

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nischarin (RAT) | BDBM50334197 (7-Chloro-3-[(imidazolidin-2-yl)imino]indazole hydr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Gdansk Curated by ChEMBL | Assay Description Displacement of [3H]2BFI from imidazoline I1 receptor in Sprague-Dawley rat brain membrane after 45 mins by liquid scintillation counting | Bioorg Med Chem 19: 321-9 (2011) Article DOI: 10.1016/j.bmc.2010.11.020 BindingDB Entry DOI: 10.7270/Q2FQ9WX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nischarin (RAT) | BDBM50334195 (4-Chloro-3-[(imidazolidin-2-yl)imino]indazole hydr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 45.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Gdansk Curated by ChEMBL | Assay Description Displacement of [3H]2BFI from imidazoline I1 receptor in Sprague-Dawley rat brain membrane after 45 mins by liquid scintillation counting | Bioorg Med Chem 19: 321-9 (2011) Article DOI: 10.1016/j.bmc.2010.11.020 BindingDB Entry DOI: 10.7270/Q2FQ9WX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nischarin (RAT) | BDBM50334196 (3-[(Imidazolidin-2-yl)imino]-4-methylindazole hydr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 133 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Gdansk Curated by ChEMBL | Assay Description Displacement of [3H]2BFI from imidazoline I1 receptor in Sprague-Dawley rat brain membrane after 45 mins by liquid scintillation counting | Bioorg Med Chem 19: 321-9 (2011) Article DOI: 10.1016/j.bmc.2010.11.020 BindingDB Entry DOI: 10.7270/Q2FQ9WX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nischarin (RAT) | BDBM50334194 (3-[(Imidazolidin-2-yl)imino]indazole hydrochloride...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 134 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Gdansk Curated by ChEMBL | Assay Description Displacement of [3H]2BFI from imidazoline I1 receptor in Sprague-Dawley rat brain membrane after 45 mins by liquid scintillation counting | Bioorg Med Chem 19: 321-9 (2011) Article DOI: 10.1016/j.bmc.2010.11.020 BindingDB Entry DOI: 10.7270/Q2FQ9WX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nischarin (RAT) | BDBM50334206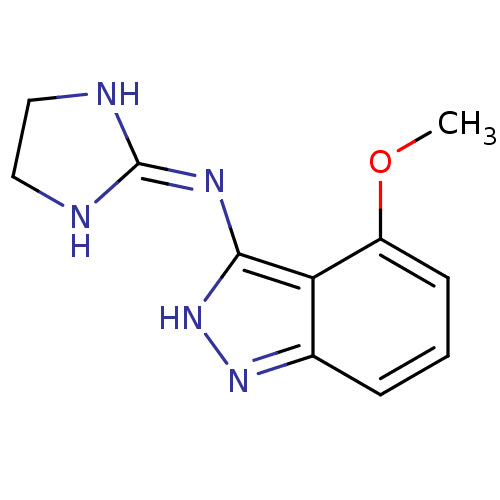 (3-[(Imidazolidin-2-yl)imino]-4-methoxyindazole hyd...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 167 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Gdansk Curated by ChEMBL | Assay Description Displacement of [3H]2BFI from imidazoline I1 receptor in Sprague-Dawley rat brain membrane after 45 mins by liquid scintillation counting | Bioorg Med Chem 19: 321-9 (2011) Article DOI: 10.1016/j.bmc.2010.11.020 BindingDB Entry DOI: 10.7270/Q2FQ9WX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nischarin (RAT) | BDBM50334204 (3-[(Imidazolidin-2-yl)imino]-6-methylindazole hydr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 