

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50293451 (1-Methyl-1-[2-(7-oxo-7H-1-aza-benzo[de]anthracen-9...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by LB plot | Eur J Med Chem 44: 2523-32 (2009) Article DOI: 10.1016/j.ejmech.2009.01.021 BindingDB Entry DOI: 10.7270/Q2Z89CFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50293452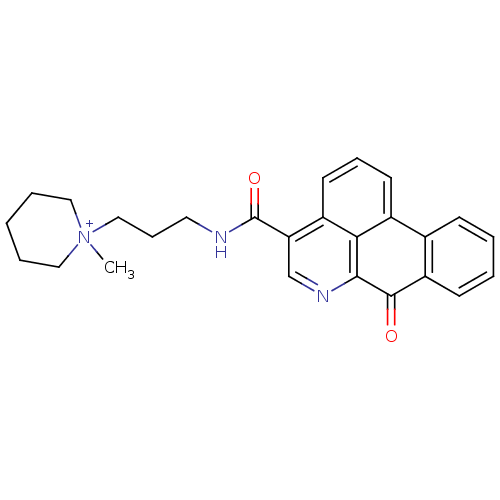 (1-methyl-1-(3-(7-oxo-7H-dibenzo[de,g]quinoline-4-c...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by LB plot | Eur J Med Chem 44: 2523-32 (2009) Article DOI: 10.1016/j.ejmech.2009.01.021 BindingDB Entry DOI: 10.7270/Q2Z89CFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50211247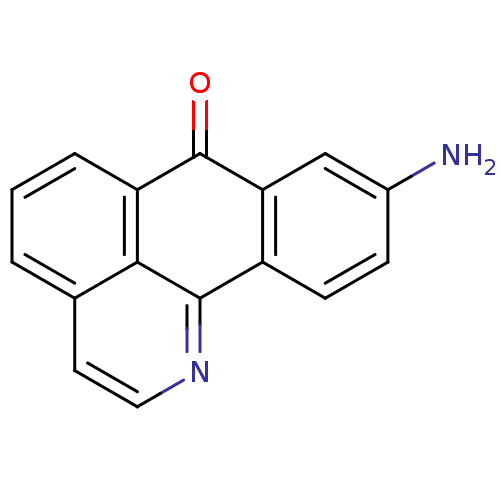 (9-Amino-1-azabenzanthrone | 9-amino-1-aza-benzo[de...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by LB plot | Eur J Med Chem 44: 2523-32 (2009) Article DOI: 10.1016/j.ejmech.2009.01.021 BindingDB Entry DOI: 10.7270/Q2Z89CFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50293443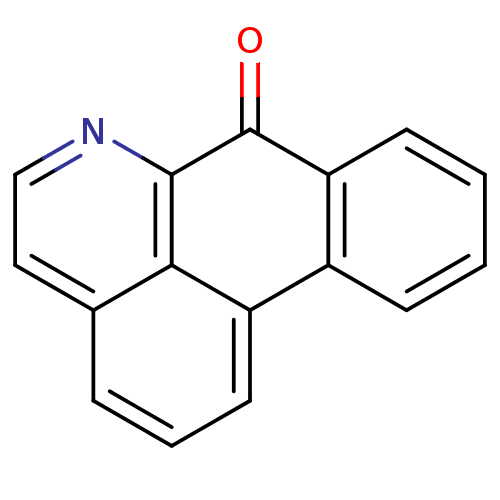 (7-Oxo-7H-dibenzo[de,g]quinoline | CHEMBL559502) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by LB plot | Eur J Med Chem 44: 2523-32 (2009) Article DOI: 10.1016/j.ejmech.2009.01.021 BindingDB Entry DOI: 10.7270/Q2Z89CFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50293451 (1-Methyl-1-[2-(7-oxo-7H-1-aza-benzo[de]anthracen-9...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Eur J Med Chem 44: 2523-32 (2009) Article DOI: 10.1016/j.ejmech.2009.01.021 BindingDB Entry DOI: 10.7270/Q2Z89CFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50293446 (CHEMBL556249 | Diethyl-methyl-[2-(7-oxo-7H-1-aza-b...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.62 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Eur J Med Chem 44: 2523-32 (2009) Article DOI: 10.1016/j.ejmech.2009.01.021 BindingDB Entry DOI: 10.7270/Q2Z89CFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50211244 (CHEMBL395926 | Trimethyl-[2-(7-oxo-7H-1-aza-benzo[...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.81 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Eur J Med Chem 44: 2523-32 (2009) Article DOI: 10.1016/j.ejmech.2009.01.021 BindingDB Entry DOI: 10.7270/Q2Z89CFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50293452 (1-methyl-1-(3-(7-oxo-7H-dibenzo[de,g]quinoline-4-c...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Eur J Med Chem 44: 2523-32 (2009) Article DOI: 10.1016/j.ejmech.2009.01.021 BindingDB Entry DOI: 10.7270/Q2Z89CFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50293440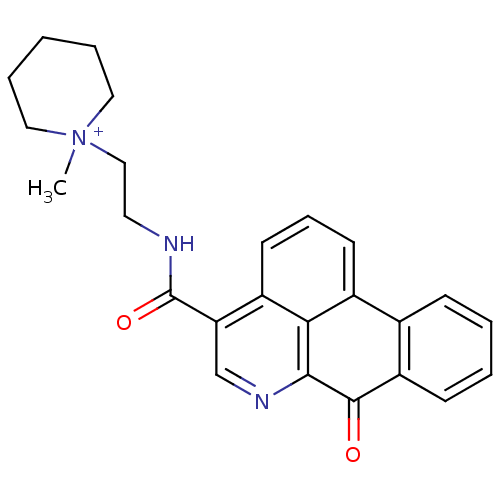 (1-methyl-1-(2-(7-oxo-7H-dibenzo[de,g]quinoline-4-c...