

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50358089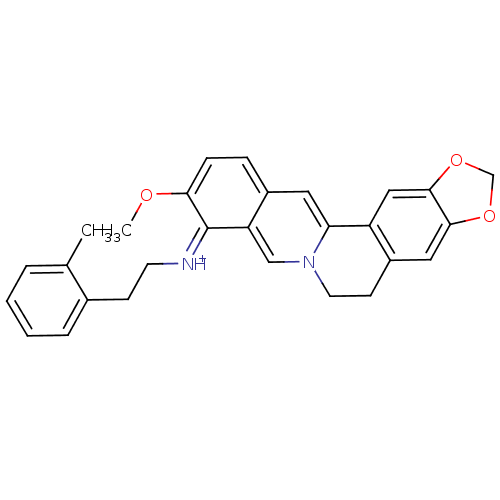 (CHEMBL1915203) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride substrate as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50358090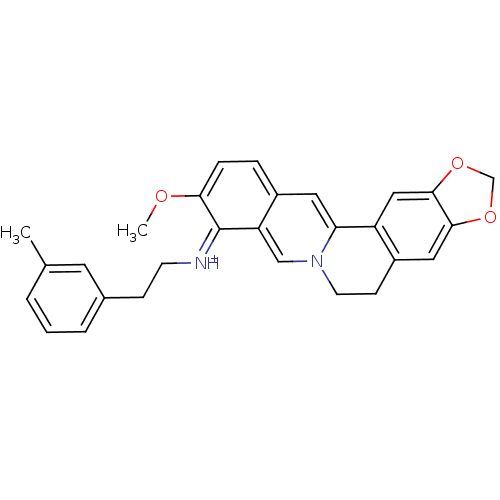 (CHEMBL1915204) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride substrate as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50358093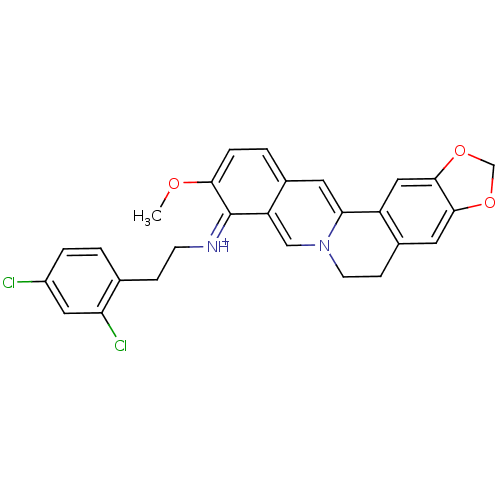 (CHEMBL1915207) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride substrate as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50358092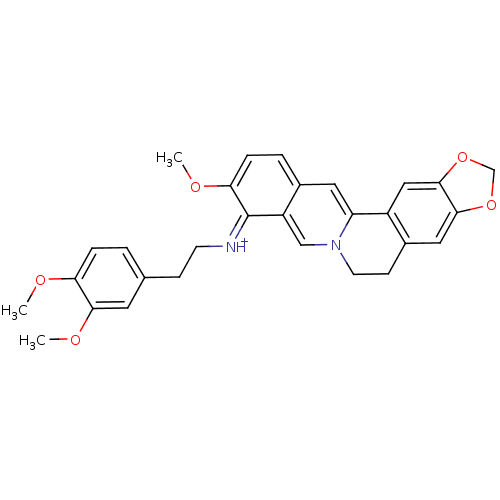 (CHEMBL1915206) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride substrate as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50358081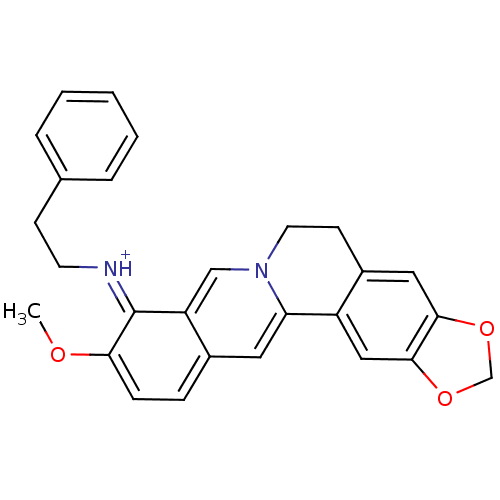 (CHEMBL541672) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 209 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride substrate as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50358074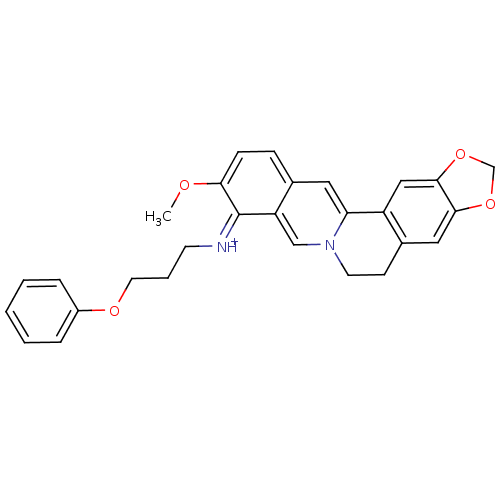 (CHEMBL1915191) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 216 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50358087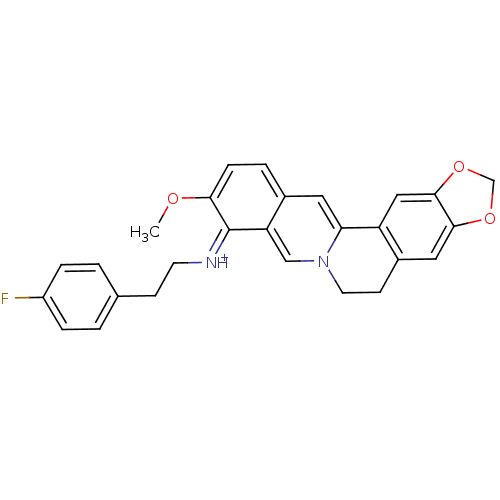 (CHEMBL1915201) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 238 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride substrate as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 311 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride substrate as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50358075 (CHEMBL1915192) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 349 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride substrate as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50203126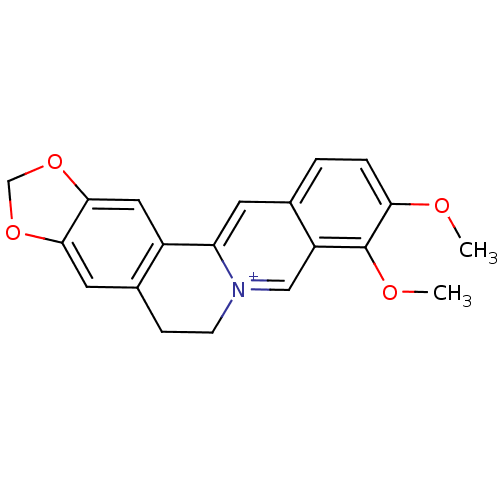 (3,4-dimethoxy-6,7-dihydro-[1,3]dioxolo[4,5-g]pyrid...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 374 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride substrate as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50358094 (CHEMBL1915208) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride substrate as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50358081 (CHEMBL541672) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 406 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50358093 (CHEMBL1915207) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 424 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50358087 (CHEMBL1915201) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 445 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50358091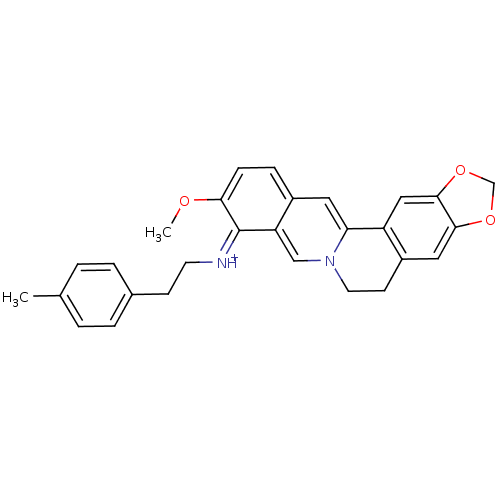 (CHEMBL1915205) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 449 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride substrate as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50358094 (CHEMBL1915208) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 511 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50358084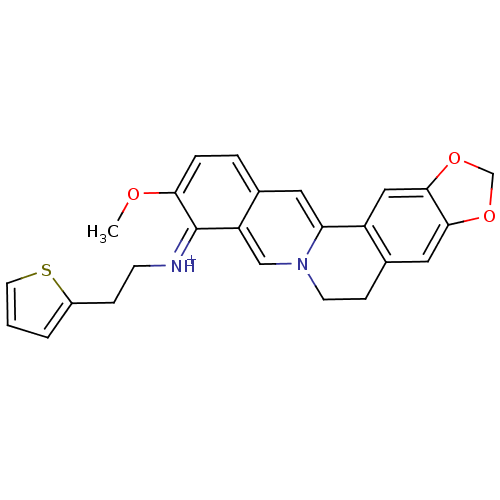 (CHEMBL1915198) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 555 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50358079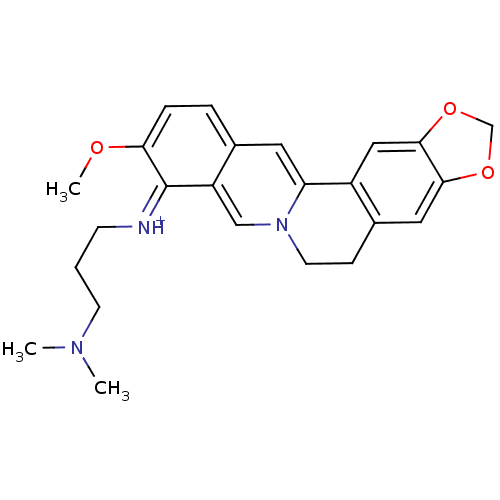 (CHEMBL539374) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 576 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride substrate as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50358074 (CHEMBL1915191) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 588 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride substrate as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50358084 (CHEMBL1915198) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 608 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride substrate as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50358090 (CHEMBL1915204) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 674 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50358082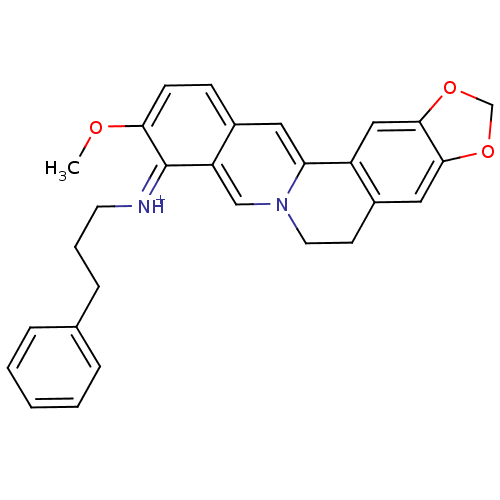 (CHEMBL1915196) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride substrate as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50358073 (CHEMBL1915190) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 688 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride substrate as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50358088 (CHEMBL1915202) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 693 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride substrate as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50358089 (CHEMBL1915203) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 713 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50358073 (CHEMBL1915190) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 727 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50358082 (CHEMBL1915196) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 785 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50358091 (CHEMBL1915205) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 821 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50358088 (CHEMBL1915202) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 841 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50358078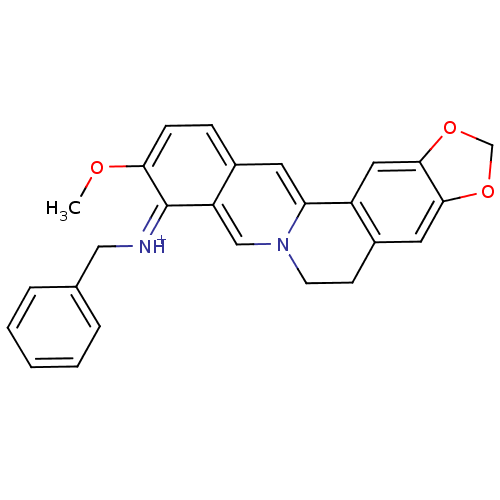 (CHEMBL1915195) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 936 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride substrate as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50358086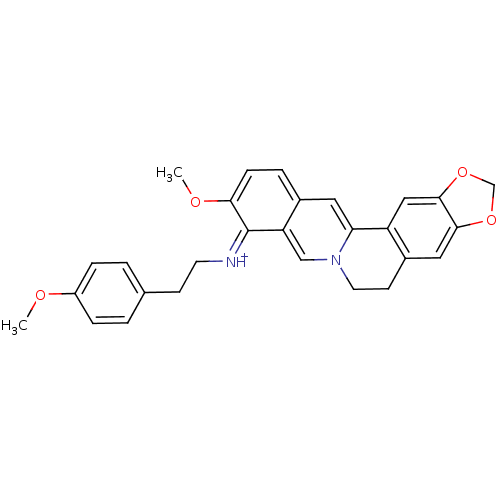 (CHEMBL1915200) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 990 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride substrate as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50358076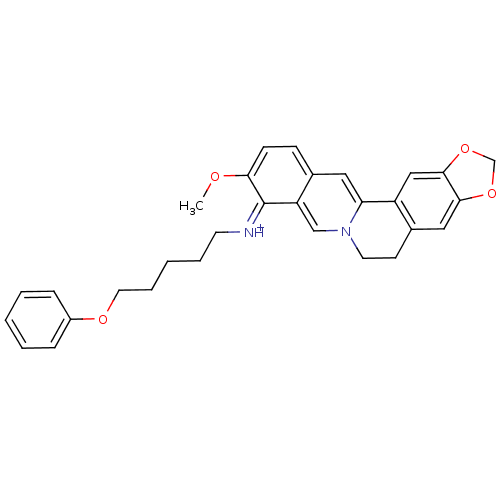 (CHEMBL1915193) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride substrate as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50358097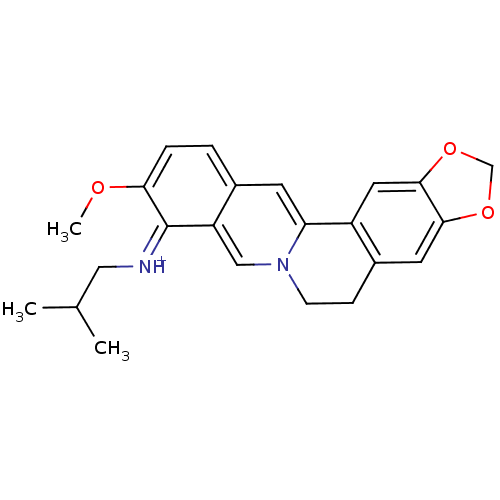 (CHEMBL1915211) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride substrate as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50358085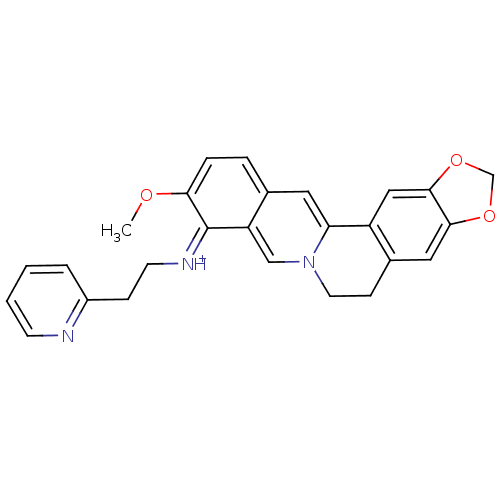 (CHEMBL1915199) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride substrate as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50358095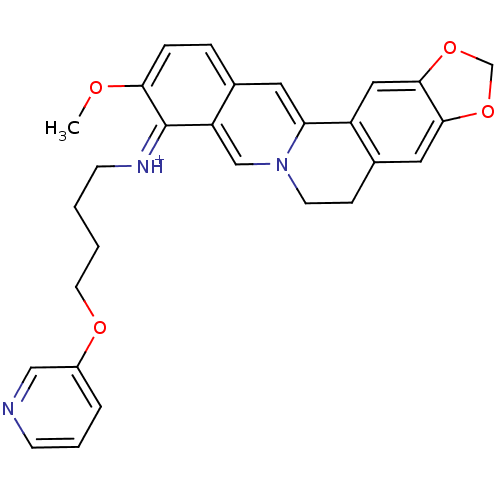 (CHEMBL1915209) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride substrate as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50358075 (CHEMBL1915192) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50358083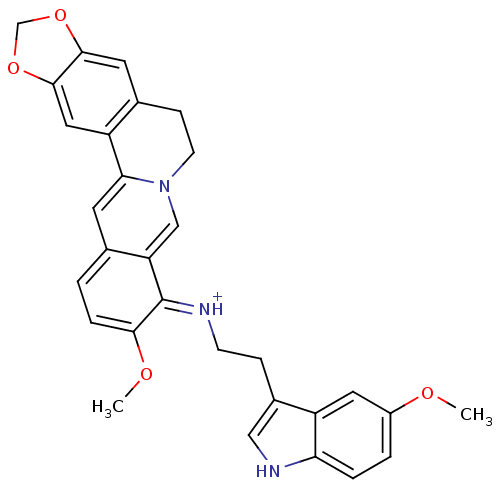 (CHEMBL1915197) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride substrate as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50358077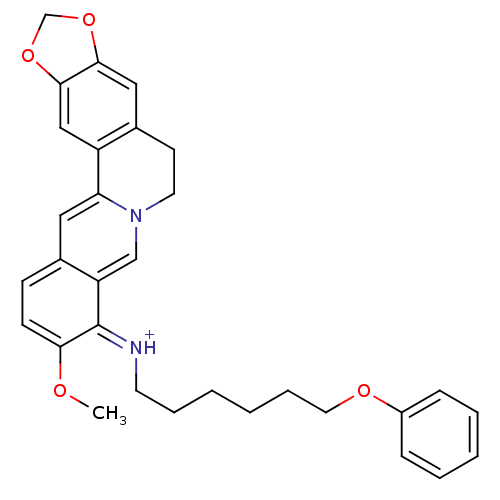 (CHEMBL1915194) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50358083 (CHEMBL1915197) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50358077 (CHEMBL1915194) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride substrate as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50358076 (CHEMBL1915193) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50358096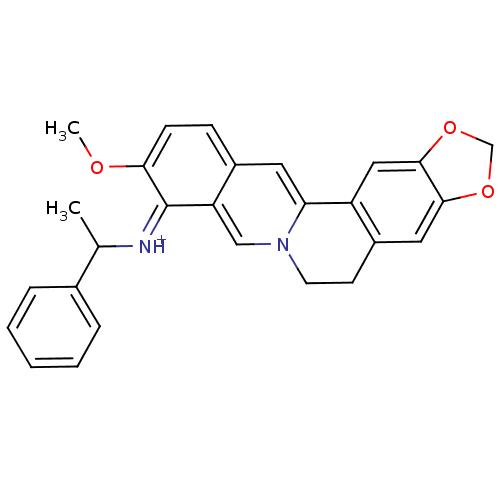 (CHEMBL1915210) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride substrate as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50358097 (CHEMBL1915211) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50358078 (CHEMBL1915195) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50358085 (CHEMBL1915199) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50358086 (CHEMBL1915200) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50358095 (CHEMBL1915209) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50358096 (CHEMBL1915210) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50358092 (CHEMBL1915206) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 15 mins by Ellman's method | Eur J Med Chem 46: 5885-93 (2011) Article DOI: 10.1016/j.ejmech.2011.09.051 BindingDB Entry DOI: 10.7270/Q2Z60PGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 52 total ) | Next | Last >> |