325 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Gdansk Curated by ChEMBL | Assay Description Displacement of [3H]2BFI from imidazoline I1 receptor in Sprague-Dawley rat brain membrane after 45 mins by liquid scintillation counting | Bioorg Med Chem 19: 321-9 (2011) Article DOI: 10.1016/j.bmc.2010.11.020 BindingDB Entry DOI: 10.7270/Q2FQ9WX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nischarin (RAT) | BDBM50334203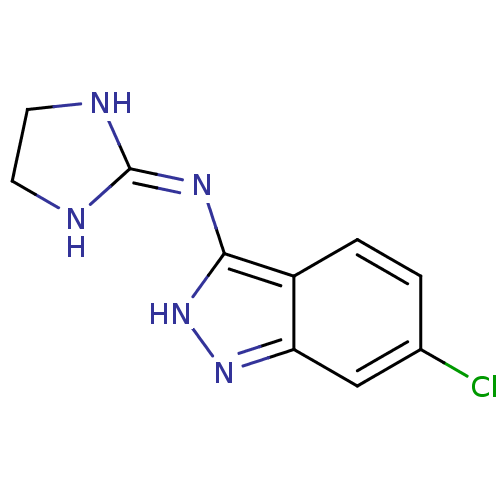 (6-Chloro-3-[(imidazolidin-2-yl)imino]indazole hydr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Gdansk Curated by ChEMBL | Assay Description Displacement of [3H]2BFI from imidazoline I1 receptor in Sprague-Dawley rat brain membrane after 45 mins by liquid scintillation counting | Bioorg Med Chem 19: 321-9 (2011) Article DOI: 10.1016/j.bmc.2010.11.020 BindingDB Entry DOI: 10.7270/Q2FQ9WX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nischarin (RAT) | BDBM50334205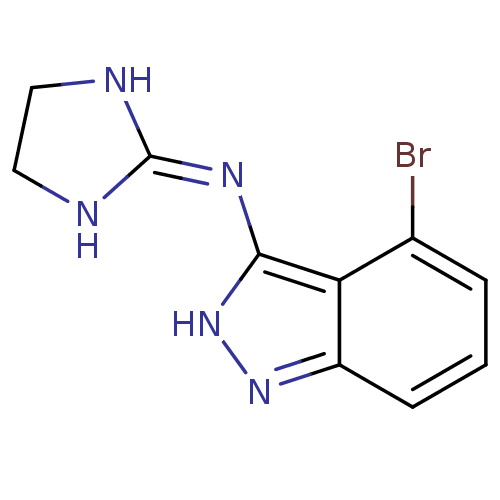 (4-Bromo-3-[(imidazolidin-2-yl)imino]indazole hydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Gdansk Curated by ChEMBL | Assay Description Displacement of [3H]2BFI from imidazoline I1 receptor in Sprague-Dawley rat brain membrane after 45 mins by liquid scintillation counting | Bioorg Med Chem 19: 321-9 (2011) Article DOI: 10.1016/j.bmc.2010.11.020 BindingDB Entry DOI: 10.7270/Q2FQ9WX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nischarin (RAT) | BDBM50334202 (3-[(Imidazolidin-2-ylo)imino]-5-methoxyindazole hy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Gdansk Curated by ChEMBL | Assay Description Displacement of [3H]2BFI from imidazoline I1 receptor in Sprague-Dawley rat brain membrane after 45 mins by liquid scintillation counting | Bioorg Med Chem 19: 321-9 (2011) Article DOI: 10.1016/j.bmc.2010.11.020 BindingDB Entry DOI: 10.7270/Q2FQ9WX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nischarin (RAT) | BDBM50334208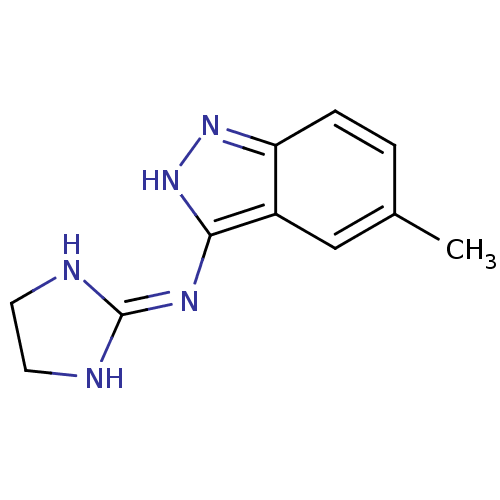 (3-[(Imidazolidin-2-yl)imino]-5-methylindazole hydr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Gdansk Curated by ChEMBL | Assay Description Displacement of [3H]2BFI from imidazoline I1 receptor in Sprague-Dawley rat brain membrane after 45 mins by liquid scintillation counting | Bioorg Med Chem 19: 321-9 (2011) Article DOI: 10.1016/j.bmc.2010.11.020 BindingDB Entry DOI: 10.7270/Q2FQ9WX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nischarin (RAT) | BDBM50334207 (5-Chloro-3-[(imidazolidin-2-yl)imino]indazole hydr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Gdansk Curated by ChEMBL | Assay Description Displacement of [3H]2BFI from imidazoline I1 receptor in Sprague-Dawley rat brain membrane after 45 mins by liquid scintillation counting | Bioorg Med Chem 19: 321-9 (2011) Article DOI: 10.1016/j.bmc.2010.11.020 