 Found 61 hits Enz. Inhib. hit(s) with all data for entry = 50037950
Found 61 hits Enz. Inhib. hit(s) with all data for entry = 50037950 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50217458
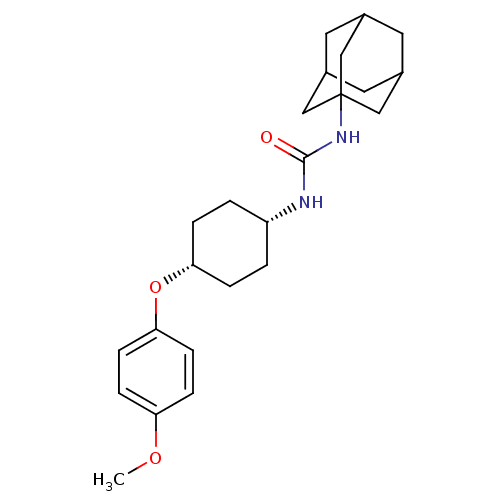
(CHEMBL244192 | cis-1-adamantan-1-yl-3-[4-(4-methox...)Show SMILES COc1ccc(O[C@@H]2CC[C@@H](CC2)NC(=O)NC23CC4CC(CC(C4)C2)C3)cc1 |wU:10.13,7.6,TLB:16:17:20.19.24:22,THB:18:19:22:26.17.25,18:17:20.19.24:22,25:17:20:24.23.22,25:23:20:26.18.17,16:17:20:24.23.22,(5.89,-12.26,;5.07,-10.96,;3.53,-11.02,;2.8,-12.38,;1.27,-12.43,;.45,-11.13,;-1.09,-11.18,;-1.81,-12.54,;-3.35,-12.6,;-4.07,-13.97,;-3.26,-15.26,;-1.71,-15.21,;-.99,-13.85,;-3.97,-16.62,;-5.51,-16.68,;-6.33,-15.38,;-6.23,-18.05,;-7.77,-18.11,;-8.78,-19.39,;-10.19,-18.82,;-11.69,-19.24,;-10.49,-17.97,;-10.5,-16.48,;-9.15,-16.01,;-10.19,-17.24,;-7.75,-16.58,;-9.17,-18.46,;1.17,-9.77,;2.7,-9.71,)| Show InChI InChI=1S/C24H34N2O3/c1-28-20-6-8-22(9-7-20)29-21-4-2-19(3-5-21)25-23(27)26-24-13-16-10-17(14-24)12-18(11-16)15-24/h6-9,16-19,21H,2-5,10-15H2,1H3,(H2,25,26,27)/t16?,17?,18?,19-,21+,24? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50217444
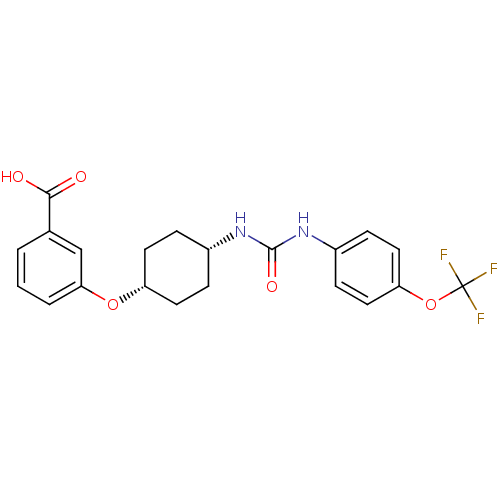
(CHEMBL244002 | cis-4-{4-[3-(4-trifluoromethoxyphen...)Show SMILES OC(=O)c1cccc(O[C@@H]2CC[C@@H](CC2)NC(=O)Nc2ccc(OC(F)(F)F)cc2)c1 |wU:12.15,9.8,(29.99,.67,;30.71,2.03,;32.25,2.08,;29.89,3.33,;30.61,4.69,;29.79,6,;28.25,5.94,;27.54,4.58,;26,4.53,;25.28,3.17,;23.74,3.11,;23.01,1.74,;23.83,.45,;25.38,.5,;26.09,1.86,;23.12,-.91,;21.58,-.97,;20.75,.33,;20.86,-2.34,;19.32,-2.4,;18.51,-1.09,;16.97,-1.15,;16.25,-2.52,;14.71,-2.58,;13.89,-1.28,;13.11,.06,;12.57,-2.08,;15.21,-.49,;17.08,-3.83,;18.62,-3.76,;28.35,3.28,)| Show InChI InChI=1S/C21H21F3N2O5/c22-21(23,24)31-17-10-6-15(7-11-17)26-20(29)25-14-4-8-16(9-5-14)30-18-3-1-2-13(12-18)19(27)28/h1-3,6-7,10-12,14,16H,4-5,8-9H2,(H,27,28)(H2,25,26,29)/t14-,16+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50217457

(CHEMBL397694 | trans-1-adamantan-1-yl-3-[4-(4-nitr...)Show SMILES [O-][N+](=O)c1ccc(O[C@H]2CC[C@@H](CC2)NC(=O)NC23CC4CC(CC(C4)C2)C3)cc1 |wU:8.7,wD:11.14,TLB:17:18:21.20.25:23,THB:19:20:23:27.18.26,19:18:21.20.25:23,26:18:21:25.24.23,26:24:21:27.19.18,17:18:21:25.24.23,(18.29,-12.77,;17.47,-11.46,;18.19,-10.1,;15.92,-11.52,;15.2,-12.88,;13.66,-12.94,;12.85,-11.63,;11.31,-11.69,;10.59,-13.05,;11.4,-14.35,;10.69,-15.71,;9.14,-15.76,;8.32,-14.47,;9.05,-13.1,;8.43,-17.13,;6.89,-17.19,;6.06,-15.89,;6.17,-18.55,;4.63,-18.61,;3.62,-19.89,;2.21,-19.33,;.71,-19.75,;1.91,-18.47,;1.9,-16.98,;3.24,-16.51,;2.2,-17.74,;4.64,-17.09,;3.23,-18.96,;13.56,-10.27,;15.1,-10.21,)| Show InChI InChI=1S/C23H31N3O4/c27-22(25-23-12-15-9-16(13-23)11-17(10-15)14-23)24-18-1-5-20(6-2-18)30-21-7-3-19(4-8-21)26(28)29/h3-4,7-8,15-18,20H,1-2,5-6,9-14H2,(H2,24,25,27)/t15?,16?,17?,18-,20-,23? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50217469
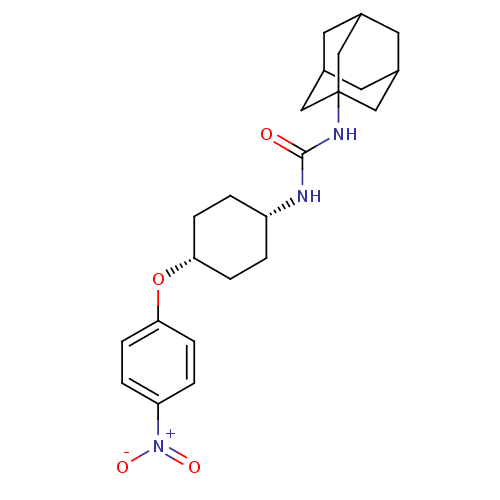
(CHEMBL244193 | cis-1-adamantan-1-yl-3-[4-(4-nitrop...)Show SMILES [O-][N+](=O)c1ccc(O[C@@H]2CC[C@@H](CC2)NC(=O)NC23CC4CC(CC(C4)C2)C3)cc1 |wU:11.14,8.7,TLB:17:18:21.20.25:23,THB:19:20:23:27.18.26,19:18:21.20.25:23,26:18:21:25.24.23,26:24:21:27.19.18,17:18:21:25.24.23,(18.33,-12.89,;17.51,-11.58,;18.23,-10.22,;15.97,-11.64,;15.24,-13,;13.71,-13.06,;12.89,-11.75,;11.35,-11.81,;10.63,-13.17,;9.09,-13.22,;8.37,-14.59,;9.18,-15.88,;10.73,-15.83,;11.45,-14.47,;8.47,-17.25,;6.93,-17.31,;6.11,-16.01,;6.21,-18.67,;4.67,-18.73,;3.66,-20.01,;2.25,-19.45,;.76,-19.87,;1.95,-18.59,;1.94,-17.1,;3.29,-16.63,;2.25,-17.86,;4.69,-17.21,;3.28,-19.08,;13.61,-10.39,;15.14,-10.33,)| Show InChI InChI=1S/C23H31N3O4/c27-22(25-23-12-15-9-16(13-23)11-17(10-15)14-23)24-18-1-5-20(6-2-18)30-21-7-3-19(4-8-21)26(28)29/h3-4,7-8,15-18,20H,1-2,5-6,9-14H2,(H2,24,25,27)/t15?,16?,17?,18-,20+,23? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50217459

