)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C33H28N6O2/c1-37(2)16-9-17-39-27-15-8-6-13-24(27)31(36-39)30-29(32(40)35-33(30)41)25-20-38(26-14-7-5-12-23(25)26)28-19-34-18-21-10-3-4-11-22(21)28/h3-8,10-15,18-20H,9,16-17H2,1-2H3,(H,35,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Potassium channel HERG |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50162993
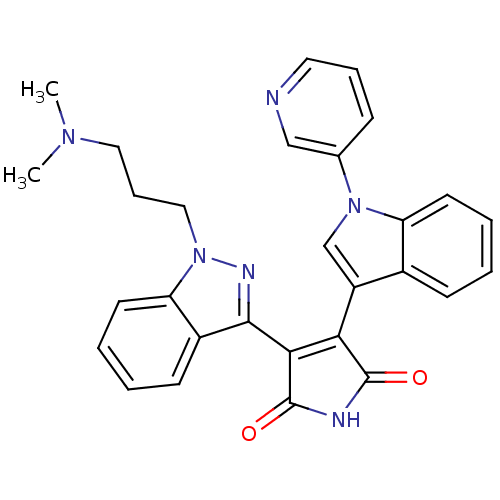
(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-(...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3cccnc3)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C29H26N6O2/c1-33(2)15-8-16-35-24-13-6-4-11-21(24)27(32-35)26-25(28(36)31-29(26)37)22-18-34(19-9-7-14-30-17-19)23-12-5-3-10-20(22)23/h3-7,9-14,17-18H,8,15-16H2,1-2H3,(H,31,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Potassium channel HERG |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50163002
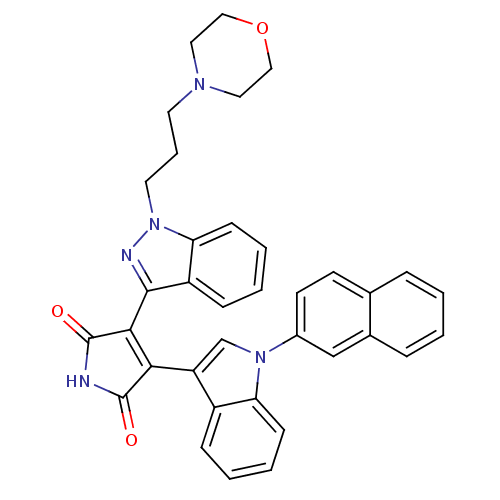
(3-(1-Morpholin-2-yl-1H-indazol-3-yl)-4-[1-(1,4,4a,...)Show SMILES O=C1NC(=O)C(=C1c1cn(-c2ccc3ccccc3c2)c2ccccc12)c1nn(CCCN2CCOCC2)c2ccccc12 |c:5| Show InChI InChI=1S/C36H31N5O3/c42-35-32(29-23-40(30-12-5-3-10-27(29)30)26-15-14-24-8-1-2-9-25(24)22-26)33(36(43)37-35)34-28-11-4-6-13-31(28)41(38-34)17-7-16-39-18-20-44-21-19-39/h1-6,8-15,22-23H,7,16-21H2,(H,37,42,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Potassium channel HERG |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Platelet-derived growth factor receptor in P19 cells |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50162990

(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-(...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3cnc4ccccc4c3)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C33H28N6O2/c1-37(2)16-9-17-39-28-15-8-5-12-24(28)31(36-39)30-29(32(40)35-33(30)41)25-20-38(27-14-7-4-11-23(25)27)22-18-21-10-3-6-13-26(21)34-19-22/h3-8,10-15,18-20H,9,16-17H2,1-2H3,(H,35,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of IL-8 release by HEK293 cells expressing PKC-beta2 |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50162996

(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-[...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3ccc4ccccc4c3)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C34H29N5O2/c1-37(2)18-9-19-39-29-15-8-6-13-26(29)32(36-39)31-30(33(40)35-34(31)41)27-21-38(28-14-7-5-12-25(27)28)24-17-16-22-10-3-4-11-23(22)20-24/h3-8,10-17,20-21H,9,18-19H2,1-2H3,(H,35,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human Protein kinase C beta 2 using [gamma-33P]-ATP |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50162993