BindingDB Entry DOI: 10.7270/Q2FQ9WX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nischarin (RAT) | BDBM50334195 (4-Chloro-3-[(imidazolidin-2-yl)imino]indazole hydr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Gdansk Curated by ChEMBL | Assay Description Displacement of [3H]clonidine from imidazoline I1 receptor in Sprague-Dawley rat kidney membrane after 45 mins by liquid scintillation counting | Bioorg Med Chem 19: 321-9 (2011) Article DOI: 10.1016/j.bmc.2010.11.020 BindingDB Entry DOI: 10.7270/Q2FQ9WX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nischarin (RAT) | BDBM50334203 (6-Chloro-3-[(imidazolidin-2-yl)imino]indazole hydr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Gdansk Curated by ChEMBL | Assay Description Displacement of [3H]clonidine from imidazoline I1 receptor in Sprague-Dawley rat kidney membrane after 45 mins by liquid scintillation counting | Bioorg Med Chem 19: 321-9 (2011) Article DOI: 10.1016/j.bmc.2010.11.020 BindingDB Entry DOI: 10.7270/Q2FQ9WX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nischarin (RAT) | BDBM50334194 (3-[(Imidazolidin-2-yl)imino]indazole hydrochloride...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Gdansk Curated by ChEMBL | Assay Description Displacement of [3H]clonidine from imidazoline I1 receptor in Sprague-Dawley rat kidney membrane after 45 mins by liquid scintillation counting | Bioorg Med Chem 19: 321-9 (2011) Article DOI: 10.1016/j.bmc.2010.11.020 BindingDB Entry DOI: 10.7270/Q2FQ9WX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nischarin (RAT) | BDBM50334197 (7-Chloro-3-[(imidazolidin-2-yl)imino]indazole hydr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Gdansk Curated by ChEMBL | Assay Description Displacement of [3H]clonidine from imidazoline I1 receptor in Sprague-Dawley rat kidney membrane after 45 mins by liquid scintillation counting | Bioorg Med Chem 19: 321-9 (2011) Article DOI: 10.1016/j.bmc.2010.11.020 BindingDB Entry DOI: 10.7270/Q2FQ9WX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nischarin (RAT) | BDBM50334205 (4-Bromo-3-[(imidazolidin-2-yl)imino]indazole hydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Gdansk Curated by ChEMBL | Assay Description Displacement of [3H]clonidine from imidazoline I1 receptor in Sprague-Dawley rat kidney membrane after 45 mins by liquid scintillation counting | Bioorg Med Chem 19: 321-9 (2011) Article DOI: 10.1016/j.bmc.2010.11.020 BindingDB Entry DOI: 10.7270/Q2FQ9WX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nischarin (RAT) | BDBM50334204 (3-[(Imidazolidin-2-yl)imino]-6-methylindazole hydr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Gdansk Curated by ChEMBL | Assay Description Displacement of [3H]clonidine from imidazoline I1 receptor in Sprague-Dawley rat kidney membrane after 45 mins by liquid scintillation