(CHEMBL243335 | trans-1-adamantan-1-yl-3-[4-(4-fluo...)Show SMILES Fc1ccc(O[C@H]2CC[C@@H](CC2)NC(=O)NC23CC4CC(CC(C4)C2)C3)cc1 |wU:6.5,wD:9.12,TLB:15:16:19.18.23:21,THB:17:18:21:25.16.24,17:16:19.18.23:21,24:16:19:23.22.21,24:22:19:25.17.16,15:16:19:23.22.21,(31.83,-10.88,;30.29,-10.94,;29.56,-12.3,;28.03,-12.36,;27.21,-11.05,;25.67,-11.11,;24.95,-12.47,;25.77,-13.77,;25.05,-15.13,;23.5,-15.18,;22.68,-13.89,;23.41,-12.52,;22.79,-16.55,;21.25,-16.61,;20.42,-15.31,;20.53,-17.97,;18.99,-18.03,;17.98,-19.31,;16.57,-18.75,;15.07,-19.17,;16.27,-17.89,;16.26,-16.4,;17.6,-15.93,;16.56,-17.16,;19,-16.51,;17.59,-18.38,;27.92,-9.69,;29.46,-9.63,)| Show InChI InChI=1S/C23H31FN2O2/c24-18-1-5-20(6-2-18)28-21-7-3-19(4-8-21)25-22(27)26-23-12-15-9-16(13-23)11-17(10-15)14-23/h1-2,5-6,15-17,19,21H,3-4,7-14H2,(H2,25,26,27)/t15?,16?,17?,19-,21-,23? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50217446
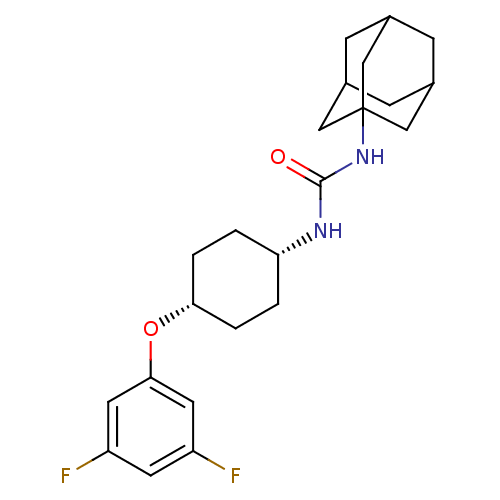
(CHEMBL396546 | cis-1-adamantan-1-yl-3-[4-(3,5-difl...)Show SMILES Fc1cc(F)cc(O[C@@H]2CC[C@@H](CC2)NC(=O)NC23CC4CC(CC(C4)C2)C3)c1 |wU:11.14,8.7,TLB:17:18:21.20.25:23,THB:19:20:23:27.18.26,19:18:21.20.25:23,26:18:21:25.24.23,26:24:21:27.19.18,17:18:21:25.24.23,(4.84,-26.81,;4.03,-25.5,;4.75,-24.14,;3.92,-22.83,;4.64,-21.47,;2.39,-22.89,;1.67,-24.25,;.14,-24.31,;-.59,-25.67,;-2.13,-25.72,;-2.85,-27.09,;-2.03,-28.38,;-.49,-28.33,;.23,-26.97,;-2.75,-29.75,;-4.29,-29.81,;-5.11,-28.51,;-5,-31.17,;-6.54,-31.23,;-7.56,-32.51,;-8.96,-31.95,;-10.46,-32.37,;-9.27,-31.09,;-9.27,-29.6,;-7.93,-29.13,;-8.97,-30.36,;-6.53,-29.71,;-7.94,-31.58,;2.49,-25.56,)| Show InChI InChI=1S/C23H30F2N2O2/c24-17-8-18(25)10-21(9-17)29-20-3-1-19(2-4-20)26-22(28)27-23-11-14-5-15(12-23)7-16(6-14)13-23/h8-10,14-16,19-20H,1-7,11-13H2,(H2,26,27,28)/t14?,15?,16?,19-,20+,23? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50217451
(CHEMBL427695 | trans-1-adamantan-1-yl-3-[4-(4-meth...)Show SMILES COc1ccc(O[C@H]2CC[C@@H](CC2)NC(=O)NC23CC4CC(CC(C4)C2)C3)cc1 |wU:7.6,wD:10.13,TLB:16:17:20.19.24:22,THB:18:19:22:26.17.25,18:17:20.19.24:22,25:17:20:24.23.22,25:23:20:26.18.17,16:17:20:24.23.22,(5.84,-12.14,;5.02,-10.84,;3.48,-10.9,;2.76,-12.26,;1.22,-12.31,;.41,-11.01,;-1.13,-11.06,;-1.85,-12.42,;-1.04,-13.73,;-1.75,-15.08,;-3.3,-15.14,;-4.12,-13.84,;-3.39,-12.48,;-4.01,-16.5,;-5.55,-16.56,;-6.38,-15.26,;-6.27,-17.93,;-7.81,-17.99,;-8.82,-19.27,;-10.23,-18.7,;-11.73,-19.12,;-10.53,-17.85,;-10.54,-16.36,;-9.2,-15.88,;-10.24,-17.12,;-7.8,-16.46,;-9.21,-18.33,;1.12,-9.65,;2.66,-9.59,)| Show InChI InChI=1S/C24H34N2O3/c1-28-20-6-8-22(9-7-20)29-21-4-2-19(3-5-21)25-23(27)26-24-13-16-10-17(14-24)12-18(11-16)15-24/h6-9,16-19,21H,2-5,10-15H2,1H3,(H2,25,26,27)/t16?,17?,18?,19-,21-,24? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50217439
(CHEMBL244405 | cis-4-[4-(3-adamantan-1-ylureido)cy...)Show SMILES OC(=O)c1ccc(O[C@@H]2CC[C@@H](CC2)NC(=O)NC23CC4CC(CC(C4)C2)C3)cc1 |wU:11.14,8.7,TLB:17:18:21.20.25:23,THB:19:20:23:27.18.26,19:18:21.20.25:23,26:18:21:25.24.23,26:24:21:27.19.18,17:18:21:25.24.23,(19.67,-26.54,;18.85,-25.24,;19.57,-23.88,;17.31,-25.3,;16.59,-26.66,;15.05,-26.71,;14.24,-25.41,;12.7,-25.46,;11.98,-26.82,;10.43,-26.88,;9.71,-28.25,;10.53,-29.54,;12.07,-29.49,;12.79,-28.13,;9.81,-30.9,;8.27,-30.96,;7.45,-29.66,;7.56,-32.33,;6.02,-32.39,;5.01,-33.67,;3.6,-33.1,;2.1,-33.52,;3.3,-32.25,;3.29,-30.76,;4.63,-30.29,;3.59,-31.52,;6.03,-30.86,;4.62,-32.74,;14.95,-24.05,;16.48,-23.99,)| Show InChI InChI=1S/C24H32N2O4/c27-22(28)18-1-5-20(6-2-18)30-21-7-3-19(4-8-21)25-23(29)26-24-12-15-9-16(13-24)11-17(10-15)14-24/h1-2,5-6,15-17,19,21H,3-4,7-14H2,(H,27,28)(H2,25,26,29)/t15?,16?,17?,19-,21+,24? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50194498
(1-[4-(4-fluoro-phenoxy)-cyclohexyl]-3-(4-trifluoro...)Show SMILES Fc1ccc(O[C@@H]2CC[C@@H](CC2)NC(=O)Nc2ccc(OC(F)(F)F)cc2)cc1 |wD:6.5,9.12,(8.62,-5.78,;7.29,-5,;5.95,-5.77,;4.62,-5,;4.63,-3.45,;3.29,-2.68,;1.96,-3.45,;1.96,-4.99,;.63,-5.75,;-.7,-4.99,;-.7,-3.45,;.63,-2.67,;-2.03,-5.76,;-3.37,-4.99,;-3.37,-3.45,;-4.7,-5.76,;-6.03,-4.99,;-6.03,-3.45,;-7.36,-2.68,;-8.7,-3.46,;-10.03,-2.69,;-10.04,-1.15,;-10.05,.4,;-8.5,-1.14,;-11.58,-1.16,;-8.69,-5,;-7.36,-5.76,;5.95,-2.69,;7.29,-3.46,)| Show InChI InChI=1S/C20H20F4N2O3/c21-13-1-7-16(8-2-13)28-17-9-3-14(4-10-17)25-19(27)26-15-5-11-18(12-6-15)29-20(22,23)24/h1-2,5-8,11-12,14,17H,3-4,9-10H2,(H2,25,26,27)/t14-,17+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50217477
(CHEMBL243793 | trans-4-{4-[3-(4-trifluoromethoxyph...)Show SMILES OC(=O)c1cccc(O[C@H]2CC[C@@H](CC2)NC(=O)Nc2ccc(OC(F)(F)F)cc2)c1 |wU:9.8,wD:12.15,(7.44,1.03,;8.16,2.39,;9.7,2.45,;7.34,3.7,;8.06,5.06,;7.23,6.37,;5.7,6.31,;4.99,4.95,;3.45,4.9,;2.73,3.53,;3.54,2.23,;2.83,.87,;1.28,.82,;.46,2.11,;1.18,3.48,;.56,-.55,;-.98,-.61,;-1.8,.7,;-1.69,-1.97,;-3.23,-2.03,;-4.05,-.73,;-5.58,-.79,;-6.3,-2.15,;-7.84,-2.21,;-8.66,-.91,;-9.45,.43,;-9.98,-1.71,;-7.34,-.13,;-5.47,-3.46,;-3.94,-3.39,;5.8,3.64,)| Show InChI InChI=1S/C21H21F3N2O5/c22-21(23,24)31-17-10-6-15(7-11-17)26-20(29)25-14-4-8-16(9-5-14)30-18-3-1-2-13(12-18)19(27)28/h1-3,6-7,10-12,14,16H,4-5,8-9H2,(H,27,28)(H2,25,26,29)/t14-,16- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50217461
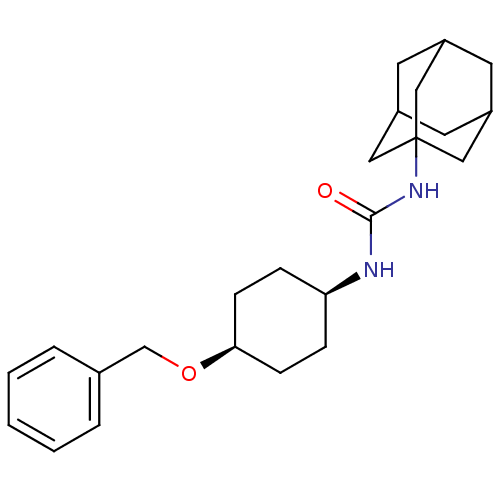
(CHEMBL242898 | cis-1-adamantan-1-yl-3-(4-benzyloxy...)Show SMILES O=C(N[C@H]1CC[C@H](CC1)OCc1ccccc1)NC12CC3CC(CC(C3)C1)C2 |wD:3.2,6.9,TLB:17:18:21.20.25:23,THB:19:20:23:27.18.26,19:18:21.20.25:23,26:18:21:25.24.23,26:24:21:27.19.18,17:18:21:25.24.23,(-5.79,-36.39,;-4.96,-37.7,;-3.42,-37.63,;-2.71,-36.27,;-1.16,-36.22,;-.45,-34.86,;-1.26,-33.55,;-2.8,-33.61,;-3.53,-34.98,;-.54,-32.19,;1,-32.14,;1.72,-30.78,;3.26,-30.73,;3.98,-29.37,;3.17,-28.06,;1.63,-28.12,;.9,-29.48,;-5.68,-39.06,;-7.22,-39.12,;-8.23,-40.4,;-9.64,-39.84,;-11.14,-40.25,;-9.94,-38.98,;-9.95,-37.49,;-8.6,-37.02,;-9.65,-38.25,;-7.21,-37.59,;-8.62,-39.47,)| Show InChI InChI=1S/C24H34N2O2/c27-23(26-24-13-18-10-19(14-24)12-20(11-18)15-24)25-21-6-8-22(9-7-21)28-16-17-4-2-1-3-5-17/h1-5,18-22H,6-16H2,(H2,25,26,27)/t18?,19?,20?,21-,22+,24? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50217473
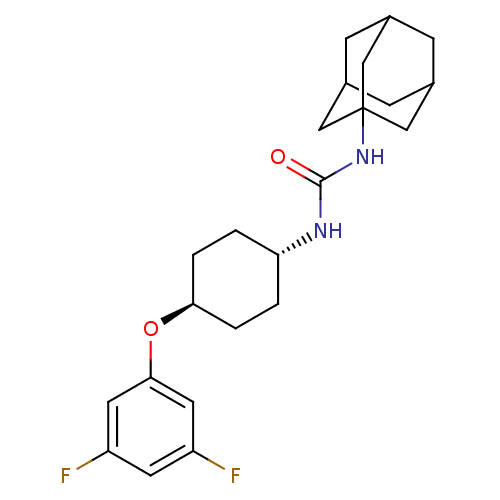
(CHEMBL243336 | trans-1-adamantan-1-yl-3-[4-(3,5-di...)Show SMILES Fc1cc(F)cc(O[C@H]2CC[C@@H](CC2)NC(=O)NC23CC4CC(CC(C4)C2)C3)c1 |wU:8.7,wD:11.14,TLB:17:18:21.20.25:23,THB:19:20:23:27.18.26,19:18:21.20.25:23,26:18:21:25.24.23,26:24:21:27.19.18,17:18:21:25.24.23,(4.8,-26.69,;3.98,-25.38,;4.71,-24.02,;3.88,-22.71,;4.6,-21.35,;2.35,-22.77,;1.63,-24.13,;.09,-24.19,;-.63,-25.55,;.19,-26.85,;-.53,-28.21,;-2.07,-28.26,;-2.89,-26.97,;-2.17,-25.61,;-2.79,-29.63,;-4.33,-29.69,;-5.15,-28.39,;-5.05,-31.05,;-6.59,-31.11,;-7.6,-32.39,;-9.01,-31.83,;-10.5,-32.25,;-9.31,-30.97,;-9.32,-29.48,;-7.97,-29.01,;-9.01,-30.24,;-6.57,-29.59,;-7.98,-31.46,;2.45,-25.44,)| Show InChI InChI=1S/C23H30F2N2O2/c24-17-8-18(25)10-21(9-17)29-20-3-1-19(2-4-20)26-22(28)27-23-11-14-5-15(12-23)7-16(6-14)13-23/h8-10,14-16,19-20H,1-7,11-13H2,(H2,26,27,28)/t14?,15?,16?,19-,20-,23? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50194500
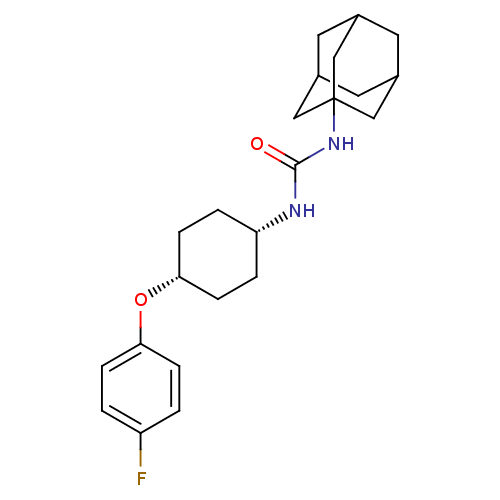
(1-adamantan-1-yl-3-[4-(4-fluoro-phenoxy)-cyclohexy...)Show SMILES Fc1ccc(O[C@@H]2CC[C@@H](CC2)NC(=O)NC23CC4CC(CC(C4)C2)C3)cc1 |wD:9.12,6.5,TLB:15:16:19:23.22.21,THB:17:18:21:25.16.24,17:16:19.18.23:21,24:16:19:23.22.21,24:22:19:25.17.16,(23.93,-16.11,;24.66,-14.74,;23.84,-13.43,;24.56,-12.07,;26.1,-12.02,;26.82,-10.66,;26,-9.35,;24.46,-9.4,;23.64,-8.1,;24.37,-6.75,;25.9,-6.67,;26.72,-7.99,;23.55,-5.45,;22,-5.51,;21.18,-4.2,;21.29,-6.87,;19.75,-6.93,;19.3,-8.5,;17.78,-8.52,;16.56,-9.49,;17.17,-7.85,;16.59,-6.47,;17.66,-5.52,;17.17,-7.05,;19.17,-5.51,;18.59,-7.79,;26.92,-13.32,;26.2,-14.68,)| Show InChI InChI=1S/C23H31FN2O2/c24-18-1-5-20(6-2-18)28-21-7-3-19(4-8-21)25-22(27)26-23-12-15-9-16(13-23)11-17(10-15)14-23/h1-2,5-6,15-17,19,21H,3-4,7-14H2,(H2,25,26,27)/t15?,16?,17?,19-,21+,23? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50217454
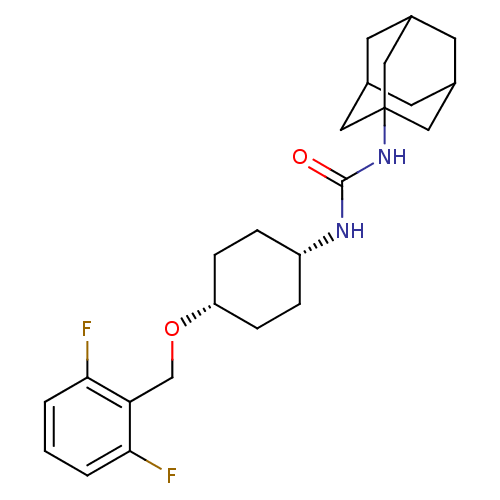
(CHEMBL395738 | cis-1-adamantan-1-yl-3-[4-(2,6-difl...)Show SMILES Fc1cccc(F)c1CO[C@@H]1CC[C@@H](CC1)NC(=O)NC12CC3CC(CC(C3)C1)C2 |wU:13.17,10.10,TLB:19:20:23.22.27:25,THB:21:22:25:29.20.28,21:20:23.22.27:25,28:20:23:27.26.25,28:26:23:29.21.20,19:20:23:27.26.25,(3.82,2.41,;3.01,3.72,;3.73,5.07,;2.92,6.38,;1.38,6.33,;.65,4.97,;-.89,4.91,;1.47,3.67,;.75,2.31,;-.79,2.25,;-1.51,.89,;-3.05,.83,;-3.77,-.53,;-2.96,-1.83,;-1.41,-1.77,;-.69,-.42,;-3.67,-3.19,;-5.21,-3.25,;-6.03,-1.95,;-5.93,-4.61,;-7.47,-4.68,;-8.48,-5.95,;-9.89,-5.39,;-11.39,-5.81,;-10.19,-4.53,;-10.2,-3.05,;-8.85,-2.57,;-9.89,-3.8,;-7.45,-3.15,;-8.86,-5.02,)| Show InChI InChI=1S/C24H32F2N2O2/c25-21-2-1-3-22(26)20(21)14-30-19-6-4-18(5-7-19)27-23(29)28-24-11-15-8-16(12-24)10-17(9-15)13-24/h1-3,15-19H,4-14H2,(H2,27,28,29)/t15?,16?,17?,18-,19+,24? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50217448
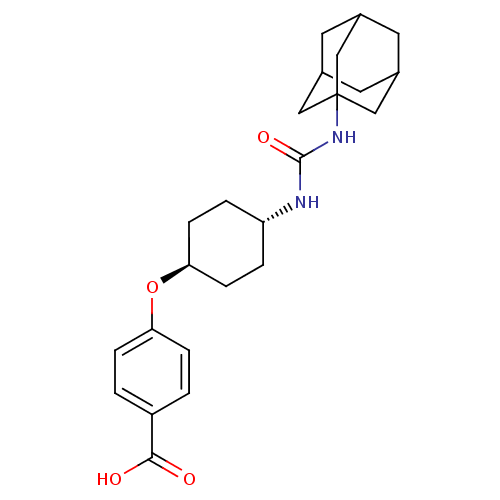
(CHEMBL242459 | US9029401, 1471 (t-AUCB) | trans-4-...)Show SMILES OC(=O)c1ccc(O[C@H]2CC[C@@H](CC2)NC(=O)NC23CC4CC(CC(C4)C2)C3)cc1 |wU:8.7,wD:11.14,TLB:17:18:21.20.25:23,THB:19:20:23:27.18.26,19:18:21.20.25:23,26:18:21:25.24.23,26:24:21:27.19.18,17:18:21:25.24.23,(19.63,-26.42,;18.81,-25.12,;19.53,-23.76,;17.27,-25.17,;16.54,-26.54,;15.01,-26.59,;14.19,-25.28,;12.65,-25.34,;11.93,-26.7,;12.75,-28.01,;12.03,-29.36,;10.48,-29.42,;9.67,-28.12,;10.39,-26.76,;9.77,-30.78,;8.23,-30.84,;7.41,-29.54,;7.51,-32.21,;5.97,-32.27,;4.96,-33.54,;3.55,-32.98,;2.06,-33.4,;3.25,-32.13,;3.24,-30.64,;4.59,-30.16,;3.55,-31.39,;5.99,-30.74,;4.58,-32.61,;14.91,-23.93,;16.44,-23.87,)| Show InChI InChI=1S/C24H32N2O4/c27-22(28)18-1-5-20(6-2-18)30-21-7-3-19(4-8-21)25-23(29)26-24-12-15-9-16(13-24)11-17(10-15)14-24/h1-2,5-6,15-17,19,21H,3-4,7-14H2,(H,27,28)(H2,25,26,29)/t15?,16?,17?,19-,21-,24? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50217456

