 Found 69 hits Enz. Inhib. hit(s) with all data for entry = 50037477
Found 69 hits Enz. Inhib. hit(s) with all data for entry = 50037477 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50032162
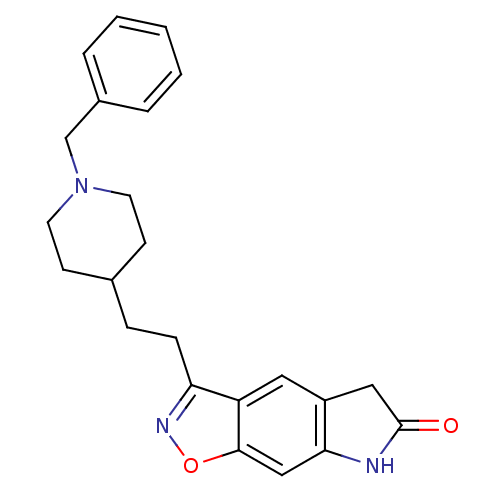
(3-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-5,7-dihydro-...)Show SMILES O=C1Cc2cc3c(CCC4CCN(Cc5ccccc5)CC4)noc3cc2N1 Show InChI InChI=1S/C23H25N3O2/c27-23-13-18-12-19-20(25-28-22(19)14-21(18)24-23)7-6-16-8-10-26(11-9-16)15-17-4-2-1-3-5-17/h1-5,12,14,16H,6-11,13,15H2,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50154795
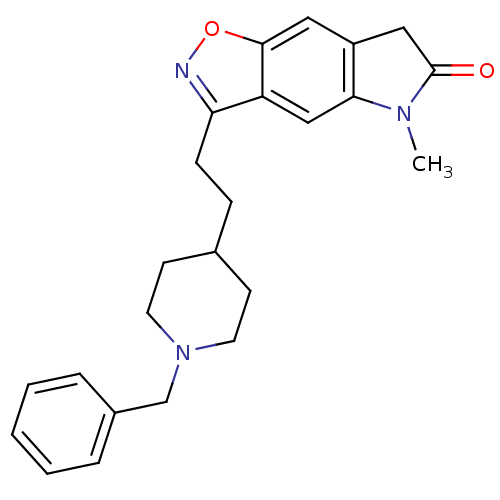
(3-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-5-methyl-5,7...)Show SMILES CN1C(=O)Cc2cc3onc(CCC4CCN(Cc5ccccc5)CC4)c3cc12 Show InChI InChI=1S/C24H27N3O2/c1-26-22-15-20-21(25-29-23(20)13-19(22)14-24(26)28)8-7-17-9-11-27(12-10-17)16-18-5-3-2-4-6-18/h2-6,13,15,17H,7-12,14,16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50032164
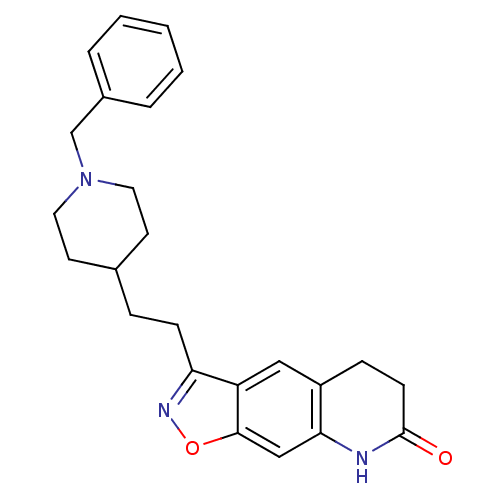
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-5,6-dihydroiso...)Show SMILES O=C1CCc2cc3c(CCC4CCN(Cc5ccccc5)CC4)noc3cc2N1 Show InChI InChI=1S/C24H27N3O2/c28-24-9-7-19-14-20-21(26-29-23(20)15-22(19)25-24)8-6-17-10-12-27(13-11-17)16-18-4-2-1-3-5-18/h1-5,14-15,17H,6-13,16H2,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039721
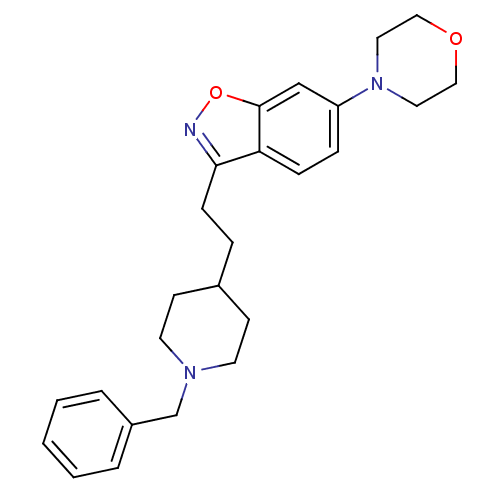
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-6-morpholinobe...)Show SMILES C(Cc1noc2cc(ccc12)N1CCOCC1)C1CCN(Cc2ccccc2)CC1 Show InChI InChI=1S/C25H31N3O2/c1-2-4-21(5-3-1)19-27-12-10-20(11-13-27)6-9-24-23-8-7-22(18-25(23)30-26-24)28-14-16-29-17-15-28/h1-5,7-8,18,20H,6,9-17,19H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50032163
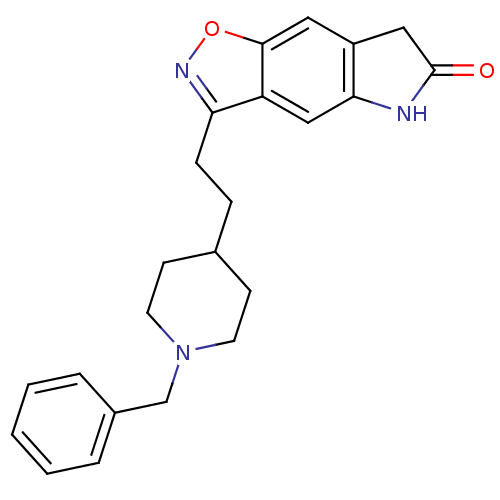
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-5H-isoxazolo[5...)Show SMILES O=C1Cc2cc3onc(CCC4CCN(Cc5ccccc5)CC4)c3cc2N1 Show InChI InChI=1S/C23H25N3O2/c27-23-13-18-12-22-19(14-21(18)24-23)20(25-28-22)7-6-16-8-10-26(11-9-16)15-17-4-2-1-3-5-17/h1-5,12,14,16H,6-11,13,15H2,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50154774
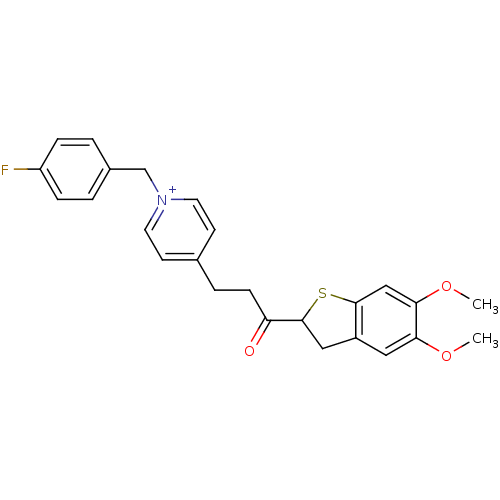
(4-[3-(5,6-Dimethoxy-2,3-dihydro-benzo[b]thiophen-2...)Show SMILES COc1cc2CC(Sc2cc1OC)C(=O)CCc1cc[n+](Cc2ccc(F)cc2)cc1 Show InChI InChI=1S/C25H25FNO3S/c1-29-22-13-19-14-25(31-24(19)15-23(22)30-2)21(28)8-5-17-9-11-27(12-10-17)16-18-3-6-20(26)7-4-18/h3-4,6-7,9-13,15,25H,5,8,14,16H2,1-2H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50032165
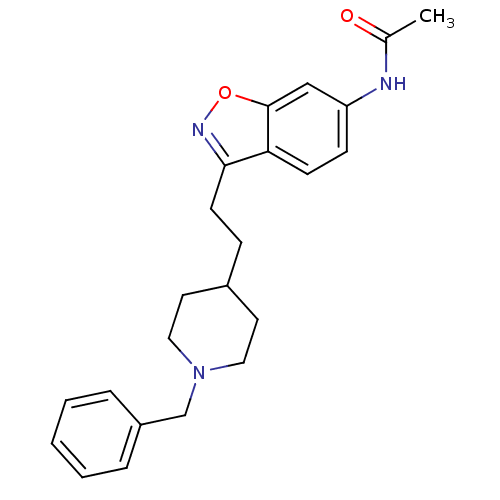