(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-(...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3cccnc3)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C29H26N6O2/c1-33(2)15-8-16-35-24-13-6-4-11-21(24)27(32-35)26-25(28(36)31-29(26)37)22-18-34(19-9-7-14-30-17-19)23-12-5-3-10-20(22)23/h3-7,9-14,17-18H,8,15-16H2,1-2H3,(H,31,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human Protein kinase C beta 2 using [gamma-33P]-ATP |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50162990

(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-(...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3cnc4ccccc4c3)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C33H28N6O2/c1-37(2)16-9-17-39-28-15-8-5-12-24(28)31(36-39)30-29(32(40)35-33(30)41)25-20-38(27-14-7-4-11-23(25)27)22-18-21-10-3-6-13-26(21)34-19-22/h3-8,10-15,18-20H,9,16-17H2,1-2H3,(H,35,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human Protein kinase C beta 1 using [gamma-33P]-ATP |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Mus musculus) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Cyclin-dependent kinase 1 in P19 cells |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human Protein kinase C beta 2 using [gamma-33P]-ATP |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50162998
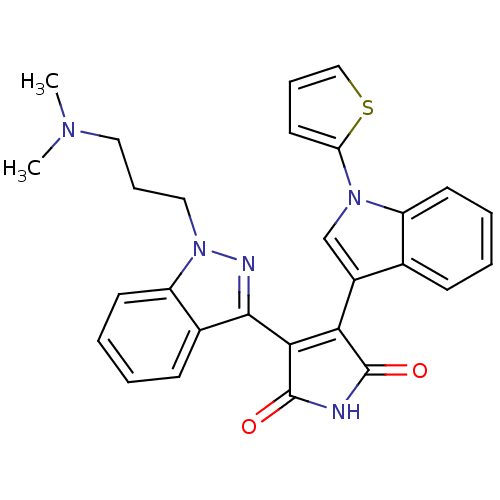
(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-(...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3cccs3)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C28H25N5O2S/c1-31(2)14-8-15-33-22-12-6-4-10-19(22)26(30-33)25-24(27(34)29-28(25)35)20-17-32(23-13-7-16-36-23)21-11-5-3-9-18(20)21/h3-7,9-13,16-17H,8,14-15H2,1-2H3,(H,29,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human Protein kinase C beta 2 using [gamma-33P]-ATP |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50162990

(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-(...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3cnc4ccccc4c3)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C33H28N6O2/c1-37(2)16-9-17-39-28-15-8-5-12-24(28)31(36-39)30-29(32(40)35-33(30)41)25-20-38(27-14-7-4-11-23(25)27)22-18-21-10-3-6-13-26(21)34-19-22/h3-8,10-15,18-20H,9,16-17H2,1-2H3,(H,35,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human Protein kinase C alpha using [gamma-33P]-ATP |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50163001
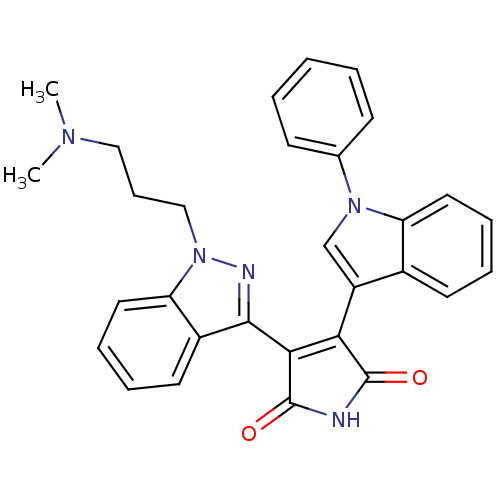
(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-(...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3ccccc3)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C30H27N5O2/c1-33(2)17-10-18-35-25-16-9-7-14-22(25)28(32-35)27-26(29(36)31-30(27)37)23-19-34(20-11-4-3-5-12-20)24-15-8-6-13-21(23)24/h3-9,11-16,19H,10,17-18H2,1-2H3,(H,31,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of IL-8 release by HEK293 cells expressing PKC-beta2 |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50162998