(CHEMBL243337 | cis-1-adamantan-1-yl-3-[4-(4-bromop...)Show SMILES Brc1ccc(O[C@@H]2CC[C@@H](CC2)NC(=O)NC23CC4CC(CC(C4)C2)C3)cc1 |wU:9.12,6.5,TLB:15:16:19.18.23:21,THB:17:18:21:25.16.24,17:16:19.18.23:21,24:16:19:23.22.21,24:22:19:25.17.16,15:16:19:23.22.21,(34.37,3.49,;32.83,3.43,;32.1,2.07,;30.57,2.01,;29.75,3.32,;28.21,3.26,;27.49,1.9,;25.95,1.84,;25.23,.48,;26.04,-.82,;27.59,-.76,;28.31,.59,;25.33,-2.18,;23.79,-2.24,;22.97,-.94,;23.07,-3.6,;21.53,-3.67,;20.52,-4.94,;19.11,-4.38,;17.61,-4.8,;18.81,-3.52,;18.8,-2.04,;20.15,-1.56,;19.11,-2.79,;21.55,-2.14,;20.13,-4.01,;30.47,4.68,;32,4.74,)| Show InChI InChI=1S/C23H31BrN2O2/c24-18-1-5-20(6-2-18)28-21-7-3-19(4-8-21)25-22(27)26-23-12-15-9-16(13-23)11-17(10-15)14-23/h1-2,5-6,15-17,19,21H,3-4,7-14H2,(H2,25,26,27)/t15?,16?,17?,19-,21+,23? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50217440
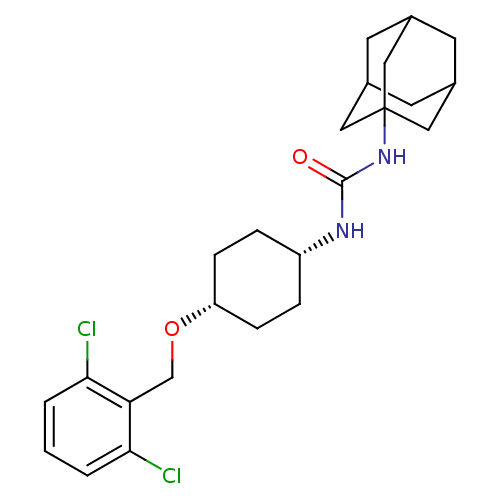
(CHEMBL242025 | cis-1-adamantan-1-yl-3-[4-(2,6-dich...)Show SMILES Clc1cccc(Cl)c1CO[C@@H]1CC[C@@H](CC1)NC(=O)NC12CC3CC(CC(C3)C1)C2 |wU:13.17,10.10,TLB:19:20:23.22.27:25,THB:21:22:25:29.20.28,21:20:23.22.27:25,28:20:23:27.26.25,28:26:23:29.21.20,19:20:23:27.26.25,(33.42,-46.78,;32.61,-45.47,;33.33,-44.12,;32.52,-42.81,;30.97,-42.86,;30.24,-44.22,;28.71,-44.28,;31.07,-45.52,;30.35,-46.88,;28.81,-46.94,;28.09,-48.3,;26.54,-48.36,;25.82,-49.72,;26.64,-51.01,;28.19,-50.96,;28.9,-49.6,;25.92,-52.38,;24.38,-52.44,;23.56,-51.14,;23.67,-53.8,;22.13,-53.86,;21.12,-55.14,;19.71,-54.58,;18.21,-55,;19.41,-53.72,;19.4,-52.23,;20.74,-51.76,;19.7,-52.99,;22.14,-52.34,;20.73,-54.21,)| Show InChI InChI=1S/C24H32Cl2N2O2/c25-21-2-1-3-22(26)20(21)14-30-19-6-4-18(5-7-19)27-23(29)28-24-11-15-8-16(12-24)10-17(9-15)13-24/h1-3,15-19H,4-14H2,(H2,27,28,29)/t15?,16?,17?,18-,19+,24? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM25732