(CHEMBL92736 | CHEMBL94217 | N-{3-[2-(1-Benzyl-pipe...)Show SMILES CC(=O)Nc1ccc2c(CCC3CCN(Cc4ccccc4)CC3)noc2c1 Show InChI InChI=1S/C23H27N3O2/c1-17(27)24-20-8-9-21-22(25-28-23(21)15-20)10-7-18-11-13-26(14-12-18)16-19-5-3-2-4-6-19/h2-6,8-9,15,18H,7,10-14,16H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50032160
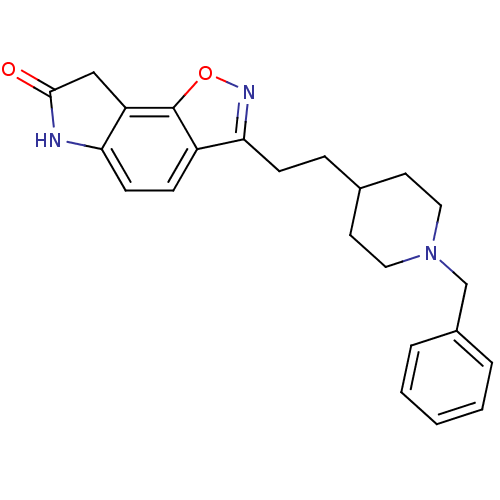
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-6H-isoxazolo[5...)Show SMILES O=C1Cc2c(N1)ccc1c(CCC3CCN(Cc4ccccc4)CC3)noc21 Show InChI InChI=1S/C23H25N3O2/c27-22-14-19-20(24-22)9-7-18-21(25-28-23(18)19)8-6-16-10-12-26(13-11-16)15-17-4-2-1-3-5-17/h1-5,7,9,16H,6,8,10-15H2,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50370522
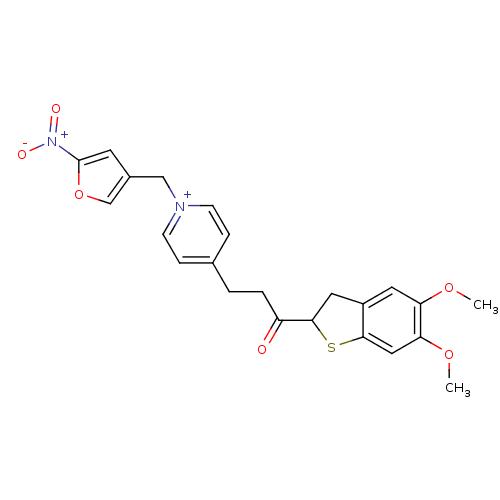
(CHEMBL610242)Show SMILES COc1cc2CC(Sc2cc1OC)C(=O)CCc1cc[n+](Cc2coc(c2)[N+]([O-])=O)cc1 Show InChI InChI=1S/C23H23N2O6S/c1-29-19-10-17-11-22(32-21(17)12-20(19)30-2)18(26)4-3-15-5-7-24(8-6-15)13-16-9-23(25(27)28)31-14-16/h5-10,12,14,22H,3-4,11,13H2,1-2H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50154771
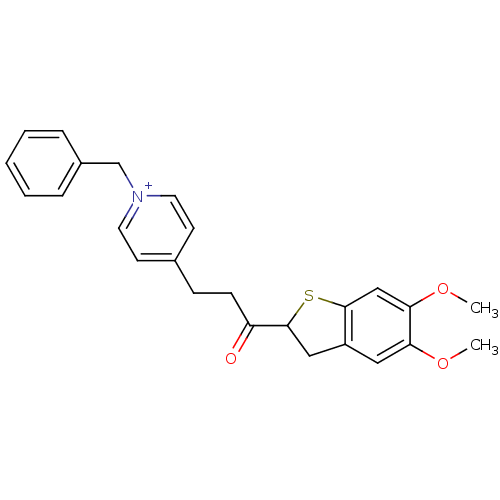
(1-Benzyl-4-[3-(5,6-dimethoxy-2,3-dihydro-benzo[b]t...)Show SMILES COc1cc2CC(Sc2cc1OC)C(=O)CCc1cc[n+](Cc2ccccc2)cc1 Show InChI InChI=1S/C25H26NO3S/c1-28-22-14-20-15-25(30-24(20)16-23(22)29-2)21(27)9-8-18-10-12-26(13-11-18)17-19-6-4-3-5-7-19/h3-7,10-14,16,25H,8-9,15,17H2,1-2H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50154794
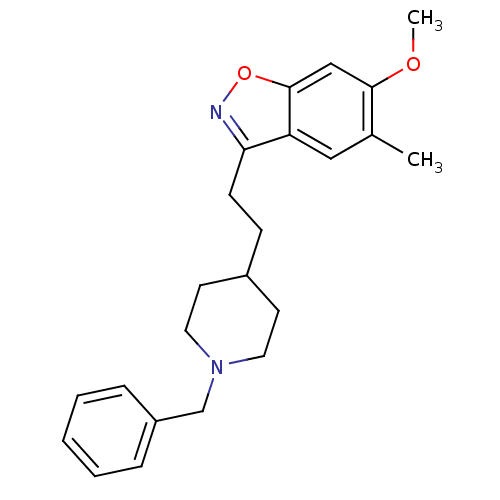
(3-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-6-methoxy-5-...)Show InChI InChI=1S/C23H28N2O2/c1-17-14-20-21(24-27-23(20)15-22(17)26-2)9-8-18-10-12-25(13-11-18)16-19-6-4-3-5-7-19/h3-7,14-15,18H,8-13,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50154799
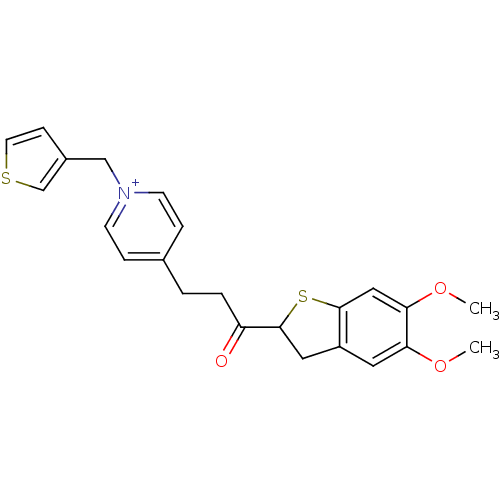
(4-[3-(5,6-Dimethoxy-2,3-dihydro-benzo[b]thiophen-2...)Show SMILES COc1cc2CC(Sc2cc1OC)C(=O)CCc1cc[n+](Cc2ccsc2)cc1 Show InChI InChI=1S/C23H24NO3S2/c1-26-20-11-18-12-23(29-22(18)13-21(20)27-2)19(25)4-3-16-5-8-24(9-6-16)14-17-7-10-28-15-17/h5-11,13,15,23H,3-4,12,14H2,1-2H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50154790
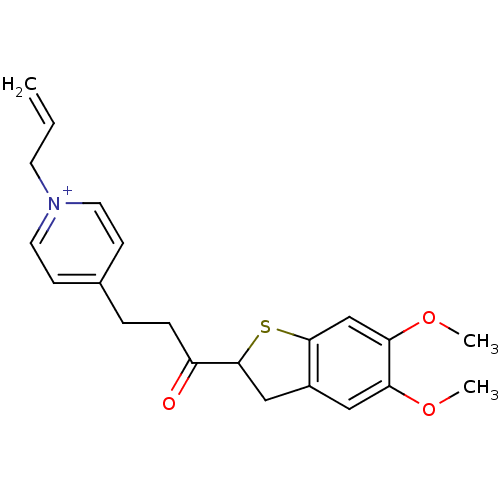
(1-Allyl-4-[3-(5,6-dimethoxy-2,3-dihydro-benzo[b]th...)Show SMILES COc1cc2CC(Sc2cc1OC)C(=O)CCc1cc[n+](CC=C)cc1 Show InChI InChI=1S/C21H24NO3S/c1-4-9-22-10-7-15(8-11-22)5-6-17(23)21-13-16-12-18(24-2)19(25-3)14-20(16)26-21/h4,7-8,10-12,14,21H,1,5-6,9,13H2,2-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.27 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50154780
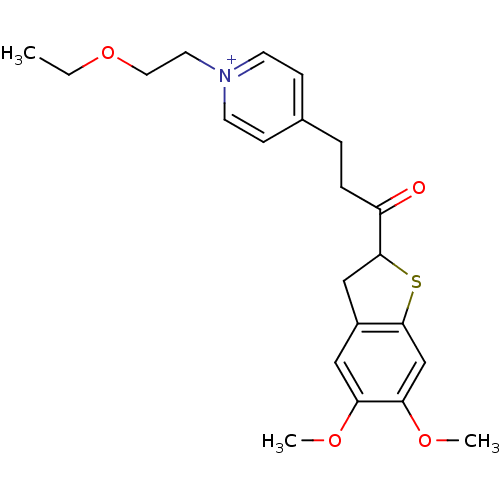