(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-(...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3cccs3)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C28H25N5O2S/c1-31(2)14-8-15-33-22-12-6-4-10-19(22)26(30-33)25-24(27(34)29-28(25)35)20-17-32(23-13-7-16-36-23)21-11-5-3-9-18(20)21/h3-7,9-13,16-17H,8,14-15H2,1-2H3,(H,29,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human Protein kinase C beta 1 using [gamma-33P]-ATP |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50162992

(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-(...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3cncc4ccccc34)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C33H28N6O2/c1-37(2)16-9-17-39-27-15-8-6-13-24(27)31(36-39)30-29(32(40)35-33(30)41)25-20-38(26-14-7-5-12-23(25)26)28-19-34-18-21-10-3-4-11-22(21)28/h3-8,10-15,18-20H,9,16-17H2,1-2H3,(H,35,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human Protein kinase C beta 2 using [gamma-33P]-ATP |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50163002

(3-(1-Morpholin-2-yl-1H-indazol-3-yl)-4-[1-(1,4,4a,...)Show SMILES O=C1NC(=O)C(=C1c1cn(-c2ccc3ccccc3c2)c2ccccc12)c1nn(CCCN2CCOCC2)c2ccccc12 |c:5| Show InChI InChI=1S/C36H31N5O3/c42-35-32(29-23-40(30-12-5-3-10-27(29)30)26-15-14-24-8-1-2-9-25(24)22-26)33(36(43)37-35)34-28-11-4-6-13-31(28)41(38-34)17-7-16-39-18-20-44-21-19-39/h1-6,8-15,22-23H,7,16-21H2,(H,37,42,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of IL-8 release by HEK293 cells expressing PKC-beta2 |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50162993

(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-(...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3cccnc3)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C29H26N6O2/c1-33(2)15-8-16-35-24-13-6-4-11-21(24)27(32-35)26-25(28(36)31-29(26)37)22-18-34(19-9-7-14-30-17-19)23-12-5-3-10-20(22)23/h3-7,9-14,17-18H,8,15-16H2,1-2H3,(H,31,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human Protein kinase C beta 1 using [gamma-33P]-ATP |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50162990

(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-(...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3cnc4ccccc4c3)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C33H28N6O2/c1-37(2)16-9-17-39-28-15-8-5-12-24(28)31(36-39)30-29(32(40)35-33(30)41)25-20-38(27-14-7-4-11-23(25)27)22-18-21-10-3-6-13-26(21)34-19-22/h3-8,10-15,18-20H,9,16-17H2,1-2H3,(H,35,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of IL-8 release by HEK293 cells expressing PKC-beta2 |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50162999
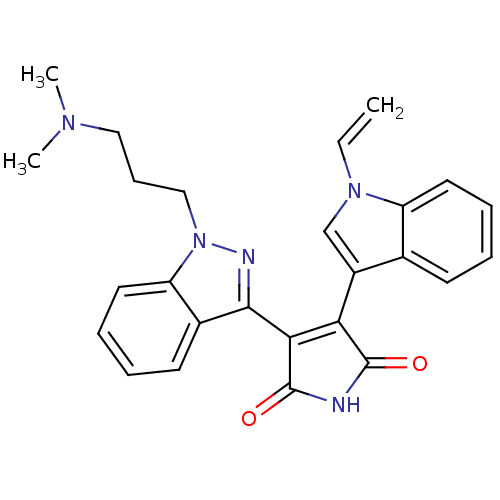
(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-(...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(C=C)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C26H25N5O2/c1-4-30-16-19(17-10-5-7-12-20(17)30)22-23(26(33)27-25(22)32)24-18-11-6-8-13-21(18)31(28-24)15-9-14-29(2)3/h4-8,10-13,16H,1,9,14-15H2,2-3H3,(H,27,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of IL-8 release by HEK293 cells expressing PKC-beta2 |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50162996