(3-adamantan-1-yl-1-cyclohexylurea | CHEMBL242255 |...)Show SMILES O=C(NC1CCCCC1)NC12CC3CC(CC(C3)C1)C2 |TLB:17:12:19:16.15.18,17:16:12.13.11:19,THB:15:14:11:16.17.18,15:16:11:14.13.19| Show InChI InChI=1S/C17H28N2O/c20-16(18-15-4-2-1-3-5-15)19-17-9-12-6-13(10-17)8-14(7-12)11-17/h12-15H,1-11H2,(H2,18,19,20) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50217449
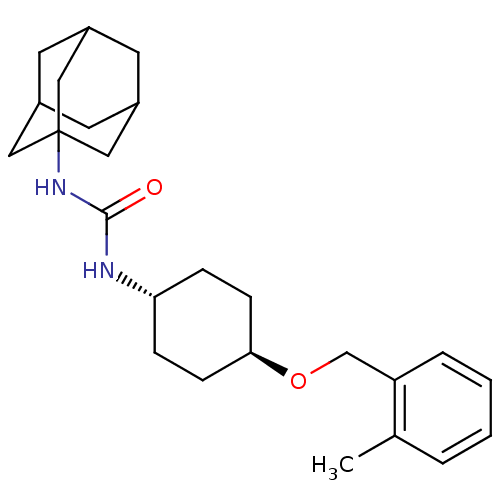
(CHEMBL395989 | trans-1-adamantan-1-yl-3-[4-(2-meth...)Show SMILES Cc1ccccc1CO[C@H]1CC[C@@H](CC1)NC(=O)NC12CC3CC(CC(C3)C1)C2 |wU:9.9,wD:12.16,TLB:18:19:22.21.26:24,THB:20:21:24:28.19.27,20:19:22.21.26:24,27:19:22:26.25.24,27:25:22:28.20.19,18:19:22:26.25.24,(3.94,-45.89,;3.12,-44.59,;3.84,-43.23,;3.03,-41.92,;1.49,-41.97,;.76,-43.34,;1.58,-44.64,;.86,-46,;-.68,-46.05,;-1.4,-47.41,;-.58,-48.72,;-1.3,-50.08,;-2.84,-50.13,;-3.66,-48.84,;-2.94,-47.47,;-3.56,-51.49,;-5.1,-51.56,;-5.92,-50.25,;-5.82,-52.92,;-7.35,-52.98,;-8.37,-54.26,;-9.78,-53.69,;-11.27,-54.11,;-10.08,-52.84,;-10.09,-51.35,;-8.74,-50.88,;-9.78,-52.11,;-7.34,-51.45,;-8.75,-53.33,)| Show InChI InChI=1S/C25H36N2O2/c1-17-4-2-3-5-21(17)16-29-23-8-6-22(7-9-23)26-24(28)27-25-13-18-10-19(14-25)12-20(11-18)15-25/h2-5,18-20,22-23H,6-16H2,1H3,(H2,26,27,28)/t18?,19?,20?,22-,23-,25? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50194504
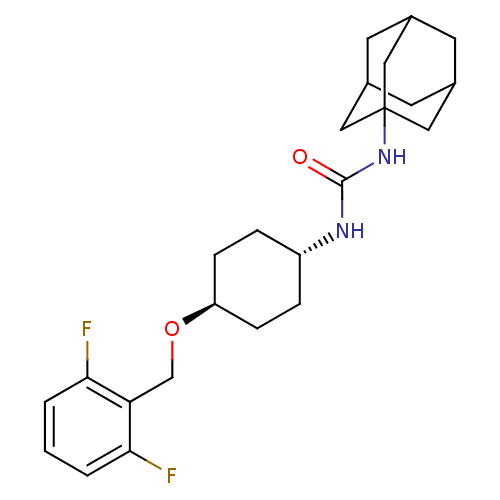
(1-adamantan-1-yl-3-[4-(2,6-difluoro-benzyloxy)-cyc...)Show SMILES Fc1cccc(F)c1CO[C@H]1CC[C@@H](CC1)NC(=O)NC12CC3CC(CC(C3)C1)C2 |wU:10.10,wD:13.17,TLB:19:20:23.22.27:25,THB:21:22:25:29.20.28,21:20:23.22.27:25,28:20:23:27.26.25,28:26:23:29.21.20,19:20:23:27.26.25,(11.93,-7.54,;13.47,-7.54,;14.61,-8.57,;16.08,-8.09,;16.39,-6.58,;15.25,-5.55,;15.51,-4.03,;13.79,-6.03,;12.65,-5,;12.97,-3.5,;11.82,-2.47,;10.36,-2.94,;9.21,-1.92,;9.54,-.41,;10.99,.08,;12.14,-.96,;8.39,.62,;6.93,.13,;5.78,1.16,;6.61,-1.37,;5.15,-1.85,;4.2,-3.18,;2.77,-2.69,;1.29,-3.19,;2.43,-1.85,;2.34,-.36,;3.67,.17,;2.69,-1.1,;5.09,-.33,;3.78,-2.27,)| Show InChI InChI=1S/C24H32F2N2O2/c25-21-2-1-3-22(26)20(21)14-30-19-6-4-18(5-7-19)27-23(29)28-24-11-15-8-16(12-24)10-17(9-15)13-24/h1-3,15-19H,4-14H2,(H2,27,28,29)/t15?,16?,17?,18-,19-,24? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50217481
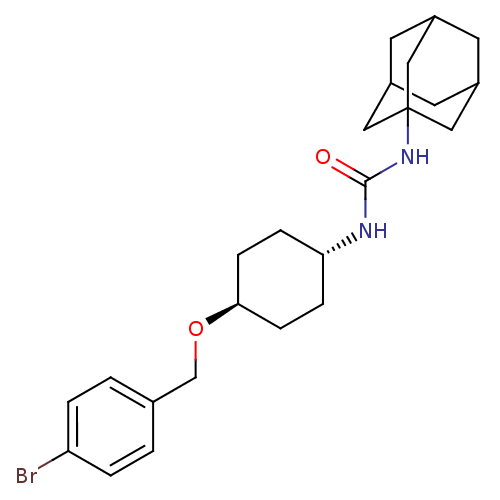
(CHEMBL242899 | trans-1-adamantan-1-yl-3-[4-(4-brom...)Show SMILES Brc1ccc(CO[C@H]2CC[C@@H](CC2)NC(=O)NC23CC4CC(CC(C4)C2)C3)cc1 |wU:7.6,wD:10.13,TLB:16:17:20.19.24:22,THB:18:19:22:26.17.25,18:17:20.19.24:22,25:17:20:24.23.22,25:23:20:26.18.17,16:17:20:24.23.22,(33.17,-23.95,;32.45,-25.31,;33.26,-26.62,;32.54,-27.97,;31,-28.02,;30.28,-29.38,;28.74,-29.44,;28.02,-30.8,;28.83,-32.11,;28.12,-33.46,;26.57,-33.51,;25.75,-32.22,;26.47,-30.86,;25.85,-34.88,;24.31,-34.94,;23.49,-33.64,;23.6,-36.3,;22.06,-36.36,;21.05,-37.64,;19.64,-37.08,;18.14,-37.5,;19.34,-36.22,;19.33,-34.74,;20.67,-34.26,;19.63,-35.49,;22.07,-34.84,;20.66,-36.71,;30.18,-26.72,;30.9,-25.36,)| Show InChI InChI=1S/C24H33BrN2O2/c25-20-3-1-16(2-4-20)15-29-22-7-5-21(6-8-22)26-23(28)27-24-12-17-9-18(13-24)11-19(10-17)14-24/h1-4,17-19,21-22H,5-15H2,(H2,26,27,28)/t17?,18?,19?,21-,22-,24? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50217453
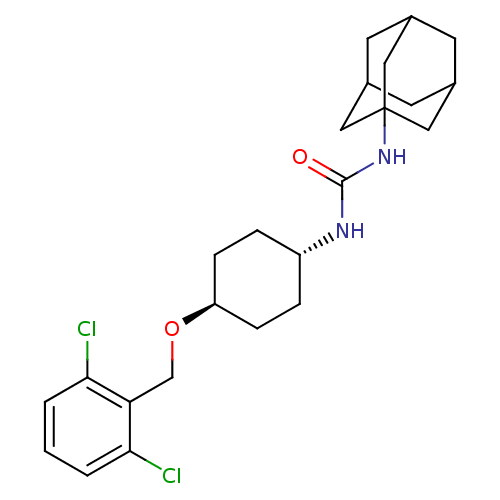
(CHEMBL243124 | trans-1-adamantan-1-yl-3-[4-(2,6-di...)Show SMILES Clc1cccc(Cl)c1CO[C@H]1CC[C@@H](CC1)NC(=O)NC12CC3CC(CC(C3)C1)C2 |wU:10.10,wD:13.17,TLB:19:20:23.22.27:25,THB:21:22:25:29.20.28,21:20:23.22.27:25,28:20:23:27.26.25,28:26:23:29.21.20,19:20:23:27.26.25,(31.75,-44.54,;30.94,-43.24,;31.66,-41.88,;30.85,-40.57,;29.31,-40.63,;28.58,-41.99,;27.04,-42.04,;29.4,-43.29,;28.68,-44.65,;27.14,-44.7,;26.42,-46.06,;27.23,-47.37,;26.52,-48.73,;24.97,-48.78,;24.15,-47.49,;24.88,-46.12,;24.25,-50.14,;22.72,-50.21,;21.89,-48.9,;22,-51.57,;20.46,-51.63,;19.45,-52.91,;18.04,-52.35,;16.54,-52.76,;17.74,-51.49,;17.73,-50,;19.07,-49.53,;18.03,-50.76,;20.47,-50.1,;19.06,-51.98,)| Show InChI InChI=1S/C24H32Cl2N2O2/c25-21-2-1-3-22(26)20(21)14-30-19-6-4-18(5-7-19)27-23(29)28-24-11-15-8-16(12-24)10-17(9-15)13-24/h1-3,15-19H,4-14H2,(H2,27,28,29)/t15?,16?,17?,18-,19-,24? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50217470
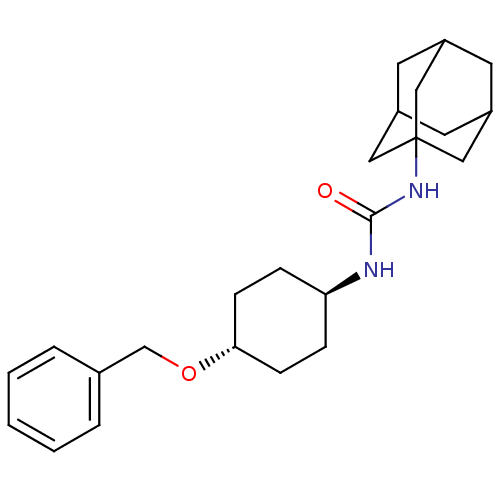
(CHEMBL395988 | trans-1-adamantan-1-yl-3-(4-benzylo...)Show SMILES O=C(N[C@H]1CC[C@@H](CC1)OCc1ccccc1)NC12CC3CC(CC(C3)C1)C2 |wU:6.9,wD:3.2,TLB:17:18:21.20.25:23,THB:19:20:23:27.18.26,19:18:21.20.25:23,26:18:21:25.24.23,26:24:21:27.19.18,17:18:21:25.24.23,(22.51,-19.43,;23.33,-20.73,;24.87,-20.67,;25.59,-19.3,;27.13,-19.25,;27.85,-17.89,;27.03,-16.59,;25.49,-16.65,;24.77,-18.01,;27.75,-15.23,;29.29,-15.17,;30.01,-13.81,;31.55,-13.76,;32.27,-12.41,;31.46,-11.1,;29.92,-11.15,;29.19,-12.51,;22.61,-22.09,;21.08,-22.15,;20.06,-23.43,;18.65,-22.87,;17.16,-23.29,;18.35,-22.01,;18.34,-20.53,;19.69,-20.05,;18.65,-21.28,;21.09,-20.63,;19.68,-22.5,)| Show InChI InChI=1S/C24H34N2O2/c27-23(26-24-13-18-10-19(14-24)12-20(11-18)15-24)25-21-6-8-22(9-7-21)28-16-17-4-2-1-3-5-17/h1-5,18-22H,6-16H2,(H2,25,26,27)/t18?,19?,20?,21-,22-,24? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50217443
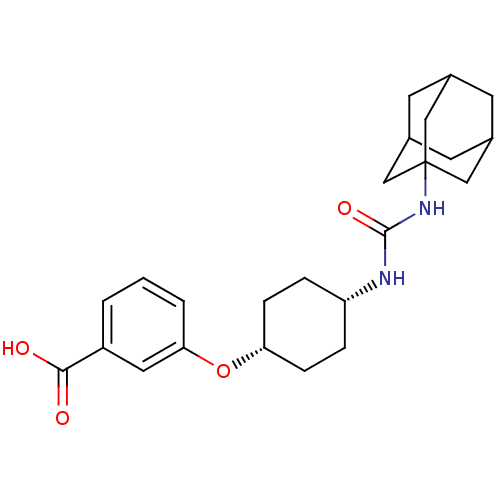
(CHEMBL244406 | cis-3-[4-(3-adamantan-1-ylureido)cy...)Show SMILES OC(=O)c1cccc(O[C@@H]2CC[C@@H](CC2)NC(=O)NC23CC4CC(CC(C4)C2)C3)c1 |wU:12.15,9.8,TLB:18:19:22.21.26:24,THB:20:21:24:28.19.27,20:19:22.21.26:24,27:19:22:26.25.24,27:25:22:28.20.19,18:19:22:26.25.24,(7.2,-43.3,;7.92,-41.94,;9.46,-41.89,;7.1,-40.64,;7.82,-39.28,;7,-37.97,;5.46,-38.03,;4.75,-39.39,;3.21,-39.44,;2.49,-40.8,;.95,-40.86,;.22,-42.22,;1.04,-43.52,;2.59,-43.46,;3.3,-42.11,;.32,-44.88,;-1.22,-44.94,;-2.04,-43.64,;-1.93,-46.31,;-3.47,-46.37,;-4.48,-47.64,;-5.89,-47.08,;-7.39,-47.5,;-6.19,-46.23,;-6.2,-44.74,;-4.86,-44.26,;-5.9,-45.49,;-3.46,-44.84,;-4.87,-46.72,;5.56,-40.69,)| Show InChI InChI=1S/C24H32N2O4/c27-22(28)18-2-1-3-21(11-18)30-20-6-4-19(5-7-20)25-23(29)26-24-12-15-8-16(13-24)10-17(9-15)14-24/h1-3,11,15-17,19-20H,4-10,12-14H2,(H,27,28)(H2,25,26,29)/t15?,16?,17?,19-,20+,24? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50217448