(4-[3-(5,6-Dimethoxy-2,3-dihydro-benzo[b]thiophen-2...)Show SMILES CCOCC[n+]1ccc(CCC(=O)C2Cc3cc(OC)c(OC)cc3S2)cc1 Show InChI InChI=1S/C22H28NO4S/c1-4-27-12-11-23-9-7-16(8-10-23)5-6-18(24)22-14-17-13-19(25-2)20(26-3)15-21(17)28-22/h7-10,13,15,22H,4-6,11-12,14H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039713
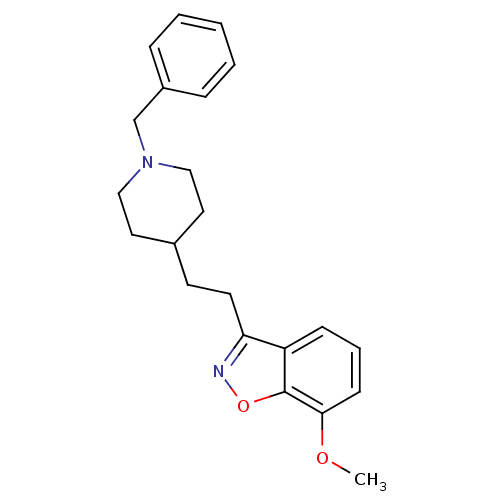
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-7-methoxybenzo...)Show InChI InChI=1S/C22H26N2O2/c1-25-21-9-5-8-19-20(23-26-22(19)21)11-10-17-12-14-24(15-13-17)16-18-6-3-2-4-7-18/h2-9,17H,10-16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039712
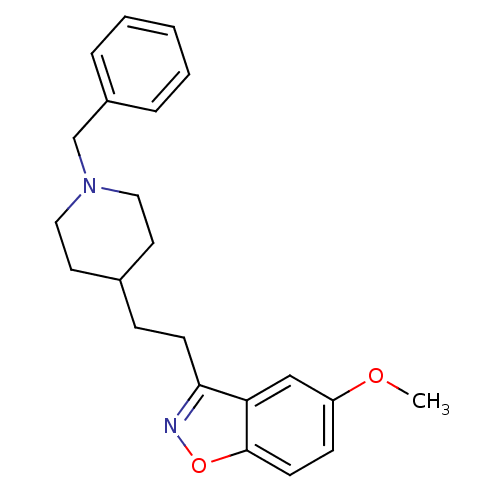
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-5-methoxybenzo...)Show InChI InChI=1S/C22H26N2O2/c1-25-19-8-10-22-20(15-19)21(23-26-22)9-7-17-11-13-24(14-12-17)16-18-5-3-2-4-6-18/h2-6,8,10,15,17H,7,9,11-14,16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039728
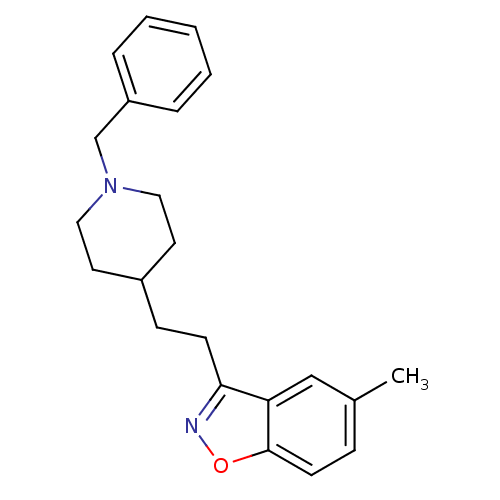
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-5-methylbenzo[...)Show InChI InChI=1S/C22H26N2O/c1-17-7-10-22-20(15-17)21(23-25-22)9-8-18-11-13-24(14-12-18)16-19-5-3-2-4-6-19/h2-7,10,15,18H,8-9,11-14,16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50154797
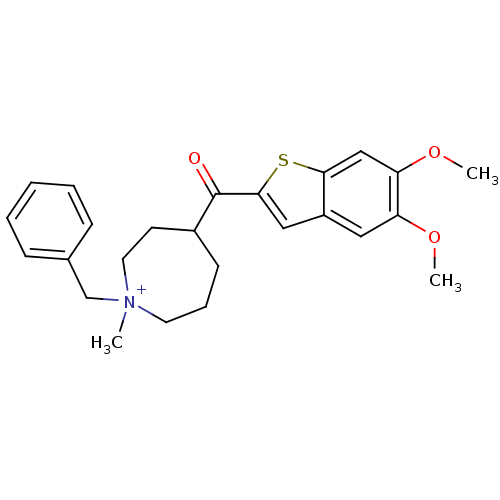
(1-Benzyl-4-(5,6-dimethoxy-benzo[b]thiophene-2-carb...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)C1CCC[N+](C)(Cc2ccccc2)CC1 Show InChI InChI=1S/C25H30NO3S/c1-26(17-18-8-5-4-6-9-18)12-7-10-19(11-13-26)25(27)24-15-20-14-21(28-2)22(29-3)16-23(20)30-24/h4-6,8-9,14-16,19H,7,10-13,17H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039717
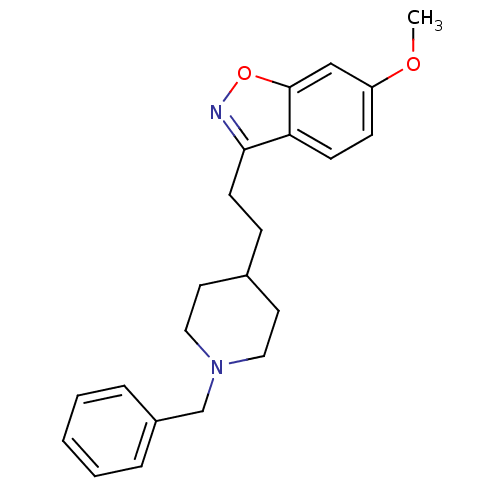
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-6-methoxybenzo...)Show InChI InChI=1S/C22H26N2O2/c1-25-19-8-9-20-21(23-26-22(20)15-19)10-7-17-11-13-24(14-12-17)16-18-5-3-2-4-6-18/h2-6,8-9,15,17H,7,10-14,16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039723
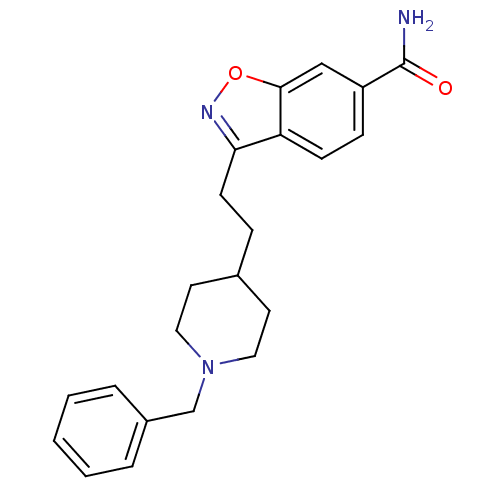
(3-(2-(1-benzylpiperidin-4-yl)ethyl)benzo[d]isoxazo...)Show InChI InChI=1S/C22H25N3O2/c23-22(26)18-7-8-19-20(24-27-21(19)14-18)9-6-16-10-12-25(13-11-16)15-17-4-2-1-3-5-17/h1-5,7-8,14,16H,6,9-13,15H2,(H2,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039730

(3-(3-(1-benzylpiperidin-4-yl)propyl)benzo[d]isoxaz...)Show InChI InChI=1S/C22H26N2O/c1-2-7-19(8-3-1)17-24-15-13-18(14-16-24)9-6-11-21-20-10-4-5-12-22(20)25-23-21/h1-5,7-8,10,12,18H,6,9,11,13-17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039727
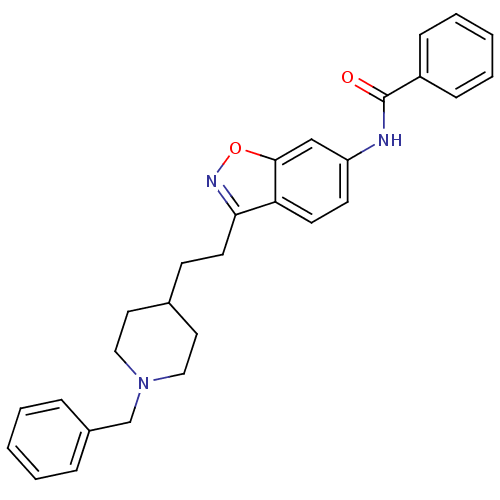