(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-[...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3ccc4ccccc4c3)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C34H29N5O2/c1-37(2)18-9-19-39-29-15-8-6-13-26(29)32(36-39)31-30(33(40)35-34(31)41)27-21-38(28-14-7-5-12-25(27)28)24-17-16-22-10-3-4-11-23(22)20-24/h3-8,10-17,20-21H,9,18-19H2,1-2H3,(H,35,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of IL-8 release by HEK293 cells expressing PKC-beta2 |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50162996

(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-[...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3ccc4ccccc4c3)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C34H29N5O2/c1-37(2)18-9-19-39-29-15-8-6-13-26(29)32(36-39)31-30(33(40)35-34(31)41)27-21-38(28-14-7-5-12-25(27)28)24-17-16-22-10-3-4-11-23(22)20-24/h3-8,10-17,20-21H,9,18-19H2,1-2H3,(H,35,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Potassium channel HERG |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human Protein kinase C alpha using [gamma-33P]-ATP |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50162994
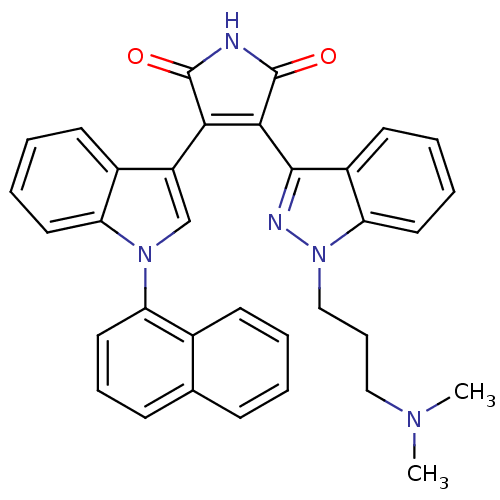
(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-[...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3cccc4ccccc34)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C34H29N5O2/c1-37(2)19-10-20-39-29-17-8-6-15-25(29)32(36-39)31-30(33(40)35-34(31)41)26-21-38(28-16-7-5-14-24(26)28)27-18-9-12-22-11-3-4-13-23(22)27/h3-9,11-18,21H,10,19-20H2,1-2H3,(H,35,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human Protein kinase C beta 2 using [gamma-33P]-ATP |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50162990

(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-(...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3cnc4ccccc4c3)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C33H28N6O2/c1-37(2)16-9-17-39-28-15-8-5-12-24(28)31(36-39)30-29(32(40)35-33(30)41)25-20-38(27-14-7-4-11-23(25)27)22-18-21-10-3-6-13-26(21)34-19-22/h3-8,10-15,18-20H,9,16-17H2,1-2H3,(H,35,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C delta |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50162996

(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-[...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3ccc4ccccc4c3)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C34H29N5O2/c1-37(2)18-9-19-39-29-15-8-6-13-26(29)32(36-39)31-30(33(40)35-34(31)41)27-21-38(28-14-7-5-12-25(27)28)24-17-16-22-10-3-4-11-23(22)20-24/h3-8,10-17,20-21H,9,18-19H2,1-2H3,(H,35,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human Protein kinase C beta 1 using [gamma-33P]-ATP |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50162991

(3-(1-Benzo[b]thiophen-2-yl-1H-indol-3-yl)-4-[1-(3-...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3csc4ccccc34)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C32H27N5O2S/c1-35(2)16-9-17-37-25-14-7-4-12-22(25)30(34-37)29-28(31(38)33-32(29)39)23-18-36(24-13-6-3-10-20(23)24)26-19-40-27-15-8-5-11-21(26)27/h3-8,10-15,18-19H,9,16-17H2,1-2H3,(H,33,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human Protein kinase C beta 2 using [gamma-33P]-ATP |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50162995

(3-[1-(1,2-Dihydro-pyridin-2-yl)-1H-indazol-3-yl]-4...)Show SMILES O=C1NC(=O)C(=C1c1cn(-c2ccc3ccccc3c2)c2ccccc12)c1nn(CCCN2CCCC2)c2ccccc12 |c:5| Show InChI InChI=1S/C36H31N5O2/c42-35-32(29-23-40(30-14-5-3-12-27(29)30)26-17-16-24-10-1-2-11-25(24)22-26)33(36(43)37-35)34-28-13-4-6-15-31(28)41(38-34)21-9-20-39-18-7-8-19-39/h1-6,10-17,22-23H,7-9,18-21H2,(H,37,42,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human Protein kinase C beta 2 using [gamma-33P]-ATP |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50163001