(CHEMBL242459 | US9029401, 1471 (t-AUCB) | trans-4-...)Show SMILES OC(=O)c1ccc(O[C@H]2CC[C@@H](CC2)NC(=O)NC23CC4CC(CC(C4)C2)C3)cc1 |wU:8.7,wD:11.14,TLB:17:18:21.20.25:23,THB:19:20:23:27.18.26,19:18:21.20.25:23,26:18:21:25.24.23,26:24:21:27.19.18,17:18:21:25.24.23,(19.63,-26.42,;18.81,-25.12,;19.53,-23.76,;17.27,-25.17,;16.54,-26.54,;15.01,-26.59,;14.19,-25.28,;12.65,-25.34,;11.93,-26.7,;12.75,-28.01,;12.03,-29.36,;10.48,-29.42,;9.67,-28.12,;10.39,-26.76,;9.77,-30.78,;8.23,-30.84,;7.41,-29.54,;7.51,-32.21,;5.97,-32.27,;4.96,-33.54,;3.55,-32.98,;2.06,-33.4,;3.25,-32.13,;3.24,-30.64,;4.59,-30.16,;3.55,-31.39,;5.99,-30.74,;4.58,-32.61,;14.91,-23.93,;16.44,-23.87,)| Show InChI InChI=1S/C24H32N2O4/c27-22(28)18-1-5-20(6-2-18)30-21-7-3-19(4-8-21)25-23(29)26-24-12-15-9-16(13-24)11-17(10-15)14-24/h1-2,5-6,15-17,19,21H,3-4,7-14H2,(H,27,28)(H2,25,26,29)/t15?,16?,17?,19-,21-,24? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of human sEH by fluorescent assay |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50217455
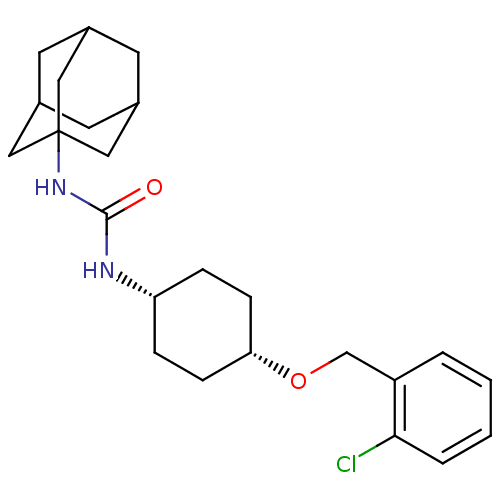
(CHEMBL245255 | cis-1-adamantan-1-yl-3-[4-(2-chloro...)Show SMILES Clc1ccccc1CO[C@@H]1CC[C@@H](CC1)NC(=O)NC12CC3CC(CC(C3)C1)C2 |wU:12.16,9.9,TLB:18:19:22.21.26:24,THB:20:21:24:28.19.27,20:19:22.21.26:24,27:19:22:26.25.24,27:25:22:28.20.19,18:19:22:26.25.24,(18.94,-47.28,;18.13,-45.97,;18.85,-44.62,;18.04,-43.31,;16.5,-43.36,;15.77,-44.72,;16.59,-46.02,;15.87,-47.38,;14.33,-47.44,;13.61,-48.8,;12.07,-48.86,;11.34,-50.22,;12.16,-51.52,;13.71,-51.46,;14.42,-50.11,;11.45,-52.88,;9.91,-52.94,;9.08,-51.64,;9.19,-54.3,;7.65,-54.37,;6.64,-55.64,;5.23,-55.08,;3.73,-55.5,;4.93,-54.22,;4.92,-52.74,;6.26,-52.26,;5.22,-53.49,;7.66,-52.84,;6.25,-54.71,)| Show InChI InChI=1S/C24H33ClN2O2/c25-22-4-2-1-3-19(22)15-29-21-7-5-20(6-8-21)26-23(28)27-24-12-16-9-17(13-24)11-18(10-16)14-24/h1-4,16-18,20-21H,5-15H2,(H2,26,27,28)/t16?,17?,18?,20-,21+,24? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50217460