(CHEMBL93123 | N-(3-(2-(1-benzylpiperidin-4-yl)ethy...)Show SMILES O=C(Nc1ccc2c(CCC3CCN(Cc4ccccc4)CC3)noc2c1)c1ccccc1 Show InChI InChI=1S/C28H29N3O2/c32-28(23-9-5-2-6-10-23)29-24-12-13-25-26(30-33-27(25)19-24)14-11-21-15-17-31(18-16-21)20-22-7-3-1-4-8-22/h1-10,12-13,19,21H,11,14-18,20H2,(H,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039725
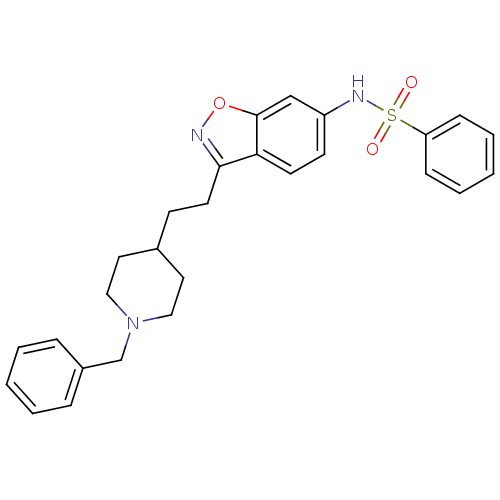
(CHEMBL92955 | N-(3-(2-(1-benzylpiperidin-4-yl)ethy...)Show SMILES O=S(=O)(Nc1ccc2c(CCC3CCN(Cc4ccccc4)CC3)noc2c1)c1ccccc1 Show InChI InChI=1S/C27H29N3O3S/c31-34(32,24-9-5-2-6-10-24)29-23-12-13-25-26(28-33-27(25)19-23)14-11-21-15-17-30(18-16-21)20-22-7-3-1-4-8-22/h1-10,12-13,19,21,29H,11,14-18,20H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039719

(3-(2-(1-benzylpiperidin-4-yl)ethyl)benzo[d]isoxazo...)Show InChI InChI=1S/C21H25N3O/c22-18-7-8-19-20(23-25-21(19)14-18)9-6-16-10-12-24(13-11-16)15-17-4-2-1-3-5-17/h1-5,7-8,14,16H,6,9-13,15,22H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117574

(1-Benzyl-1-methyl-4-[3-(5-methyl-1-phenyl-1H-pyraz...)Show SMILES Cc1cc(nn1-c1ccccc1)C(=O)CCC1CC[N+](C)(Cc2ccccc2)CC1 |(5.62,-.38,;6.31,-1.76,;7.82,-2.01,;8.06,-3.52,;6.68,-4.21,;5.61,-3.13,;4.08,-3.34,;3.13,-2.15,;1.59,-2.36,;1.03,-3.81,;2.01,-5.02,;3.53,-4.78,;9.47,-2.9,;9.64,-1.36,;10.72,-3.8,;12.12,-3.17,;13.38,-3.81,;13.38,-5.37,;14.71,-6.14,;16.04,-5.37,;17.13,-4.27,;17.37,-6.14,;18.7,-5.37,;18.7,-3.83,;20.03,-3.06,;21.37,-3.83,;21.37,-5.37,;20.03,-6.14,;16.05,-3.83,;14.72,-3.06,)| Show InChI InChI=1S/C26H32N3O/c1-21-19-25(27-28(21)24-11-7-4-8-12-24)26(30)14-13-22-15-17-29(2,18-16-22)20-23-9-5-3-6-10-23/h3-12,19,22H,13-18,20H2,1-2H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039731

(3-(2-(1-benzylpiperidin-4-yl)ethyl)benzo[d]isoxazo...)Show InChI InChI=1S/C21H24N2O2/c24-18-7-8-19-20(22-25-21(19)14-18)9-6-16-10-12-23(13-11-16)15-17-4-2-1-3-5-17/h1-5,7-8,14,16,24H,6,9-13,15H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50154789

(4-(5,6-Dimethoxy-benzo[b]thiophene-2-carbonyl)-1-m...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)C1CCC[N+](C)(CC2CCCCO2)CC1 Show InChI InChI=1S/C24H34NO4S/c1-25(16-19-8-4-5-12-29-19)10-6-7-17(9-11-25)24(26)23-14-18-13-20(27-2)21(28-3)15-22(18)30-23/h13-15,17,19H,4-12,16H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50154768
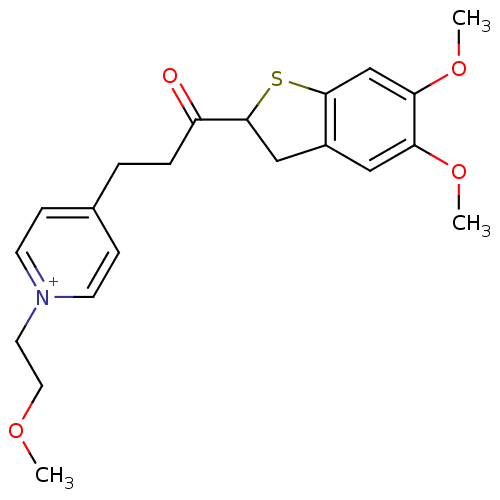
(4-[3-(5,6-Dimethoxy-2,3-dihydro-benzo[b]thiophen-2...)Show SMILES COCC[n+]1ccc(CCC(=O)C2Cc3cc(OC)c(OC)cc3S2)cc1 Show InChI InChI=1S/C21H26NO4S/c1-24-11-10-22-8-6-15(7-9-22)4-5-17(23)21-13-16-12-18(25-2)19(26-3)14-20(16)27-21/h6-9,12,14,21H,4-5,10-11,13H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50370523
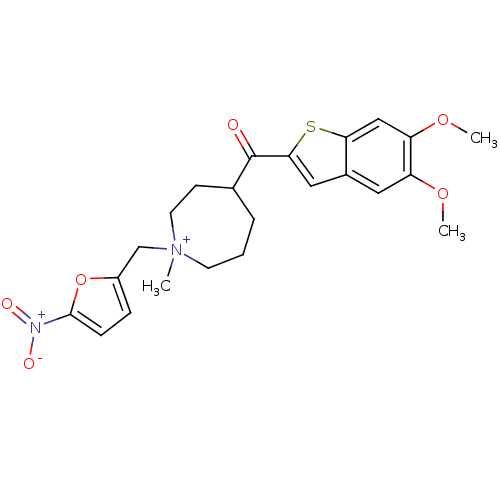