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Eur J Med Chem 44: 2523-32 (2009) Article DOI: 10.1016/j.ejmech.2009.01.021 BindingDB Entry DOI: 10.7270/Q2Z89CFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Eur J Med Chem 44: 2523-32 (2009) Article DOI: 10.1016/j.ejmech.2009.01.021 BindingDB Entry DOI: 10.7270/Q2Z89CFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50293451 (1-Methyl-1-[2-(7-oxo-7H-1-aza-benzo[de]anthracen-9...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by Ellman's method | Eur J Med Chem 44: 2523-32 (2009) Article DOI: 10.1016/j.ejmech.2009.01.021 BindingDB Entry DOI: 10.7270/Q2Z89CFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50293447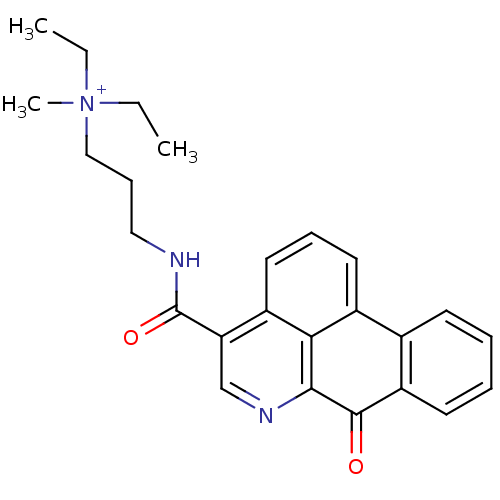 (CHEMBL549935 | N,N-diethyl-N-methyl-3-(7-oxo-7H-di...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Eur J Med Chem 44: 2523-32 (2009) Article DOI: 10.1016/j.ejmech.2009.01.021 BindingDB Entry DOI: 10.7270/Q2Z89CFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50293441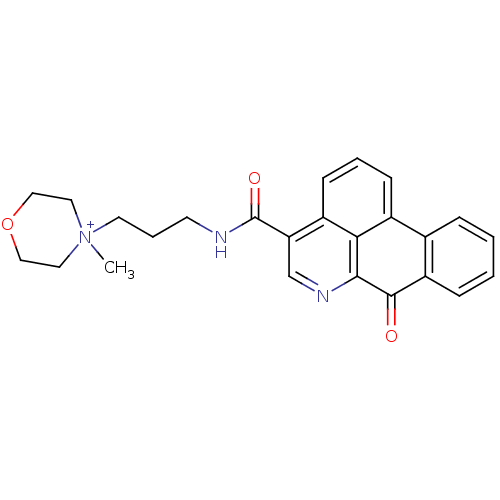 (4-methyl-4-(3-(7-oxo-7H-dibenzo[de,g]quinoline-4-c...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Eur J Med Chem 44: 2523-32 (2009) Article DOI: 10.1016/j.ejmech.2009.01.021 BindingDB Entry DOI: 10.7270/Q2Z89CFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50293442 (4-methyl-4-(2-(7-oxo-7H-dibenzo[de,g]quinoline-4-c...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Eur J Med Chem 44: 2523-32 (2009) Article DOI: 10.1016/j.ejmech.2009.01.021 BindingDB Entry DOI: 10.7270/Q2Z89CFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50293448 (CHEMBL550076 | N,N-diethyl-N-methyl-2-(7-oxo-7H-di...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Eur J Med Chem 44: 2523-32 (2009) Article DOI: 10.1016/j.ejmech.2009.01.021 BindingDB Entry DOI: 10.7270/Q2Z89CFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50293449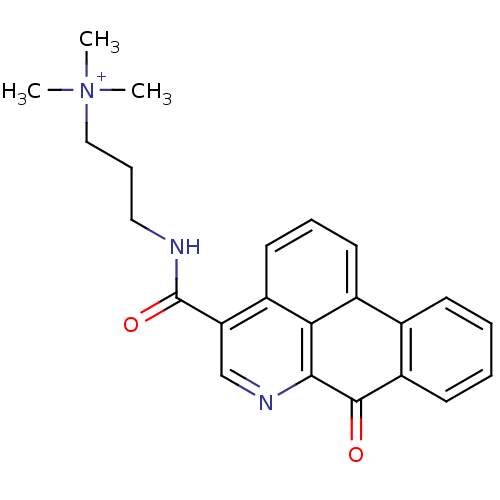 (CHEMBL559266 | N,N,N-trimethyl-3-(7-oxo-7H-dibenzo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by Ellman's method | Eur J Med Chem 44: 2523-32 (2009) Article DOI: 10.1016/j.ejmech.2009.01.021 BindingDB Entry DOI: 10.7270/Q2Z89CFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50211244 (CHEMBL395926 | Trimethyl-[2-(7-oxo-7H-1-aza-benzo[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by Ellman's method | Eur J Med Chem 44: 2523-32 (2009) Article DOI: 10.1016/j.ejmech.2009.01.021 BindingDB Entry DOI: 10.7270/Q2Z89CFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50293449 (CHEMBL559266 | N,N,N-trimethyl-3-(7-oxo-7H-dibenzo...