(CHEMBL243125 | trans-1-adamantan-1-yl-3-[4-(4-brom...)Show SMILES Brc1ccc(O[C@H]2CC[C@@H](CC2)NC(=O)NC23CC4CC(CC(C4)C2)C3)cc1 |wU:6.5,wD:9.12,TLB:15:16:19.18.23:21,THB:17:18:21:25.16.24,17:16:19.18.23:21,24:16:19:23.22.21,24:22:19:25.17.16,15:16:19:23.22.21,(34.33,3.61,;32.79,3.55,;32.06,2.19,;30.53,2.13,;29.71,3.44,;28.17,3.38,;27.45,2.02,;28.27,.71,;27.55,-.64,;26,-.7,;25.18,.6,;25.91,1.96,;25.29,-2.06,;23.75,-2.12,;22.92,-.82,;23.03,-3.49,;21.49,-3.55,;20.48,-4.82,;19.07,-4.26,;17.57,-4.68,;18.77,-3.41,;18.76,-1.92,;20.1,-1.44,;19.06,-2.67,;21.5,-2.02,;20.09,-3.89,;30.42,4.8,;31.96,4.86,)| Show InChI InChI=1S/C23H31BrN2O2/c24-18-1-5-20(6-2-18)28-21-7-3-19(4-8-21)25-22(27)26-23-12-15-9-16(13-23)11-17(10-15)14-23/h1-2,5-6,15-17,19,21H,3-4,7-14H2,(H2,25,26,27)/t15?,16?,17?,19-,21-,23? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50217466
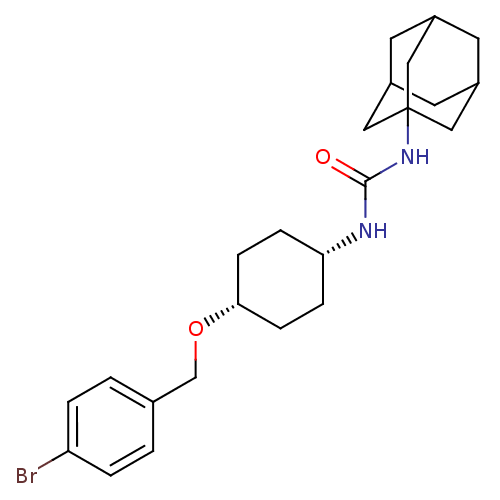
(CHEMBL242460 | cis-1-adamantan-1-yl-3-[4-(4-bromob...)Show SMILES Brc1ccc(CO[C@@H]2CC[C@@H](CC2)NC(=O)NC23CC4CC(CC(C4)C2)C3)cc1 |wU:10.13,7.6,TLB:16:17:20.19.24:22,THB:18:19:22:26.17.25,18:17:20.19.24:22,25:17:20:24.23.22,25:23:20:26.18.17,16:17:20:24.23.22,(26.78,-26.25,;26.06,-27.61,;26.87,-28.92,;26.15,-30.28,;24.61,-30.33,;23.89,-31.69,;22.35,-31.74,;21.63,-33.1,;20.09,-33.16,;19.36,-34.53,;20.18,-35.82,;21.73,-35.77,;22.44,-34.41,;19.46,-37.18,;17.93,-37.25,;17.1,-35.94,;17.21,-38.61,;15.67,-38.67,;14.66,-39.95,;13.25,-39.39,;11.75,-39.8,;12.95,-38.53,;12.94,-37.04,;14.28,-36.57,;13.24,-37.8,;15.68,-37.14,;14.27,-39.02,;23.79,-29.03,;24.52,-27.66,)| Show InChI InChI=1S/C24H33BrN2O2/c25-20-3-1-16(2-4-20)15-29-22-7-5-21(6-8-22)26-23(28)27-24-12-17-9-18(13-24)11-19(10-17)14-24/h1-4,17-19,21-22H,5-15H2,(H2,26,27,28)/t17?,18?,19?,21-,22+,24? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50217472
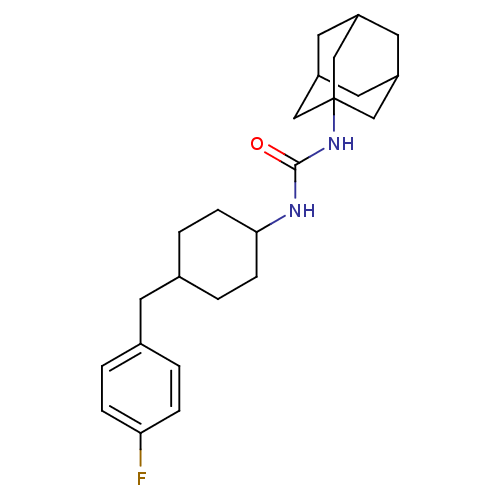
(1-adamantan-1-yl-3-[4-(4-fluorobenzyl)cyclohexyl]u...)Show SMILES Fc1ccc(CC2CCC(CC2)NC(=O)NC23CC4CC(CC(C4)C2)C3)cc1 |TLB:15:16:19.18.23:21,THB:17:18:21:25.16.24,17:16:19.18.23:21,24:16:19:23.22.21,24:22:19:25.17.16,15:16:19:23.22.21,(35.36,-29.17,;33.82,-29.23,;33.11,-30.59,;31.57,-30.65,;30.75,-29.34,;29.21,-29.4,;28.49,-30.76,;29.31,-32.07,;28.59,-33.42,;27.05,-33.47,;26.22,-32.18,;26.95,-30.82,;26.33,-34.83,;24.79,-34.89,;23.97,-33.6,;24.07,-36.26,;22.53,-36.32,;21.52,-37.6,;20.12,-37.04,;18.62,-37.46,;19.81,-36.18,;19.8,-34.69,;21.15,-34.22,;20.11,-35.45,;22.55,-34.79,;21.14,-36.67,;31.47,-27.98,;33,-27.92,)| Show InChI InChI=1S/C24H33FN2O/c25-21-5-1-16(2-6-21)9-17-3-7-22(8-4-17)26-23(28)27-24-13-18-10-19(14-24)12-20(11-18)15-24/h1-2,5-6,17-20,22H,3-4,7-15H2,(H2,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50217464
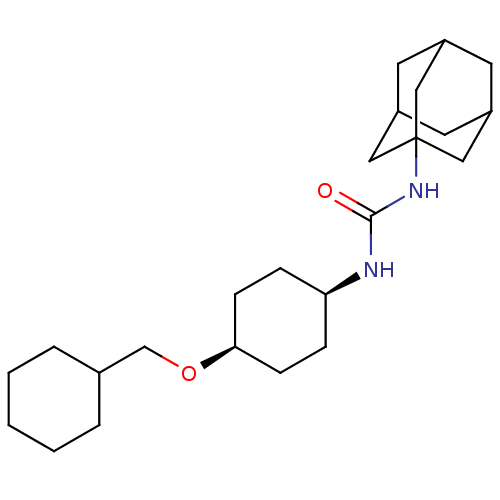
(CHEMBL395987 | cis-1-adamantan-1-yl-3-(4-cyclohexy...)Show SMILES O=C(N[C@H]1CC[C@H](CC1)OCC1CCCCC1)NC12CC3CC(CC(C3)C1)C2 |wD:3.2,6.9,TLB:17:18:21.20.25:23,THB:19:20:23:27.18.26,19:18:21.20.25:23,26:18:21:25.24.23,26:24:21:27.19.18,17:18:21:25.24.23,(6.65,-19.46,;7.47,-20.76,;9.01,-20.7,;9.72,-19.34,;11.27,-19.29,;11.99,-17.93,;11.17,-16.62,;9.63,-16.68,;8.9,-18.05,;11.89,-15.26,;13.43,-15.21,;14.15,-13.85,;15.69,-13.79,;16.41,-12.44,;15.6,-11.13,;14.06,-11.18,;13.33,-12.55,;6.75,-22.13,;5.21,-22.19,;4.2,-23.47,;2.79,-22.9,;1.29,-23.32,;2.49,-22.05,;2.48,-20.56,;3.83,-20.08,;2.79,-21.32,;5.23,-20.66,;3.81,-22.54,)| Show InChI InChI=1S/C24H40N2O2/c27-23(26-24-13-18-10-19(14-24)12-20(11-18)15-24)25-21-6-8-22(9-7-21)28-16-17-4-2-1-3-5-17/h17-22H,1-16H2,(H2,25,26,27)/t18?,19?,20?,21-,22+,24? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50217479
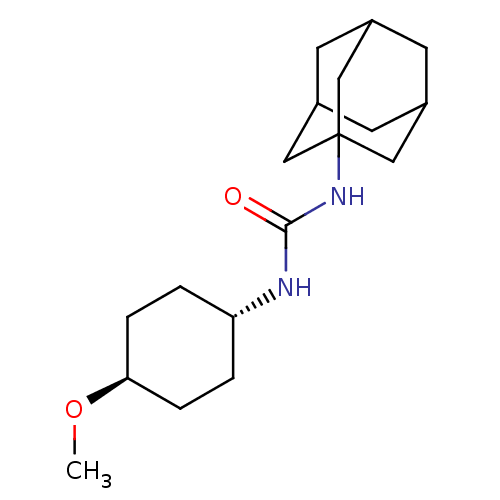
(CHEMBL242256 | trans-1-adamantan-1-yl-3-(4-methoxy...)Show SMILES CO[C@H]1CC[C@@H](CC1)NC(=O)NC12CC3CC(CC(C3)C1)C2 |wU:2.1,wD:5.8,TLB:11:12:15.14.19:17,THB:13:14:17:21.12.20,13:12:15.14.19:17,20:12:15:19.18.17,20:18:15:21.13.12,11:12:15:19.18.17,(16.68,-2.51,;15.14,-2.57,;14.42,-3.93,;15.24,-5.23,;14.52,-6.59,;12.97,-6.64,;12.16,-5.35,;12.88,-3.98,;12.26,-8.01,;10.72,-8.07,;9.9,-6.77,;10,-9.43,;8.46,-9.49,;7.45,-10.77,;6.04,-10.21,;4.55,-10.63,;5.74,-9.35,;5.73,-7.86,;7.08,-7.39,;6.04,-8.62,;8.48,-7.96,;7.07,-9.84,)| Show InChI InChI=1S/C18H30N2O2/c1-22-16-4-2-15(3-5-16)19-17(21)20-18-9-12-6-13(10-18)8-14(7-12)11-18/h12-16H,2-11H2,1H3,(H2,19,20,21)/t12?,13?,14?,15-,16-,18? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50217476
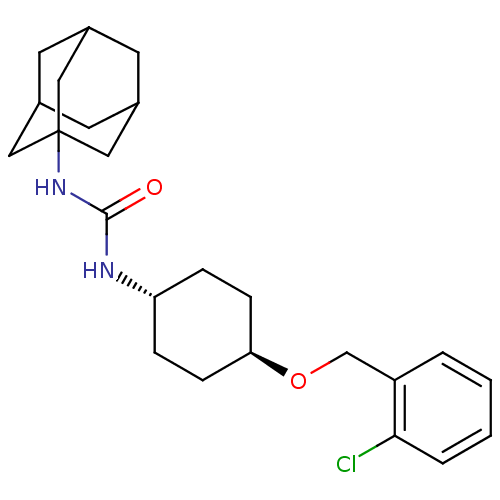
(CHEMBL242900 | trans-1-adamantan-1-yl-3-[4-(2-chlo...)Show SMILES Clc1ccccc1CO[C@H]1CC[C@@H](CC1)NC(=O)NC12CC3CC(CC(C3)C1)C2 |wU:9.9,wD:12.16,TLB:18:19:22.21.26:24,THB:20:21:24:28.19.27,20:19:22.21.26:24,27:19:22:26.25.24,27:25:22:28.20.19,18:19:22:26.25.24,(17.27,-45.05,;16.46,-43.74,;17.18,-42.39,;16.37,-41.08,;14.83,-41.13,;14.1,-42.49,;14.92,-43.79,;14.2,-45.15,;12.66,-45.21,;11.94,-46.57,;12.76,-47.87,;12.04,-49.23,;10.49,-49.28,;9.67,-47.99,;10.4,-46.63,;9.78,-50.65,;8.24,-50.71,;7.42,-49.41,;7.52,-52.07,;5.98,-52.13,;4.97,-53.41,;3.56,-52.85,;2.06,-53.27,;3.26,-51.99,;3.25,-50.5,;4.6,-50.03,;3.56,-51.26,;6,-50.61,;4.58,-52.48,)| Show InChI InChI=1S/C24H33ClN2O2/c25-22-4-2-1-3-19(22)15-29-21-7-5-20(6-8-21)26-23(28)27-24-12-16-9-17(13-24)11-18(10-16)14-24/h1-4,16-18,20-21H,5-15H2,(H2,26,27,28)/t16?,17?,18?,20-,21-,24? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50217468

(1-adamantan-1-yl-3-[4-(4-fluorophenoxy)phenyl]urea...)Show SMILES Fc1ccc(Oc2ccc(NC(=O)NC34CC5CC(CC(C5)C3)C4)cc2)cc1 |TLB:13:14:17.16.21:19,THB:15:16:19:23.14.22,15:14:17.16.21:19,22:14:17:21.20.19,22:20:17:23.15.14,13:14:17:21.20.19| Show InChI InChI=1S/C23H25FN2O2/c24-18-1-5-20(6-2-18)28-21-7-3-19(4-8-21)25-22(27)26-23-12-15-9-16(13-23)11-17(10-15)14-23/h1-8,15-17H,9-14H2,(H2,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Mus musculus (Mouse)) | BDBM25738
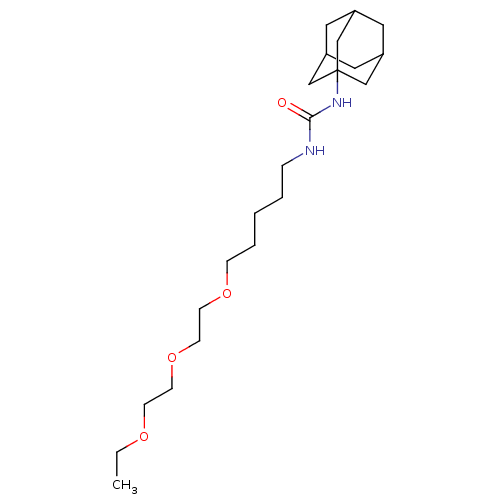
(1-adamantan-1-yl-3-{5-[2-(2-ethoxyethoxy)ethoxy]pe...)Show SMILES CCOCCOCCOCCCCCNC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:25:20:27:24.23.26,25:24:20.21.19:27,THB:23:22:19:24.25.26,23:24:19:22.21.27| Show InChI InChI=1S/C22H40N2O4/c1-2-26-8-9-28-11-10-27-7-5-3-4-6-23-21(25)24-22-15-18-12-19(16-22)14-20(13-18)17-22/h18-20H,2-17H2,1H3,(H2,23,24,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant soluble epoxide hydrolase by fluorescent assay |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM25737