counting | Bioorg Med Chem 19: 321-9 (2011) Article DOI: 10.1016/j.bmc.2010.11.020 BindingDB Entry DOI: 10.7270/Q2FQ9WX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nischarin (RAT) | BDBM50334208 (3-[(Imidazolidin-2-yl)imino]-5-methylindazole hydr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Gdansk Curated by ChEMBL | Assay Description Displacement of [3H]clonidine from imidazoline I1 receptor in Sprague-Dawley rat kidney membrane after 45 mins by liquid scintillation counting | Bioorg Med Chem 19: 321-9 (2011) Article DOI: 10.1016/j.bmc.2010.11.020 BindingDB Entry DOI: 10.7270/Q2FQ9WX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nischarin (RAT) | BDBM50334206 (3-[(Imidazolidin-2-yl)imino]-4-methoxyindazole hyd...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Gdansk Curated by ChEMBL | Assay Description Displacement of [3H]clonidine from imidazoline I1 receptor in Sprague-Dawley rat kidney membrane after 45 mins by liquid scintillation counting | Bioorg Med Chem 19: 321-9 (2011) Article DOI: 10.1016/j.bmc.2010.11.020 BindingDB Entry DOI: 10.7270/Q2FQ9WX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nischarin (RAT) | BDBM50334196 (3-[(Imidazolidin-2-yl)imino]-4-methylindazole hydr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Gdansk Curated by ChEMBL | Assay Description Displacement of [3H]clonidine from imidazoline I1 receptor in Sprague-Dawley rat kidney membrane after 45 mins by liquid scintillation counting | Bioorg Med Chem 19: 321-9 (2011) Article DOI: 10.1016/j.bmc.2010.11.020 BindingDB Entry DOI: 10.7270/Q2FQ9WX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nischarin (RAT) | BDBM50334202 (3-[(Imidazolidin-2-ylo)imino]-5-methoxyindazole hy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Gdansk Curated by ChEMBL | Assay Description Displacement of [3H]clonidine from imidazoline I1 receptor in Sprague-Dawley rat kidney membrane after 45 mins by liquid scintillation counting | Bioorg Med Chem 19: 321-9 (2011) Article DOI: 10.1016/j.bmc.2010.11.020 BindingDB Entry DOI: 10.7270/Q2FQ9WX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nischarin (RAT) | BDBM50334207 (5-Chloro-3-[(imidazolidin-2-yl)imino]indazole hydr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Gdansk Curated by ChEMBL | Assay Description Displacement of [3H]clonidine from imidazoline I1 receptor in Sprague-Dawley rat kidney membrane after 45 mins by liquid scintillation counting | Bioorg Med Chem 19: 321-9 (2011) Article DOI: 10.1016/j.bmc.2010.11.020 BindingDB Entry DOI: 10.7270/Q2FQ9WX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50334197 (7-Chloro-3-[(imidazolidin-2-yl)imino]indazole hydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 73 | n/a | n/a | n/a | n/a |