(CHEMBL610243)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)C1CCC[N+](C)(Cc2ccc(o2)[N+]([O-])=O)CC1 Show InChI InChI=1S/C23H27N2O6S/c1-25(14-17-6-7-22(31-17)24(27)28)9-4-5-15(8-10-25)23(26)21-12-16-11-18(29-2)19(30-3)13-20(16)32-21/h6-7,11-13,15H,4-5,8-10,14H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50154779
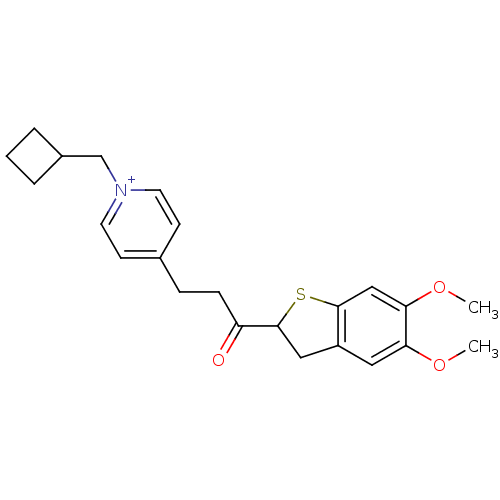
(1-Cyclobutylmethyl-4-[3-(5,6-dimethoxy-2,3-dihydro...)Show SMILES COc1cc2CC(Sc2cc1OC)C(=O)CCc1cc[n+](CC2CCC2)cc1 Show InChI InChI=1S/C23H28NO3S/c1-26-20-12-18-13-23(28-22(18)14-21(20)27-2)19(25)7-6-16-8-10-24(11-9-16)15-17-4-3-5-17/h8-12,14,17,23H,3-7,13,15H2,1-2H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117591
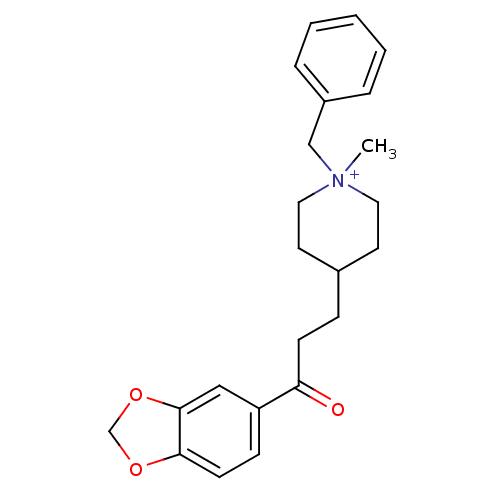
(4-(3-Benzo[1,3]dioxol-5-yl-3-oxo-propyl)-1-benzyl-...)Show SMILES C[N+]1(Cc2ccccc2)CCC(CCC(=O)c2ccc3OCOc3c2)CC1 |(20.14,-5.28,;19.05,-6.38,;20.38,-7.15,;21.72,-6.38,;21.71,-4.84,;23.04,-4.07,;24.38,-4.84,;24.38,-6.38,;23.05,-7.15,;17.72,-7.15,;16.39,-6.37,;16.4,-4.83,;15.14,-4.18,;13.73,-4.81,;12.48,-3.9,;12.65,-2.36,;11.07,-4.53,;10.92,-6.05,;9.52,-6.68,;8.26,-5.79,;6.73,-6.1,;5.97,-4.74,;7.01,-3.6,;8.43,-4.25,;9.83,-3.62,;17.74,-4.07,;19.05,-4.84,)| Show InChI InChI=1S/C23H28NO3/c1-24(16-19-5-3-2-4-6-19)13-11-18(12-14-24)7-9-21(25)20-8-10-22-23(15-20)27-17-26-22/h2-6,8,10,15,18H,7,9,11-14,16-17H2,1H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50154775
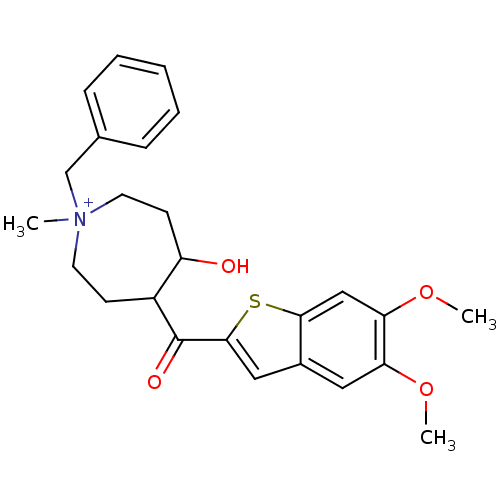
(1-Benzyl-4-(5,6-dimethoxy-benzo[b]thiophene-2-carb...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)C1CC[N+](C)(Cc2ccccc2)CCC1O Show InChI InChI=1S/C25H30NO4S/c1-26(16-17-7-5-4-6-8-17)11-9-19(20(27)10-12-26)25(28)24-14-18-13-21(29-2)22(30-3)15-23(18)31-24/h4-8,13-15,19-20,27H,9-12,16H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039734

(3-(2-(1-benzylpiperidin-4-yl)ethyl)-6-bromobenzo[d...)Show InChI InChI=1S/C21H23BrN2O/c22-18-7-8-19-20(23-25-21(19)14-18)9-6-16-10-12-24(13-11-16)15-17-4-2-1-3-5-17/h1-5,7-8,14,16H,6,9-13,15H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117576
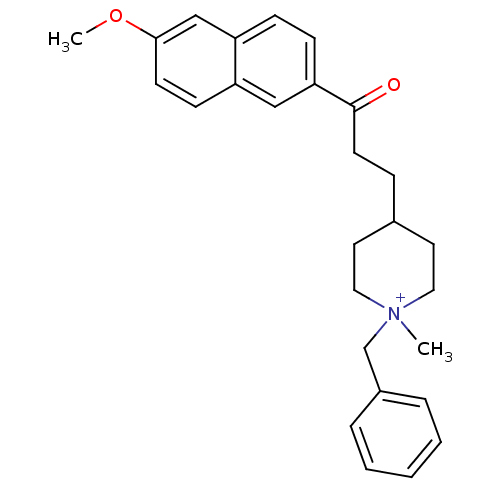
(1-Benzyl-4-[3-(6-methoxy-naphthalen-2-yl)-3-oxo-pr...)Show SMILES COc1ccc2cc(ccc2c1)C(=O)CCC1CC[N+](C)(Cc2ccccc2)CC1 |(-3.21,-6.68,;-1.98,-7.59,;-.58,-6.98,;-.39,-5.42,;1.03,-4.81,;2.27,-5.74,;3.67,-5.12,;4.91,-6.03,;4.76,-7.54,;3.36,-8.17,;2.08,-7.28,;.68,-7.89,;6.32,-5.4,;6.49,-3.86,;7.57,-6.3,;8.98,-5.68,;10.24,-6.32,;11.58,-5.56,;12.9,-6.33,;12.89,-7.87,;13.98,-6.77,;14.22,-8.64,;15.56,-7.87,;16.89,-8.64,;18.22,-7.87,;18.22,-6.33,;16.88,-5.56,;15.55,-6.33,;11.56,-8.64,;10.23,-7.86,)| Show InChI InChI=1S/C27H32NO2/c1-28(20-22-6-4-3-5-7-22)16-14-21(15-17-28)8-13-27(29)25-10-9-24-19-26(30-2)12-11-23(24)18-25/h3-7,9-12,18-19,21H,8,13-17,20H2,1-2H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50154777