(12-[(adamantan-1-ylcarbamoyl)amino]dodecanoic acid...)Show SMILES OC(=O)CCCCCCCCCCCNC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:25:20:27:24.23.26,25:24:20.21.19:27,THB:23:22:19:24.25.26,23:24:19:22.21.27| Show InChI InChI=1S/C23H40N2O3/c26-21(27)10-8-6-4-2-1-3-5-7-9-11-24-22(28)25-23-15-18-12-19(16-23)14-20(13-18)17-23/h18-20H,1-17H2,(H,26,27)(H2,24,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of human sEH by fluorescent assay |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50217450
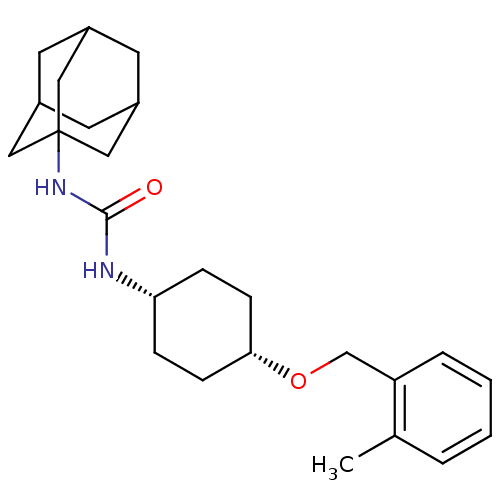
(CHEMBL397426 | cis-1-adamantan-1-yl-3-[4-(2-methyl...)Show SMILES Cc1ccccc1CO[C@@H]1CC[C@@H](CC1)NC(=O)NC12CC3CC(CC(C3)C1)C2 |wU:12.16,9.9,TLB:18:19:22.21.26:24,THB:20:21:24:28.19.27,20:19:22.21.26:24,27:19:22:26.25.24,27:25:22:28.20.19,18:19:22:26.25.24,(5.6,-48.13,;4.79,-46.82,;5.51,-45.47,;4.7,-44.16,;3.16,-44.21,;2.43,-45.57,;3.25,-46.87,;2.53,-48.23,;.99,-48.29,;.27,-49.65,;-1.27,-49.7,;-2,-51.07,;-1.18,-52.36,;.37,-52.31,;1.09,-50.95,;-1.89,-53.73,;-3.43,-53.79,;-4.25,-52.49,;-4.15,-55.15,;-5.69,-55.21,;-6.7,-56.49,;-8.11,-55.93,;-9.61,-56.35,;-8.41,-55.07,;-8.42,-53.58,;-7.07,-53.11,;-8.11,-54.34,;-5.67,-53.69,;-7.09,-55.56,)| Show InChI InChI=1S/C25H36N2O2/c1-17-4-2-3-5-21(17)16-29-23-8-6-22(7-9-23)26-24(28)27-25-13-18-10-19(14-25)12-20(11-18)15-25/h2-5,18-20,22-23H,6-16H2,1H3,(H2,26,27,28)/t18?,19?,20?,22-,23+,25? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50217467
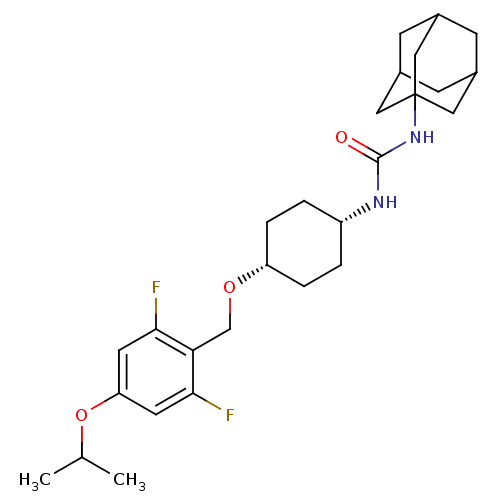
(CHEMBL242026 | cis-1-adamantan-1-yl-3-[4-(2,6-difl...)Show SMILES CC(C)Oc1cc(F)c(CO[C@@H]2CC[C@@H](CC2)NC(=O)NC23CC4CC(CC(C4)C2)C3)c(F)c1 |wU:11.10,14.17,TLB:20:21:24.23.28:26,THB:22:23:26:30.21.29,22:21:24.23.28:26,29:21:24:28.27.26,29:27:24:30.22.21,20:21:24:28.27.26,(18.09,8,;17.37,6.64,;18.18,5.33,;15.83,6.59,;15.1,5.24,;15.91,3.93,;15.19,2.57,;16.01,1.27,;13.65,2.52,;12.93,1.16,;11.39,1.1,;10.67,-.26,;9.13,-.31,;8.41,-1.68,;9.23,-2.97,;10.77,-2.92,;11.49,-1.56,;8.51,-4.33,;6.97,-4.4,;6.15,-3.09,;6.26,-5.76,;4.72,-5.82,;3.7,-7.1,;2.3,-6.54,;.8,-6.96,;1.99,-5.68,;1.99,-4.19,;3.33,-3.72,;2.29,-4.95,;4.73,-4.29,;3.32,-6.17,;12.83,3.82,;11.29,3.77,;13.56,5.18,)| Show InChI InChI=1S/C27H38F2N2O3/c1-16(2)34-22-10-24(28)23(25(29)11-22)15-33-21-5-3-20(4-6-21)30-26(32)31-27-12-17-7-18(13-27)9-19(8-17)14-27/h10-11,16-21H,3-9,12-15H2,1-2H3,(H2,30,31,32)/t17?,18?,19?,20-,21+,27? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50217462
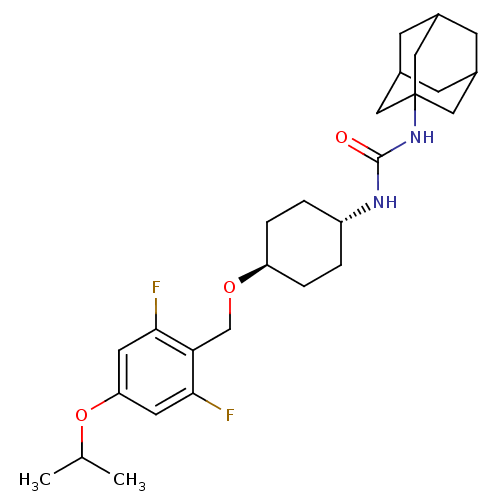
(CHEMBL397693 | trans-1-adamantan-1-yl-3-[4-(2,6-di...)Show SMILES CC(C)Oc1cc(F)c(CO[C@H]2CC[C@@H](CC2)NC(=O)NC23CC4CC(CC(C4)C2)C3)c(F)c1 |wU:11.10,wD:14.17,TLB:20:21:24.23.28:26,THB:22:23:26:30.21.29,22:21:24.23.28:26,29:21:24:28.27.26,29:27:24:30.22.21,20:21:24:28.27.26,(18.05,8.12,;17.33,6.76,;18.14,5.45,;15.79,6.71,;15.06,5.36,;15.87,4.05,;15.15,2.69,;15.97,1.39,;13.61,2.64,;12.89,1.28,;11.35,1.22,;10.63,-.14,;11.45,-1.44,;10.73,-2.8,;9.18,-2.85,;8.37,-1.56,;9.09,-.19,;8.47,-4.22,;6.93,-4.28,;6.11,-2.97,;6.21,-5.64,;4.67,-5.7,;3.66,-6.98,;2.25,-6.42,;.76,-6.84,;1.95,-5.56,;1.94,-4.07,;3.29,-3.6,;2.25,-4.83,;4.69,-4.17,;3.28,-6.05,;12.79,3.94,;11.25,3.89,;13.52,5.3,)| Show InChI InChI=1S/C27H38F2N2O3/c1-16(2)34-22-10-24(28)23(25(29)11-22)15-33-21-5-3-20(4-6-21)30-26(32)31-27-12-17-7-18(13-27)9-19(8-17)14-27/h10-11,16-21H,3-9,12-15H2,1-2H3,(H2,30,31,32)/t17?,18?,19?,20-,21-,27? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50217441
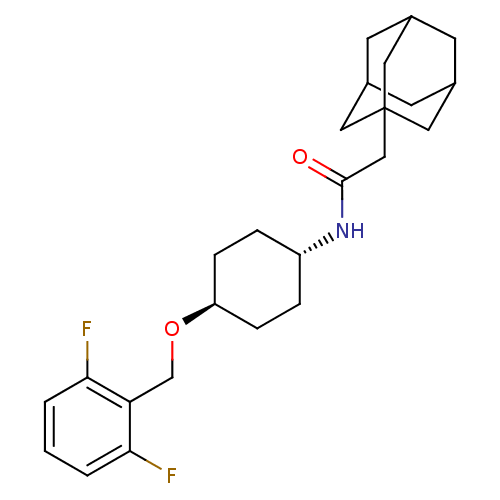
(CHEMBL245225 | trans-2-adamantan-1-yl-N-[4-(2,6-di...)Show SMILES Fc1cccc(F)c1CO[C@H]1CC[C@@H](CC1)NC(=O)CC12CC3CC(CC(C3)C1)C2 |wU:10.10,wD:13.17,TLB:19:20:23.22.27:25,THB:21:22:25:29.20.28,21:20:23.22.27:25,28:20:23:27.26.25,28:26:23:29.21.20,19:20:23:27.26.25,(22.93,-9.7,;22.12,-8.39,;22.84,-7.03,;22.02,-5.72,;20.47,-5.78,;19.76,-7.14,;18.22,-7.2,;20.58,-8.44,;19.86,-9.8,;18.32,-9.86,;17.59,-11.22,;18.41,-12.52,;17.7,-13.88,;16.15,-13.93,;15.33,-12.63,;16.05,-11.28,;15.43,-15.29,;13.89,-15.36,;13.07,-14.05,;13.18,-16.72,;11.64,-16.78,;10.62,-18.06,;9.21,-17.5,;7.71,-17.92,;8.91,-16.64,;8.91,-15.15,;10.25,-14.68,;9.21,-15.91,;11.65,-15.25,;10.24,-17.13,)| Show InChI InChI=1S/C25H33F2NO2/c26-22-2-1-3-23(27)21(22)15-30-20-6-4-19(5-7-20)28-24(29)14-25-11-16-8-17(12-25)10-18(9-16)13-25/h1-3,16-20H,4-15H2,(H,28,29)/t16?,17?,18?,19-,20-,25? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50217475

(1-adamantan-1-yl-3-[4-(4-fluorophenoxy)butyl]urea ...)Show SMILES Fc1ccc(OCCCCNC(=O)NC23CC4CC(CC(C4)C2)C3)cc1 |TLB:13:14:17.16.21:19,THB:15:16:19:23.14.22,15:14:17.16.21:19,22:14:17:21.20.19,22:20:17:23.15.14,13:14:17:21.20.19| Show InChI InChI=1S/C21H29FN2O2/c22-18-3-5-19(6-4-18)26-8-2-1-7-23-20(25)24-21-12-15-9-16(13-21)11-17(10-15)14-21/h3-6,15-17H,1-2,7-14H2,(H2,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50217465
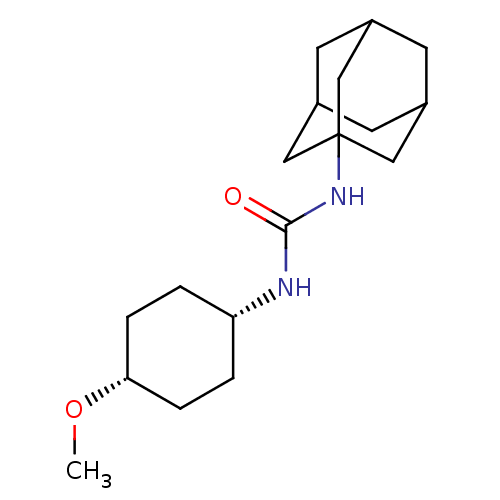
(CHEMBL242470 | trans-1-adamantan-1-yl-3-(4-cyclohe...)Show SMILES CO[C@@H]1CC[C@@H](CC1)NC(=O)NC12CC3CC(CC(C3)C1)C2 |wD:5.8,2.1,TLB:11:12:15.14.19:17,THB:13:14:17:21.12.20,13:12:15.14.19:17,20:12:15:19.18.17,20:18:15:21.13.12,11:12:15:19.18.17,(32.43,-1.35,;30.89,-1.4,;30.17,-2.76,;30.99,-4.07,;30.27,-5.42,;28.73,-5.48,;27.91,-4.18,;28.63,-2.82,;28.01,-6.84,;26.47,-6.9,;25.65,-5.6,;25.75,-8.27,;24.22,-8.33,;23.2,-9.6,;21.79,-9.04,;20.3,-9.46,;21.49,-8.19,;21.48,-6.7,;22.83,-6.22,;21.79,-7.46,;24.23,-6.8,;22.82,-8.67,)| Show InChI InChI=1S/C18H30N2O2/c1-22-16-4-2-15(3-5-16)19-17(21)20-18-9-12-6-13(10-18)8-14(7-12)11-18/h12-16H,2-11H2,1H3,(H2,19,20,21)/t12?,13?,14?,15-,16+,18? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50217445