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Eur J Med Chem 44: 2523-32 (2009) Article DOI: 10.1016/j.ejmech.2009.01.021 BindingDB Entry DOI: 10.7270/Q2Z89CFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50293450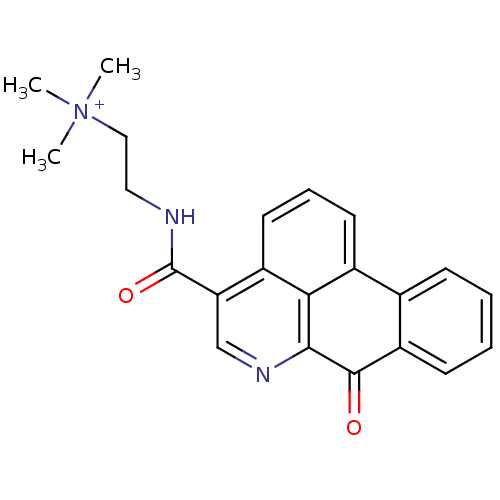 (CHEMBL551226 | N,N,N-trimethyl-2-(7-oxo-7H-dibenzo...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Eur J Med Chem 44: 2523-32 (2009) Article DOI: 10.1016/j.ejmech.2009.01.021 BindingDB Entry DOI: 10.7270/Q2Z89CFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50293443 (7-Oxo-7H-dibenzo[de,g]quinoline | CHEMBL559502) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by Ellman's method | Eur J Med Chem 44: 2523-32 (2009) Article DOI: 10.1016/j.ejmech.2009.01.021 BindingDB Entry DOI: 10.7270/Q2Z89CFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50293441 (4-methyl-4-(3-(7-oxo-7H-dibenzo[de,g]quinoline-4-c...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by Ellman's method | Eur J Med Chem 44: 2523-32 (2009) Article DOI: 10.1016/j.ejmech.2009.01.021 BindingDB Entry DOI: 10.7270/Q2Z89CFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50293450 (CHEMBL551226 | N,N,N-trimethyl-2-(7-oxo-7H-dibenzo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by Ellman's method | Eur J Med Chem 44: 2523-32 (2009) Article DOI: 10.1016/j.ejmech.2009.01.021 BindingDB Entry DOI: 10.7270/Q2Z89CFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50293446 (CHEMBL556249 | Diethyl-methyl-[2-(7-oxo-7H-1-aza-b...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by Ellman's method | Eur J Med Chem 44: 2523-32 (2009) Article DOI: 10.1016/j.ejmech.2009.01.021 BindingDB Entry DOI: 10.7270/Q2Z89CFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50293452 (1-methyl-1-(3-(7-oxo-7H-dibenzo[de,g]quinoline-4-c...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by Ellman's method | Eur J Med Chem 44: 2523-32 (2009) Article DOI: 10.1016/j.ejmech.2009.01.021 BindingDB Entry DOI: 10.7270/Q2Z89CFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50293440 (1-methyl-1-(2-(7-oxo-7H-dibenzo[de,g]quinoline-4-c...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by Ellman's method | Eur J Med Chem 44: 2523-32 (2009) Article DOI: 10.1016/j.ejmech.2009.01.021 BindingDB Entry DOI: 10.7270/Q2Z89CFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50293448 (CHEMBL550076 | N,N-diethyl-N-methyl-2-(7-oxo-7H-di...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by Ellman's method | Eur J Med Chem 44: 2523-32 (2009) Article DOI: 10.1016/j.ejmech.2009.01.021 BindingDB Entry DOI: 10.7270/Q2Z89CFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50293447 (CHEMBL549935 | N,N-diethyl-N-methyl-3-(7-oxo-7H-di...