(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-(...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3ccccc3)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C30H27N5O2/c1-33(2)17-10-18-35-25-16-9-7-14-22(25)28(32-35)27-26(29(36)31-30(27)37)23-19-34(20-11-4-3-5-12-20)24-15-8-6-13-21(23)24/h3-9,11-16,19H,10,17-18H2,1-2H3,(H,31,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human Protein kinase C beta 1 using [gamma-33P]-ATP |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Protein kinase C gamma type
(Homo sapiens (Human)) | BDBM50162990

(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-(...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3cnc4ccccc4c3)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C33H28N6O2/c1-37(2)16-9-17-39-28-15-8-5-12-24(28)31(36-39)30-29(32(40)35-33(30)41)25-20-38(27-14-7-4-11-23(25)27)22-18-21-10-3-6-13-26(21)34-19-22/h3-8,10-15,18-20H,9,16-17H2,1-2H3,(H,35,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human Protein kinase C gamma using [gamma-33P]-ATP |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50162999

(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-(...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(C=C)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C26H25N5O2/c1-4-30-16-19(17-10-5-7-12-20(17)30)22-23(26(33)27-25(22)32)24-18-11-6-8-13-21(18)31(28-24)15-9-14-29(2)3/h4-8,10-13,16H,1,9,14-15H2,2-3H3,(H,27,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of rabbit Glycogen synthase kinase-3 beta |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C delta |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50162996

(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-[...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3ccc4ccccc4c3)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C34H29N5O2/c1-37(2)18-9-19-39-29-15-8-6-13-26(29)32(36-39)31-30(33(40)35-34(31)41)27-21-38(28-14-7-5-12-25(27)28)24-17-16-22-10-3-4-11-23(22)20-24/h3-8,10-17,20-21H,9,18-19H2,1-2H3,(H,35,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human Protein kinase C alpha using [gamma-33P]-ATP |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Protein kinase C gamma type
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human Protein kinase C gamma using [gamma-33P]-ATP |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50163000

(3-(1-Piperazin-1-yl-1H-indazol-3-yl)-4-[1-(1,4,4a,...)Show SMILES CN1CCN(CCCn2nc(C3=C(C(=O)NC3=O)c3cn(-c4ccc5ccccc5c4)c4ccccc34)c3ccccc23)CC1 |t:11| Show InChI InChI=1S/C37H34N6O2/c1-40-19-21-41(22-20-40)17-8-18-43-32-14-7-5-12-29(32)35(39-43)34-33(36(44)38-37(34)45)30-24-42(31-13-6-4-11-28(30)31)27-16-15-25-9-2-3-10-26(25)23-27/h2-7,9-16,23-24H,8,17-22H2,1H3,(H,38,44,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human Protein kinase C beta 1 using [gamma-33P]-ATP |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human Protein kinase C beta 1 using [gamma-33P]-ATP |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50163002

(3-(1-Morpholin-2-yl-1H-indazol-3-yl)-4-[1-(1,4,4a,...)Show SMILES O=C1NC(=O)C(=C1c1cn(-c2ccc3ccccc3c2)c2ccccc12)c1nn(CCCN2CCOCC2)c2ccccc12 |c:5| Show InChI InChI=1S/C36H31N5O3/c42-35-32(29-23-40(30-12-5-3-10-27(29)30)26-15-14-24-8-1-2-9-25(24)22-26)33(36(43)37-35)34-28-11-4-6-13-31(28)41(38-34)17-7-16-39-18-20-44-21-19-39/h1-6,8-15,22-23H,7,16-21H2,(H,37,42,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of IL-8 release by HEK293 cells expressing PKC-beta2 |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50162998