Medical University of Gdansk Curated by ChEMBL | Assay Description Agonist activity at human recombinant alpha2A adrenergic receptor expressed in CHO cells assessed as induction of [35S]GTPgammaS binding after 60 min... | Bioorg Med Chem 19: 321-9 (2011) Article DOI: 10.1016/j.bmc.2010.11.020 BindingDB Entry DOI: 10.7270/Q2FQ9WX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50334196 (3-[(Imidazolidin-2-yl)imino]-4-methylindazole hydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Medical University of Gdansk Curated by ChEMBL | Assay Description Agonist activity at human recombinant alpha2A adrenergic receptor expressed in CHO cells assessed as induction of [35S]GTPgammaS binding after 60 min... | Bioorg Med Chem 19: 321-9 (2011) Article DOI: 10.1016/j.bmc.2010.11.020 BindingDB Entry DOI: 10.7270/Q2FQ9WX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50334195 (4-Chloro-3-[(imidazolidin-2-yl)imino]indazole hydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 240 | n/a | n/a | n/a | n/a |
Medical University of Gdansk Curated by ChEMBL | Assay Description Agonist activity at human recombinant alpha2A adrenergic receptor expressed in CHO cells assessed as induction of [35S]GTPgammaS binding after 60 min... | Bioorg Med Chem 19: 321-9 (2011) Article DOI: 10.1016/j.bmc.2010.11.020 BindingDB Entry DOI: 10.7270/Q2FQ9WX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50334194 (3-[(Imidazolidin-2-yl)imino]indazole hydrochloride...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 82 | n/a | n/a | n/a | n/a |
Medical University of Gdansk Curated by ChEMBL | Assay Description Agonist activity at human recombinant alpha2A adrenergic receptor expressed in CHO cells assessed as induction of [35S]GTPgammaS binding after 60 min... | Bioorg Med Chem 19: 321-9 (2011) Article DOI: 10.1016/j.bmc.2010.11.020 BindingDB Entry DOI: 10.7270/Q2FQ9WX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50029051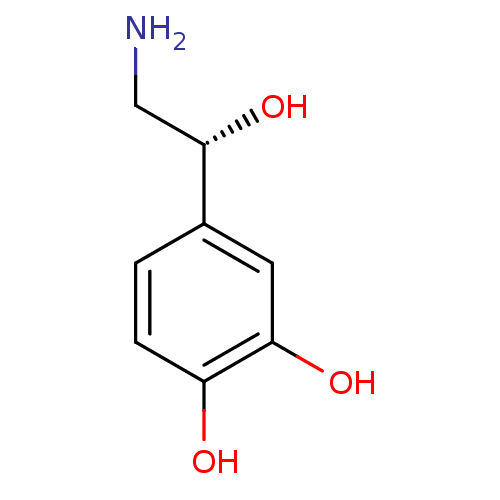 ((-)-arterenol | (-)-noradrenaline | (-)-norepineph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a |
Medical University of Gdansk Curated by ChEMBL | Assay Description Agonist activity at human recombinant alpha2A adrenergic receptor expressed in CHO cells assessed as induction of [35S]GTPgammaS binding after 60 min... | Bioorg Med Chem 19: 321-9 (2011) Article DOI: 10.1016/j.bmc.2010.11.020 BindingDB Entry DOI: 10.7270/Q2FQ9WX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50085683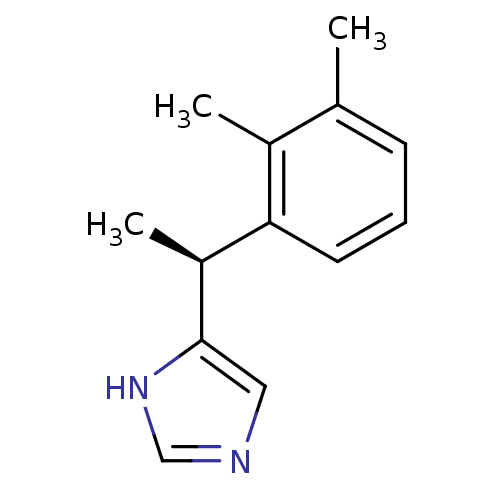 ((+)-4-((S)-alpha,2,3-trimethylbenzyl)imidazole | 4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a |
Medical University of Gdansk Curated by ChEMBL | Assay Description Agonist activity at human recombinant alpha2A adrenergic receptor expressed in CHO cells assessed as induction of [35S]GTPgammaS binding after 60 min... | Bioorg Med Chem 19: 321-9 (2011) Article DOI: 10.1016/j.bmc.2010.11.020 BindingDB Entry DOI: 10.7270/Q2FQ9WX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50016897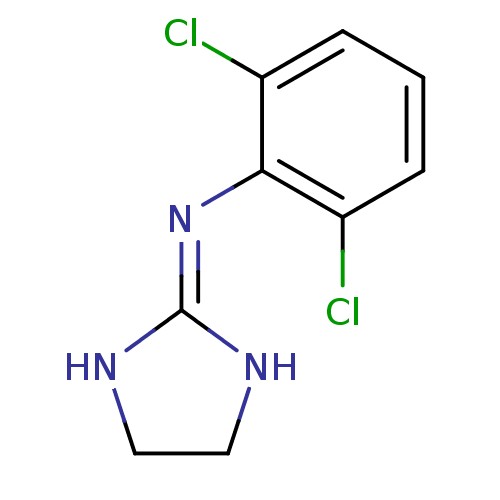 (2-(2,6-dichloroanilino)-1,3-diazacyclopentene-(2) ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 28 | n/a | n/a | n/a | n/a |