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by Ellman's method | Eur J Med Chem 44: 2523-32 (2009) Article DOI: 10.1016/j.ejmech.2009.01.021 BindingDB Entry DOI: 10.7270/Q2Z89CFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50293443 (7-Oxo-7H-dibenzo[de,g]quinoline | CHEMBL559502) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Eur J Med Chem 44: 2523-32 (2009) Article DOI: 10.1016/j.ejmech.2009.01.021 BindingDB Entry DOI: 10.7270/Q2Z89CFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50293442 (4-methyl-4-(2-(7-oxo-7H-dibenzo[de,g]quinoline-4-c...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by Ellman's method | Eur J Med Chem 44: 2523-32 (2009) Article DOI: 10.1016/j.ejmech.2009.01.021 BindingDB Entry DOI: 10.7270/Q2Z89CFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50293444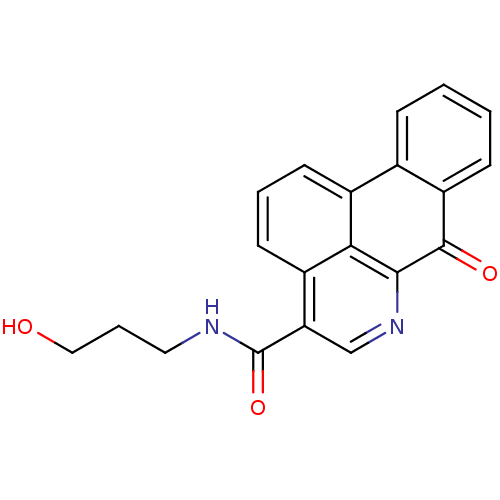 (CHEMBL549936 | N-(3-hydroxypropyl)-7-oxo-7H-benzo[...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Eur J Med Chem 44: 2523-32 (2009) Article DOI: 10.1016/j.ejmech.2009.01.021 BindingDB Entry DOI: 10.7270/Q2Z89CFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by Ellman's method | Eur J Med Chem 44: 2523-32 (2009) Article DOI: 10.1016/j.ejmech.2009.01.021 BindingDB Entry DOI: 10.7270/Q2Z89CFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50293445 (CHEMBL551430 | N-(2-hydroxyethyl)-7-oxo-7H-benzo[d...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Eur J Med Chem 44: 2523-32 (2009) Article DOI: 10.1016/j.ejmech.2009.01.021 BindingDB Entry DOI: 10.7270/Q2Z89CFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50211247 (9-Amino-1-azabenzanthrone | 9-amino-1-aza-benzo[de...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Eur J Med Chem 44: 2523-32 (2009) Article DOI: 10.1016/j.ejmech.2009.01.021 BindingDB Entry DOI: 10.7270/Q2Z89CFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50293444 (CHEMBL549936 | N-(3-hydroxypropyl)-7-oxo-7H-benzo[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by Ellman's method | Eur J Med Chem 44: 2523-32 (2009) Article DOI: 10.1016/j.ejmech.2009.01.021 BindingDB Entry DOI: 10.7270/Q2Z89CFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50293445 (CHEMBL551430 | N-(2-hydroxyethyl)-7-oxo-7H-benzo[d...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by Ellman's method | Eur J Med Chem 44: 2523-32 (2009) Article DOI: 10.1016/j.ejmech.2009.01.021 BindingDB Entry DOI: 10.7270/Q2Z89CFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50211247 (9-Amino-1-azabenzanthrone | 9-amino-1-aza-benzo[de...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by Ellman's method | Eur J Med Chem 44: 2523-32 (2009) Article DOI: 10.1016/j.ejmech.2009.01.021 BindingDB Entry DOI: 10.7270/Q2Z89CFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||