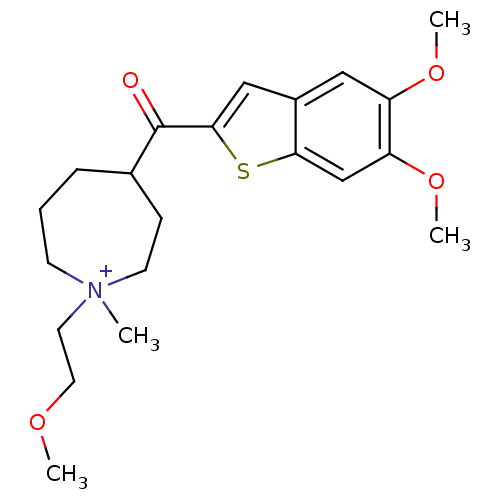
(4-(5,6-Dimethoxy-benzo[b]thiophene-2-carbonyl)-1-(...)Show SMILES COCC[N+]1(C)CCCC(CC1)C(=O)c1cc2cc(OC)c(OC)cc2s1 Show InChI InChI=1S/C21H30NO4S/c1-22(10-11-24-2)8-5-6-15(7-9-22)21(23)20-13-16-12-17(25-3)18(26-4)14-19(16)27-20/h12-15H,5-11H2,1-4H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50154782

(4-[3-(5,6-Dimethoxy-2,3-dihydro-benzo[b]thiophen-2...)Show SMILES COC(=O)C[N+]1(C)CCC(CCC(=O)C2Cc3cc(OC)c(OC)cc3S2)CC1 Show InChI InChI=1S/C22H32NO5S/c1-23(14-22(25)28-4)9-7-15(8-10-23)5-6-17(24)21-12-16-11-18(26-2)19(27-3)13-20(16)29-21/h11,13,15,21H,5-10,12,14H2,1-4H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039716
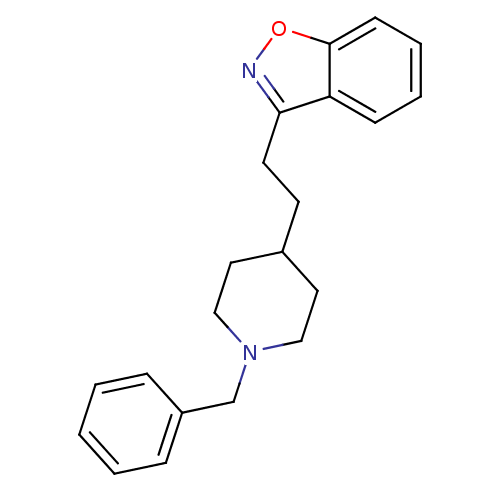
(3-(2-(1-benzylpiperidin-4-yl)ethyl)benzo[d]isoxazo...)Show InChI InChI=1S/C21H24N2O/c1-2-6-18(7-3-1)16-23-14-12-17(13-15-23)10-11-20-19-8-4-5-9-21(19)24-22-20/h1-9,17H,10-16H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039716

(3-(2-(1-benzylpiperidin-4-yl)ethyl)benzo[d]isoxazo...)Show InChI InChI=1S/C21H24N2O/c1-2-6-18(7-3-1)16-23-14-12-17(13-15-23)10-11-20-19-8-4-5-9-21(19)24-22-20/h1-9,17H,10-16H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50154778
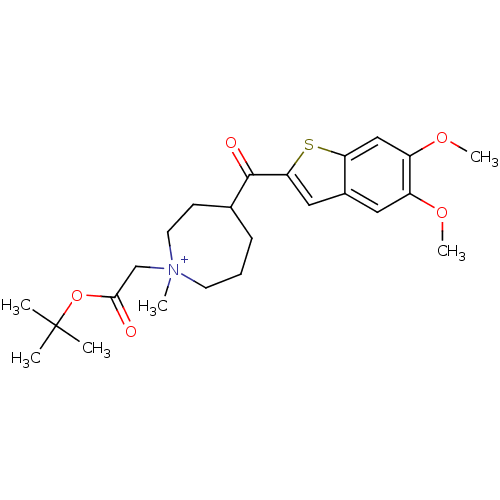
(1-tert-Butoxycarbonylmethyl-4-(5,6-dimethoxy-benzo...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)C1CCC[N+](C)(CC(=O)OC(C)(C)C)CC1 Show InChI InChI=1S/C24H34NO5S/c1-24(2,3)30-22(26)15-25(4)10-7-8-16(9-11-25)23(27)21-13-17-12-18(28-5)19(29-6)14-20(17)31-21/h12-14,16H,7-11,15H2,1-6H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50154786
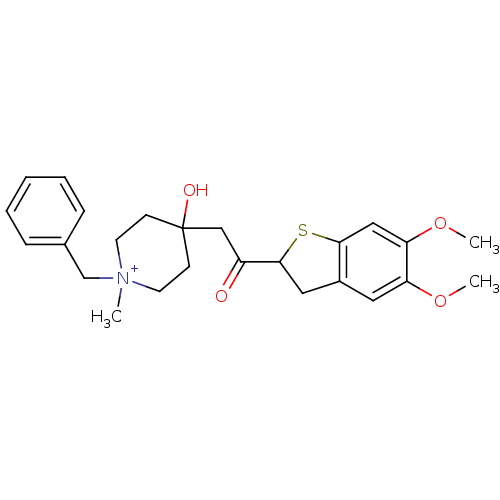
(1-Benzyl-4-[2-(5,6-dimethoxy-2,3-dihydro-benzo[b]t...)Show SMILES COc1cc2CC(Sc2cc1OC)C(=O)CC1(O)CC[N+](C)(Cc2ccccc2)CC1 |(-7.92,2.81,;-6.38,2.86,;-5.57,1.53,;-4.03,1.58,;-3.23,.27,;-1.72,-.01,;-1.51,-1.54,;-2.91,-2.2,;-3.96,-1.1,;-5.5,-1.14,;-6.31,.16,;-7.85,.13,;-8.57,-1.24,;-.16,-2.27,;-.11,-3.8,;1.15,-1.45,;2.52,-2.19,;2.57,-3.73,;2.57,-.65,;3.94,.09,;5.23,-.75,;6.02,-2.08,;6,.6,;5.21,1.93,;3.67,1.9,;2.88,3.23,;3.65,4.59,;5.18,4.59,;5.98,3.29,;5.18,-2.29,;3.81,-2.99,)| Show InChI InChI=1S/C25H32NO4S/c1-26(17-18-7-5-4-6-8-18)11-9-25(28,10-12-26)16-20(27)24-14-19-13-21(29-2)22(30-3)15-23(19)31-24/h4-8,13,15,24,28H,9-12,14,16-17H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039715
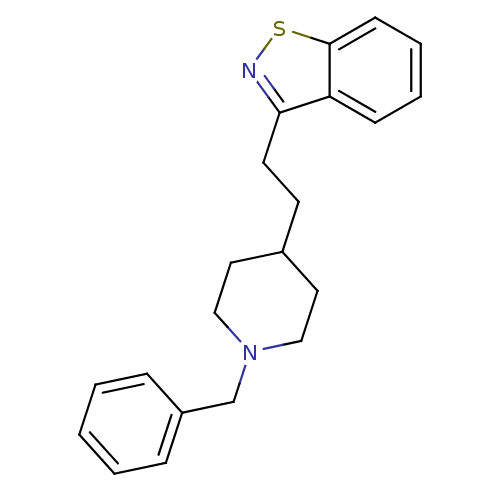