Medical University of Gdansk Curated by ChEMBL | Assay Description Agonist activity at human recombinant alpha2A adrenergic receptor expressed in CHO cells assessed as induction of [35S]GTPgammaS binding after 60 min... | Bioorg Med Chem 19: 321-9 (2011) Article DOI: 10.1016/j.bmc.2010.11.020 BindingDB Entry DOI: 10.7270/Q2FQ9WX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50271255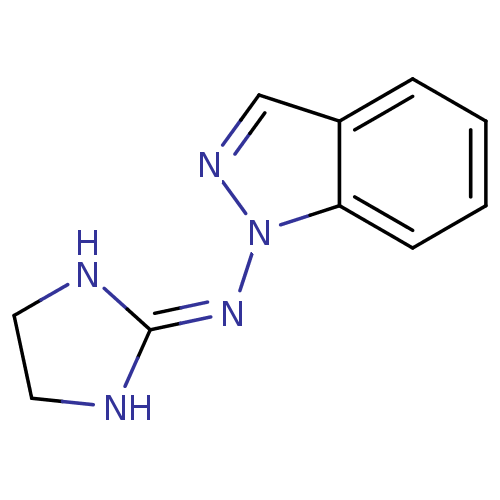 (1-[(Imidazolidin-2-yl)imino]indazole hydrochloride...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 160 | n/a | n/a | n/a | n/a |
Medical University of Gdansk Curated by ChEMBL | Assay Description Agonist activity at human recombinant alpha2A adrenergic receptor expressed in CHO cells assessed as induction of [35S]GTPgammaS binding after 60 min... | Bioorg Med Chem 19: 321-9 (2011) Article DOI: 10.1016/j.bmc.2010.11.020 BindingDB Entry DOI: 10.7270/Q2FQ9WX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50334201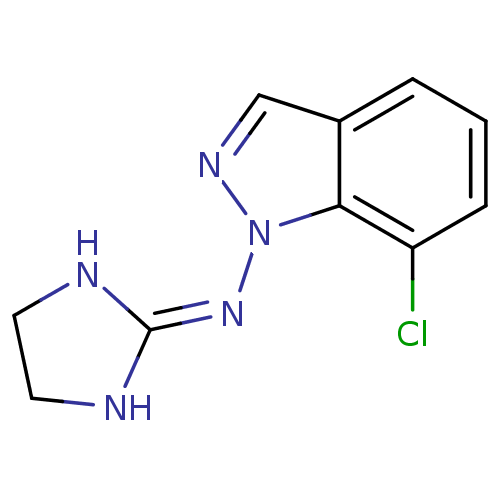 (7-Cl-Marsanidine | CHEMBL1641690) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
Medical University of Gdansk Curated by ChEMBL | Assay Description Agonist activity at human recombinant alpha2A adrenergic receptor expressed in CHO cells assessed as induction of [35S]GTPgammaS binding after 60 min... | Bioorg Med Chem 19: 321-9 (2011) Article DOI: 10.1016/j.bmc.2010.11.020 BindingDB Entry DOI: 10.7270/Q2FQ9WX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50270556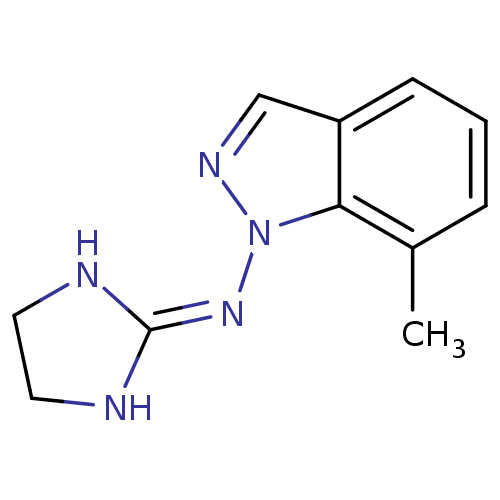 (1-[(Imidazolidin-2-yl)imino]-7-methylindazole hydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 37 | n/a | n/a | n/a | n/a |
Medical University of Gdansk Curated by ChEMBL | Assay Description Agonist activity at human recombinant alpha2A adrenergic receptor expressed in CHO cells assessed as induction of [35S]GTPgammaS binding after 60 min... | Bioorg Med Chem 19: 321-9 (2011) Article DOI: 10.1016/j.bmc.2010.11.020 BindingDB Entry DOI: 10.7270/Q2FQ9WX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50271294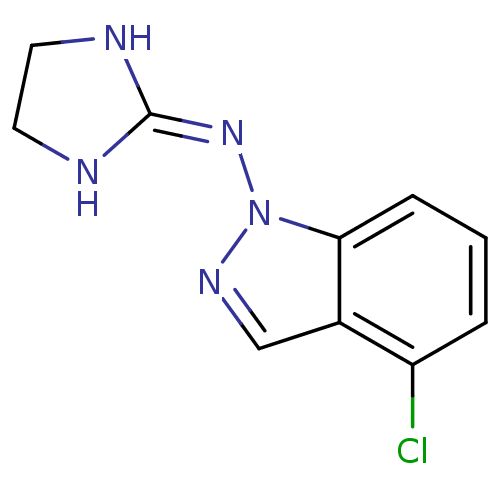 (4-Chloro-1-[(imidazolidin-2-yl)imino]indazole hydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 180 | n/a | n/a | n/a | n/a |
Medical University of Gdansk Curated by ChEMBL | Assay Description Agonist activity at human recombinant alpha2A adrenergic receptor expressed in CHO cells assessed as induction of [35S]GTPgammaS binding after 60 min... | Bioorg Med Chem 19: 321-9 (2011) Article DOI: 10.1016/j.bmc.2010.11.020 BindingDB Entry DOI: 10.7270/Q2FQ9WX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||