(CHEMBL397669 | cis-1-adamantan-1-yl-3-(4-methoxycy...)Show SMILES O=C(N[C@H]1CC[C@@H](CC1)OCC1CCCCC1)NC12CC3CC(CC(C3)C1)C2 |wU:6.9,wD:3.2,TLB:17:18:21.20.25:23,THB:19:20:23:27.18.26,19:18:21.20.25:23,26:18:21:25.24.23,26:24:21:27.19.18,17:18:21:25.24.23,(-5.73,-20.09,;-4.9,-21.4,;-3.36,-21.34,;-2.65,-19.97,;-1.1,-19.92,;-.39,-18.56,;-1.2,-17.26,;-2.74,-17.31,;-3.47,-18.68,;-.48,-15.9,;1.06,-15.84,;1.78,-14.48,;3.32,-14.43,;4.04,-13.07,;3.23,-11.76,;1.69,-11.82,;.96,-13.18,;-5.62,-22.76,;-7.16,-22.82,;-8.17,-24.1,;-9.58,-23.54,;-11.08,-23.96,;-9.88,-22.68,;-9.89,-21.19,;-8.54,-20.72,;-9.59,-21.95,;-7.15,-21.29,;-8.56,-23.17,)| Show InChI InChI=1S/C24H40N2O2/c27-23(26-24-13-18-10-19(14-24)12-20(11-18)15-24)25-21-6-8-22(9-7-21)28-16-17-4-2-1-3-5-17/h17-22H,1-16H2,(H2,25,26,27)/t18?,19?,20?,21-,22-,24? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM25738

(1-adamantan-1-yl-3-{5-[2-(2-ethoxyethoxy)ethoxy]pe...)Show SMILES CCOCCOCCOCCCCCNC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:25:20:27:24.23.26,25:24:20.21.19:27,THB:23:22:19:24.25.26,23:24:19:22.21.27| Show InChI InChI=1S/C22H40N2O4/c1-2-26-8-9-28-11-10-27-7-5-3-4-6-23-21(25)24-22-15-18-12-19(16-22)14-20(13-18)17-22/h18-20H,2-17H2,1H3,(H2,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant soluble epoxide hydrolase by fluorescent assay |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50191854
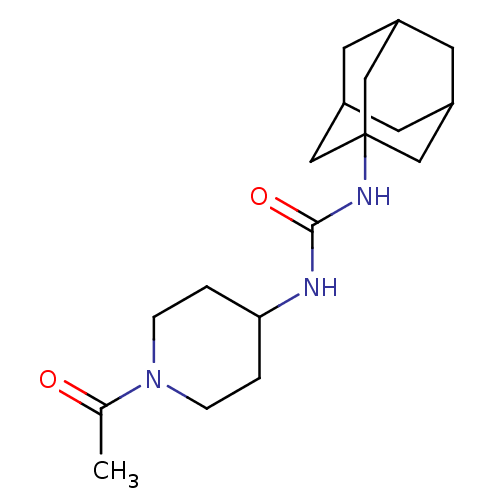
(CHEMBL436774 | N-(1-acetyl-piperidin-4-yl)-N'-(ada...)Show SMILES CC(=O)N1CCC(CC1)NC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:12:13:16.15.20:18,THB:14:15:18:22.13.21,14:13:16.15.20:18,21:13:16:20.19.18,21:19:16:22.14.13,12:13:16:20.19.18| Show InChI InChI=1S/C18H29N3O2/c1-12(22)21-4-2-16(3-5-21)19-17(23)20-18-9-13-6-14(10-18)8-15(7-13)11-18/h13-16H,2-11H2,1H3,(H2,19,20,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant soluble epoxide hydrolase by fluorescent assay |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Mus musculus (Mouse)) | BDBM50217448

(CHEMBL242459 | US9029401, 1471 (t-AUCB) | trans-4-...)Show SMILES OC(=O)c1ccc(O[C@H]2CC[C@@H](CC2)NC(=O)NC23CC4CC(CC(C4)C2)C3)cc1 |wU:8.7,wD:11.14,TLB:17:18:21.20.25:23,THB:19:20:23:27.18.26,19:18:21.20.25:23,26:18:21:25.24.23,26:24:21:27.19.18,17:18:21:25.24.23,(19.63,-26.42,;18.81,-25.12,;19.53,-23.76,;17.27,-25.17,;16.54,-26.54,;15.01,-26.59,;14.19,-25.28,;12.65,-25.34,;11.93,-26.7,;12.75,-28.01,;12.03,-29.36,;10.48,-29.42,;9.67,-28.12,;10.39,-26.76,;9.77,-30.78,;8.23,-30.84,;7.41,-29.54,;7.51,-32.21,;5.97,-32.27,;4.96,-33.54,;3.55,-32.98,;2.06,-33.4,;3.25,-32.13,;3.24,-30.64,;4.59,-30.16,;3.55,-31.39,;5.99,-30.74,;4.58,-32.61,;14.91,-23.93,;16.44,-23.87,)| Show InChI InChI=1S/C24H32N2O4/c27-22(28)18-1-5-20(6-2-18)30-21-7-3-19(4-8-21)25-23(29)26-24-12-15-9-16(13-24)11-17(10-15)14-24/h1-2,5-6,15-17,19,21H,3-4,7-14H2,(H,27,28)(H2,25,26,29)/t15?,16?,17?,19-,21-,24? | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant soluble epoxide hydrolase by fluorescent assay |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50217448

(CHEMBL242459 | US9029401, 1471 (t-AUCB) | trans-4-...)Show SMILES OC(=O)c1ccc(O[C@H]2CC[C@@H](CC2)NC(=O)NC23CC4CC(CC(C4)C2)C3)cc1 |wU:8.7,wD:11.14,TLB:17:18:21.20.25:23,THB:19:20:23:27.18.26,19:18:21.20.25:23,26:18:21:25.24.23,26:24:21:27.19.18,17:18:21:25.24.23,(19.63,-26.42,;18.81,-25.12,;19.53,-23.76,;17.27,-25.17,;16.54,-26.54,;15.01,-26.59,;14.19,-25.28,;12.65,-25.34,;11.93,-26.7,;12.75,-28.01,;12.03,-29.36,;10.48,-29.42,;9.67,-28.12,;10.39,-26.76,;9.77,-30.78,;8.23,-30.84,;7.41,-29.54,;7.51,-32.21,;5.97,-32.27,;4.96,-33.54,;3.55,-32.98,;2.06,-33.4,;3.25,-32.13,;3.24,-30.64,;4.59,-30.16,;3.55,-31.39,;5.99,-30.74,;4.58,-32.61,;14.91,-23.93,;16.44,-23.87,)| Show InChI InChI=1S/C24H32N2O4/c27-22(28)18-1-5-20(6-2-18)30-21-7-3-19(4-8-21)25-23(29)26-24-12-15-9-16(13-24)11-17(10-15)14-24/h1-2,5-6,15-17,19,21H,3-4,7-14H2,(H,27,28)(H2,25,26,29)/t15?,16?,17?,19-,21-,24? | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant soluble epoxide hydrolase by fluorescent assay |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Mus musculus (Mouse)) | BDBM50191854

(CHEMBL436774 | N-(1-acetyl-piperidin-4-yl)-N'-(ada...)Show SMILES CC(=O)N1CCC(CC1)NC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:12:13:16.15.20:18,THB:14:15:18:22.13.21,14:13:16.15.20:18,21:13:16:20.19.18,21:19:16:22.14.13,12:13:16:20.19.18| Show InChI InChI=1S/C18H29N3O2/c1-12(22)21-4-2-16(3-5-21)19-17(23)20-18-9-13-6-14(10-18)8-15(7-13)11-18/h13-16H,2-11H2,1H3,(H2,19,20,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant soluble epoxide hydrolase by fluorescent assay |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50217471
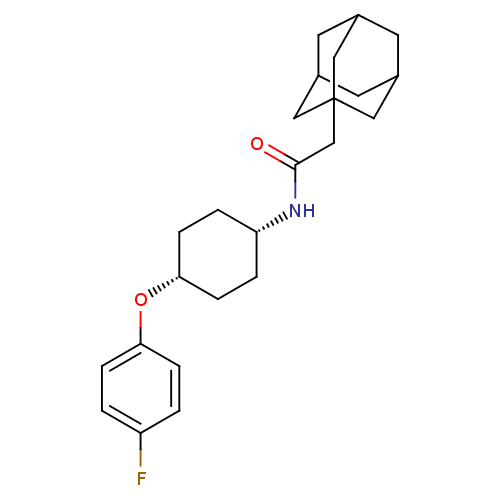
(CHEMBL243302 | cis-2-adamantan-1-yl-N-[4-(4-fluoro...)Show SMILES Fc1ccc(O[C@@H]2CC[C@@H](CC2)NC(=O)CC23CC4CC(CC(C4)C2)C3)cc1 |wD:9.12,6.5,TLB:15:16:19.18.23:21,THB:17:18:21:25.16.24,17:16:19.18.23:21,24:16:19:23.22.21,24:22:19:25.17.16,15:16:19:23.22.21,(36.25,-9.09,;34.71,-9.15,;33.99,-10.51,;32.45,-10.57,;31.63,-9.26,;30.09,-9.32,;29.38,-10.68,;30.2,-11.99,;29.48,-13.33,;27.93,-13.39,;27.11,-12.1,;27.84,-10.73,;27.22,-14.75,;25.68,-14.81,;24.85,-13.52,;24.96,-16.18,;23.42,-16.24,;22.4,-17.52,;21,-16.96,;19.5,-17.37,;20.69,-16.1,;20.69,-14.61,;22.03,-14.14,;21,-15.37,;23.43,-14.71,;22.02,-16.59,;32.35,-7.9,;33.89,-7.84,)| Show InChI InChI=1S/C24H32FNO2/c25-19-1-5-21(6-2-19)28-22-7-3-20(4-8-22)26-23(27)15-24-12-16-9-17(13-24)11-18(10-16)14-24/h1-2,5-6,16-18,20,22H,3-4,7-15H2,(H,26,27)/t16?,17?,18?,20-,22+,24? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Mus musculus (Mouse)) | BDBM25737

(12-[(adamantan-1-ylcarbamoyl)amino]dodecanoic acid...)Show SMILES OC(=O)CCCCCCCCCCCNC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:25:20:27:24.23.26,25:24:20.21.19:27,THB:23:22:19:24.25.26,23:24:19:22.21.27| Show InChI InChI=1S/C23H40N2O3/c26-21(27)10-8-6-4-2-1-3-5-7-9-11-24-22(28)25-23-15-18-12-19(16-23)14-20(13-18)17-23/h18-20H,1-17H2,(H,26,27)(H2,24,25,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant soluble epoxide hydrolase by fluorescent assay |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM25737

(12-[(adamantan-1-ylcarbamoyl)amino]dodecanoic acid...)Show SMILES OC(=O)CCCCCCCCCCCNC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:25:20:27:24.23.26,25:24:20.21.19:27,THB:23:22:19:24.25.26,23:24:19:22.21.27| Show InChI InChI=1S/C23H40N2O3/c26-21(27)10-8-6-4-2-1-3-5-7-9-11-24-22(28)25-23-15-18-12-19(16-23)14-20(13-18)17-23/h18-20H,1-17H2,(H,26,27)(H2,24,25,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant soluble epoxide hydrolase by fluorescent assay |
J Med Chem 50: 3825-40 (2007)
Article DOI: 10.1021/jm070270t
BindingDB Entry DOI: 10.7270/Q2TQ62BW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data