(3-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-benzo[d]isot...)Show InChI InChI=1S/C21H24N2S/c1-2-6-18(7-3-1)16-23-14-12-17(13-15-23)10-11-20-19-8-4-5-9-21(19)24-22-20/h1-9,17H,10-16H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117578
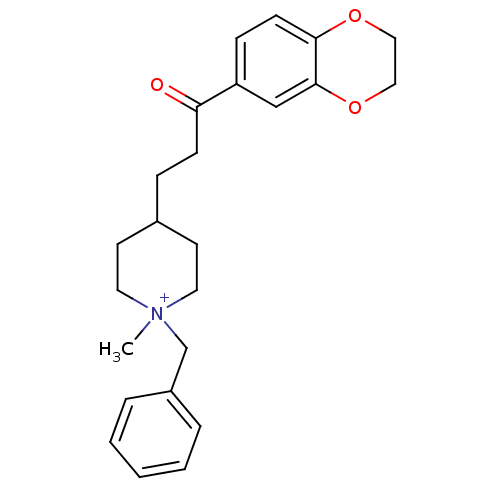
(1-Benzyl-4-[3-(2,3-dihydro-benzo[1,4]dioxin-6-yl)-...)Show SMILES C[N+]1(Cc2ccccc2)CCC(CCC(=O)c2ccc3OCCOc3c2)CC1 |(20.14,-5.28,;19.05,-6.38,;20.38,-7.15,;21.72,-6.38,;23.05,-7.15,;24.38,-6.38,;24.38,-4.84,;23.04,-4.07,;21.71,-4.84,;19.05,-4.84,;17.74,-4.07,;16.4,-4.83,;15.14,-4.18,;13.73,-4.81,;12.48,-3.9,;12.65,-2.36,;11.07,-4.53,;10.92,-6.05,;9.52,-6.68,;8.26,-5.79,;6.84,-6.42,;5.58,-5.49,;5.75,-3.94,;7.19,-3.32,;8.43,-4.25,;9.83,-3.62,;16.39,-6.37,;17.72,-7.15,)| Show InChI InChI=1S/C24H30NO3/c1-25(18-20-5-3-2-4-6-20)13-11-19(12-14-25)7-9-22(26)21-8-10-23-24(17-21)28-16-15-27-23/h2-6,8,10,17,19H,7,9,11-16,18H2,1H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039718

(3-(2-(1-benzylpiperidin-4-yl)ethyl)benzo[d]isoxazo...)Show InChI InChI=1S/C22H23N3O/c23-15-19-6-8-20-21(24-26-22(20)14-19)9-7-17-10-12-25(13-11-17)16-18-4-2-1-3-5-18/h1-6,8,14,17H,7,9-13,16H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50154804
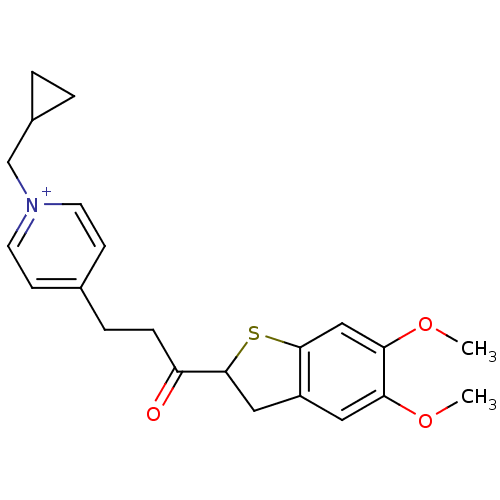
(1-Cyclopropylmethyl-4-[3-(5,6-dimethoxy-2,3-dihydr...)Show SMILES COc1cc2CC(Sc2cc1OC)C(=O)CCc1cc[n+](CC2CC2)cc1 Show InChI InChI=1S/C22H26NO3S/c1-25-19-11-17-12-22(27-21(17)13-20(19)26-2)18(24)6-5-15-7-9-23(10-8-15)14-16-3-4-16/h7-11,13,16,22H,3-6,12,14H2,1-2H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50154796
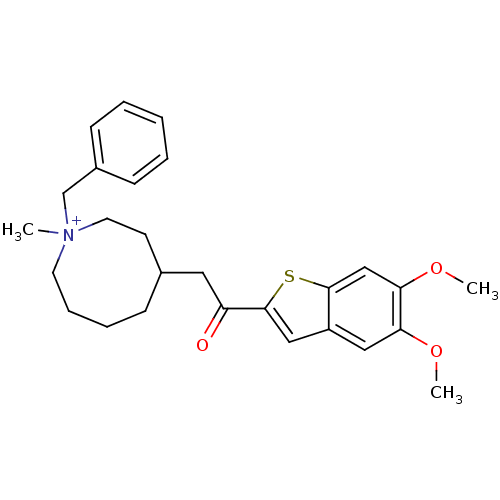
(1-Benzyl-4-[2-(5,6-dimethoxy-benzo[b]thiophen-2-yl...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CC1CCCC[N+](C)(Cc2ccccc2)CC1 Show InChI InChI=1S/C27H34NO3S/c1-28(19-21-10-5-4-6-11-21)13-8-7-9-20(12-14-28)15-23(29)27-17-22-16-24(30-2)25(31-3)18-26(22)32-27/h4-6,10-11,16-18,20H,7-9,12-15,19H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117585
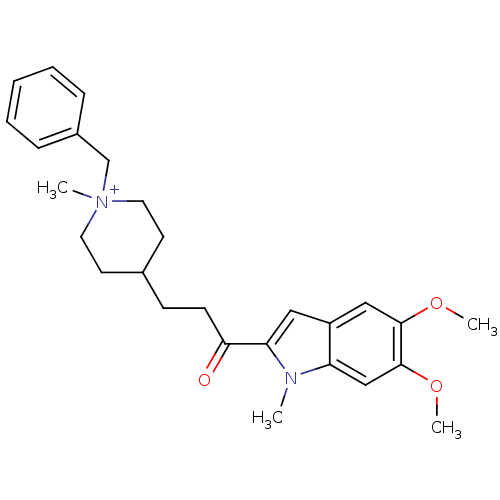
(1-Benzyl-4-[3-(5,6-dimethoxy-1-methyl-1H-indol-2-y...)Show SMILES COc1cc2cc(C(=O)CCC3CC[N+](C)(Cc4ccccc4)CC3)n(C)c2cc1OC |(-2.15,-5.79,;-1.52,-7.21,;.01,-7.37,;.92,-6.12,;2.45,-6.28,;3.58,-5.26,;4.91,-6.03,;6.32,-5.4,;6.49,-3.86,;7.57,-6.3,;8.98,-5.68,;10.24,-6.32,;11.58,-5.56,;12.9,-6.33,;12.89,-7.87,;13.98,-6.77,;14.22,-8.64,;15.56,-7.87,;16.89,-8.64,;18.22,-7.87,;18.22,-6.33,;16.88,-5.56,;15.55,-6.33,;11.56,-8.64,;10.23,-7.86,;4.6,-7.54,;5.63,-8.68,;3.06,-7.68,;2.17,-8.94,;.64,-8.78,;-.25,-10.02,;-1.79,-9.87,)| Show InChI InChI=1S/C27H35N2O3/c1-28-23-18-27(32-4)26(31-3)17-22(23)16-24(28)25(30)11-10-20-12-14-29(2,15-13-20)19-21-8-6-5-7-9-21/h5-9,16-18,20H,10-15,19H2,1-4H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039720