(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-(...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3cccs3)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C28H25N5O2S/c1-31(2)14-8-15-33-22-12-6-4-10-19(22)26(30-33)25-24(27(34)29-28(25)35)20-17-32(23-13-7-16-36-23)21-11-5-3-9-18(20)21/h3-7,9-13,16-17H,8,14-15H2,1-2H3,(H,29,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of IL-8 release by HEK293 cells expressing PKC-beta2 |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of IL-8 release by HEK293 cells expressing PKC-beta2 |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50162993

(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-(...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3cccnc3)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C29H26N6O2/c1-33(2)15-8-16-35-24-13-6-4-11-21(24)27(32-35)26-25(28(36)31-29(26)37)22-18-34(19-9-7-14-30-17-19)23-12-5-3-10-20(22)23/h3-7,9-14,17-18H,8,15-16H2,1-2H3,(H,31,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of IL-8 release by HEK293 cells expressing PKC-beta2 |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50162991

(3-(1-Benzo[b]thiophen-2-yl-1H-indol-3-yl)-4-[1-(3-...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3csc4ccccc34)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C32H27N5O2S/c1-35(2)16-9-17-37-25-14-7-4-12-22(25)30(34-37)29-28(31(38)33-32(29)39)23-18-36(24-13-6-3-10-20(23)24)26-19-40-27-15-8-5-11-21(26)27/h3-8,10-15,18-19H,9,16-17H2,1-2H3,(H,33,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human Protein kinase C beta 1 using [gamma-33P]-ATP |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50162998

(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-(...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3cccs3)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C28H25N5O2S/c1-31(2)14-8-15-33-22-12-6-4-10-19(22)26(30-33)25-24(27(34)29-28(25)35)20-17-32(23-13-7-16-36-23)21-11-5-3-9-18(20)21/h3-7,9-13,16-17H,8,14-15H2,1-2H3,(H,29,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C delta |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50162992

(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-(...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3cncc4ccccc34)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C33H28N6O2/c1-37(2)16-9-17-39-27-15-8-6-13-24(27)31(36-39)30-29(32(40)35-33(30)41)25-20-38(26-14-7-5-12-23(25)26)28-19-34-18-21-10-3-4-11-22(21)28/h3-8,10-15,18-20H,9,16-17H2,1-2H3,(H,35,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of IL-8 release by HEK293 cells expressing PKC-beta2 |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50162994

(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-[...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3cccc4ccccc34)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C34H29N5O2/c1-37(2)19-10-20-39-29-17-8-6-15-25(29)32(36-39)31-30(33(40)35-34(31)41)26-21-38(28-16-7-5-14-24(26)28)27-18-9-12-22-11-3-4-13-23(22)27/h3-9,11-18,21H,10,19-20H2,1-2H3,(H,35,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human Protein kinase C beta 1 using [gamma-33P]-ATP |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50162992

(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-(...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3cncc4ccccc34)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C33H28N6O2/c1-37(2)16-9-17-39-27-15-8-6-13-24(27)31(36-39)30-29(32(40)35-33(30)41)25-20-38(26-14-7-5-12-23(25)26)28-19-34-18-21-10-3-4-11-22(21)28/h3-8,10-15,18-20H,9,16-17H2,1-2H3,(H,35,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human Protein kinase C beta 1 using [gamma-33P]-ATP |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50163000

(3-(1-Piperazin-1-yl-1H-indazol-3-yl)-4-[1-(1,4,4a,...)Show SMILES CN1CCN(CCCn2nc(C3=C(C(=O)NC3=O)c3cn(-c4ccc5ccccc5c4)c4ccccc34)c3ccccc23)CC1 |t:11| Show InChI InChI=1S/C37H34N6O2/c1-40-19-21-41(22-20-40)17-8-18-43-32-14-7-5-12-29(32)35(39-43)34-33(36(44)38-37(34)45)30-24-42(31-13-6-4-11-28(30)31)27-16-15-25-9-2-3-10-26(25)23-27/h2-7,9-16,23-24H,8,17-22H2,1H3,(H,38,44,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human Protein kinase C beta 2 using [gamma-33P]-ATP |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data