(3-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-1H-indazole ...)Show InChI InChI=1S/C21H25N3/c1-2-6-18(7-3-1)16-24-14-12-17(13-15-24)10-11-21-19-8-4-5-9-20(19)22-23-21/h1-9,17H,10-16H2,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50154798
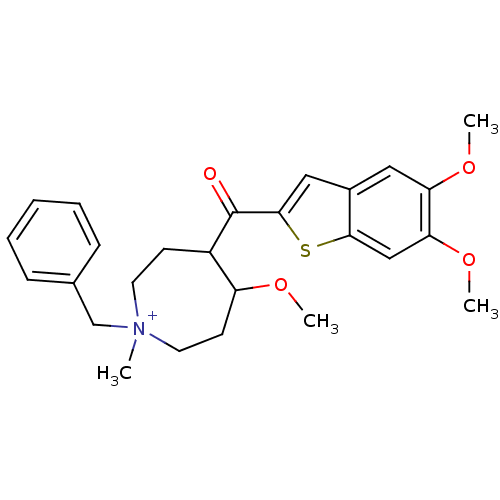
(1-Benzyl-4-(5,6-dimethoxy-benzo[b]thiophene-2-carb...)Show SMILES COC1CC[N+](C)(Cc2ccccc2)CCC1C(=O)c1cc2cc(OC)c(OC)cc2s1 Show InChI InChI=1S/C26H32NO4S/c1-27(17-18-8-6-5-7-9-18)12-10-20(21(29-2)11-13-27)26(28)25-15-19-14-22(30-3)23(31-4)16-24(19)32-25/h5-9,14-16,20-21H,10-13,17H2,1-4H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117579
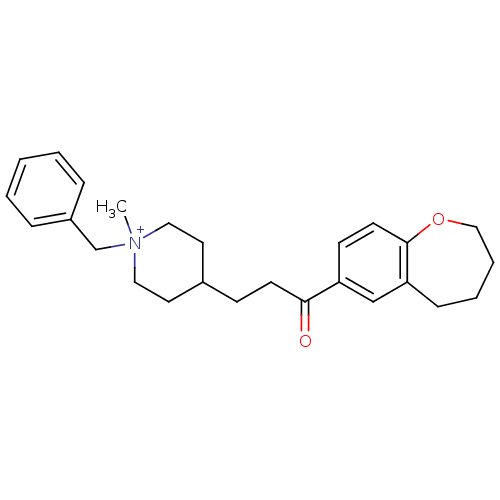
(1-Benzyl-1-methyl-4-[3-oxo-3-(2,3,4,5-tetrahydro-b...)Show SMILES C[N+]1(Cc2ccccc2)CCC(CCC(=O)c2ccc3OCCCCc3c2)CC1 |(13.98,-6.77,;12.89,-7.87,;14.22,-8.64,;15.56,-7.87,;15.55,-6.33,;16.88,-5.56,;18.22,-6.33,;18.22,-7.87,;16.89,-8.64,;11.56,-8.64,;10.23,-7.86,;10.24,-6.32,;8.98,-5.68,;7.57,-6.3,;6.32,-5.4,;6.49,-3.86,;4.91,-6.03,;4.76,-7.54,;3.36,-8.17,;2.08,-7.28,;.75,-8.13,;-.79,-7.68,;-1.38,-6.11,;-.53,-4.72,;1.08,-4.53,;2.27,-5.74,;3.67,-5.12,;11.58,-5.56,;12.9,-6.33,)| Show InChI InChI=1S/C26H34NO2/c1-27(20-22-7-3-2-4-8-22)16-14-21(15-17-27)10-12-25(28)23-11-13-26-24(19-23)9-5-6-18-29-26/h2-4,7-8,11,13,19,21H,5-6,9-10,12,14-18,20H2,1H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 139 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50154801
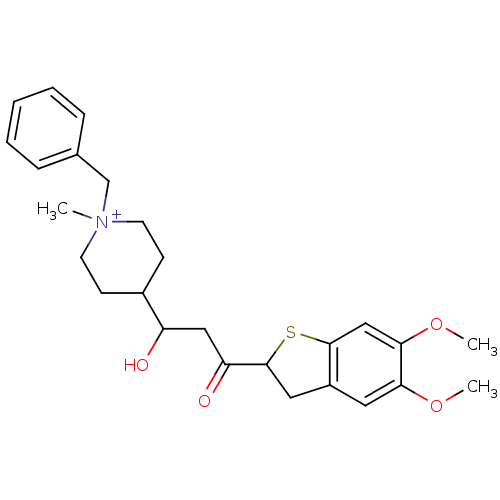
(1-Benzyl-4-[3-(5,6-dimethoxy-2,3-dihydro-benzo[b]t...)Show SMILES COc1cc2CC(Sc2cc1OC)C(=O)CC(O)C1CC[N+](C)(Cc2ccccc2)CC1 |(-8.57,2.53,;-7.02,2.58,;-6.23,1.25,;-4.69,1.28,;-3.89,-.02,;-2.36,-.29,;-2.17,-1.82,;-3.56,-2.5,;-4.62,-1.38,;-6.16,-1.41,;-6.97,-.1,;-8.51,-.15,;-9.25,-1.52,;-.81,-2.55,;-.75,-4.09,;.5,-1.75,;1.87,-2.46,;1.91,-4,;3.17,-1.66,;3.24,-.12,;4.59,.6,;5.89,-.22,;6.68,-1.56,;6.65,1.12,;5.86,2.46,;6.63,3.79,;5.85,5.12,;4.29,5.12,;3.52,3.77,;4.32,2.43,;5.83,-1.76,;4.48,-2.46,)| Show InChI InChI=1S/C26H34NO4S/c1-27(17-18-7-5-4-6-8-18)11-9-19(10-12-27)21(28)15-22(29)26-14-20-13-23(30-2)24(31-3)16-25(20)32-26/h4-8,13,16,19,21,26,28H,9-12,14-15,17H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase enzyme using human AChE assay |
J Med Chem 47: 5492-500 (2004)
Article DOI: 10.1021/jm049695v
BindingDB Entry DOI: 10.7270/Q2765G3W |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data