

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50034137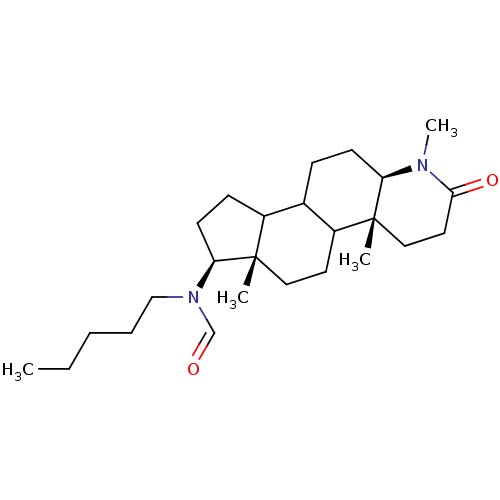 (CHEMBL275153 | N-Pentyl-N-((4aR,6aS,7S,11aR)-1,4a,...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of human steroid 5-alpha-reductase type I in Du-145 cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50034151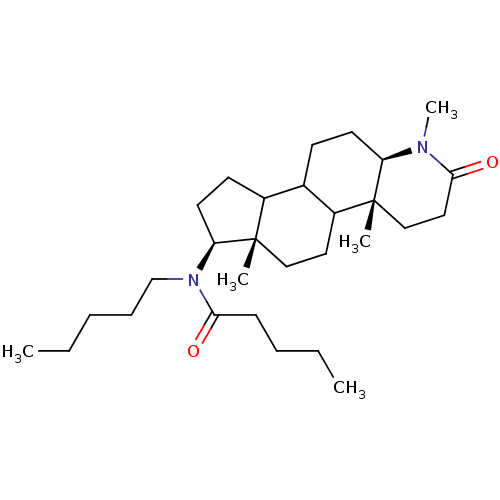 (CHEMBL280015 | Pentanoic acid pentyl-((4aR,6aS,7S,...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of human steroid 5-alpha-reductase type I in Du-145 cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50034161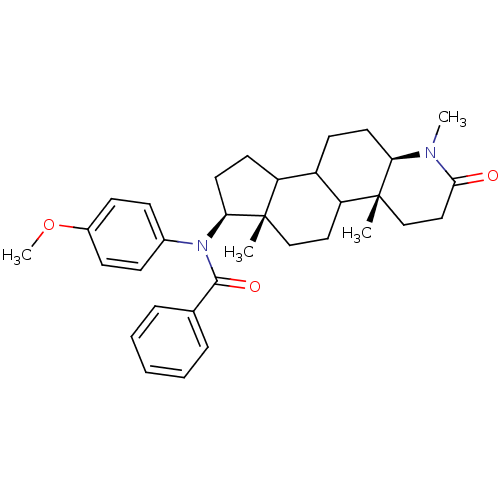 (CHEMBL17245 | N-(4-Methoxy-phenyl)-N-((4aR,6aS,7S,...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of human steroid 5-alpha-reductase type I in Du-145 cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50034134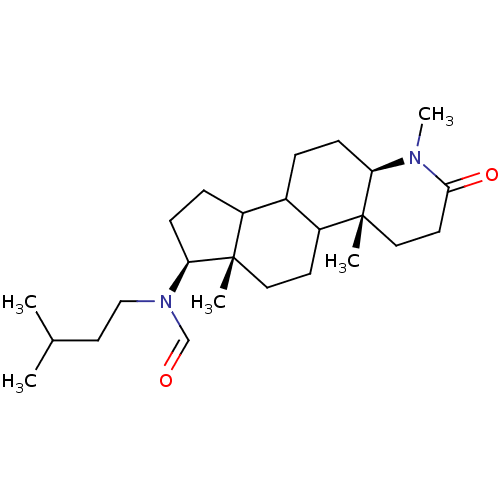 (CHEMBL17222 | N-(3-Methyl-butyl)-N-((4aR,6aS,7S,11...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of human steroid 5-alpha-reductase type I in Du-145 cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50034142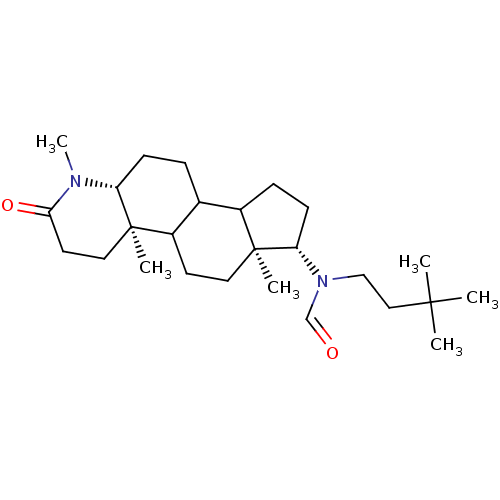 (CHEMBL16863 | N-(3,3-Dimethyl-butyl)-N-((4aR,6aS,7...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of human steroid 5-alpha-reductase type I in Du-145 cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50034135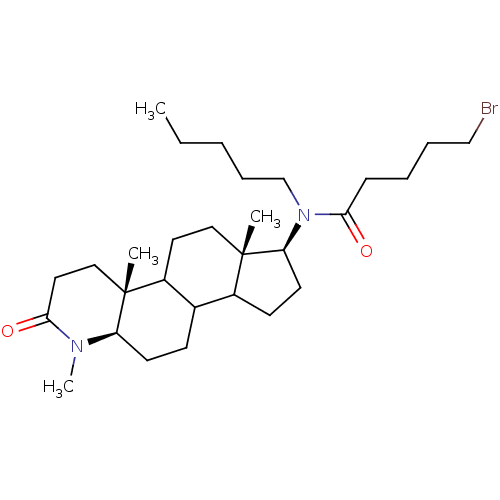 (5-Bromo-pentanoic acid pentyl-((4aR,6aS,7S,11aR)-1...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of human steroid 5-alpha-reductase type I in Du-145 cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50034157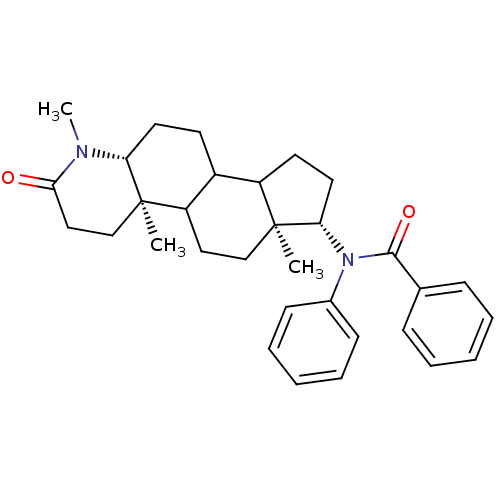 (CHEMBL276320 | N-Phenyl-N-((4aR,6aS,7S,11aR)-1,4a,...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of human steroid 5-alpha-reductase type I in Du-145 cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50034143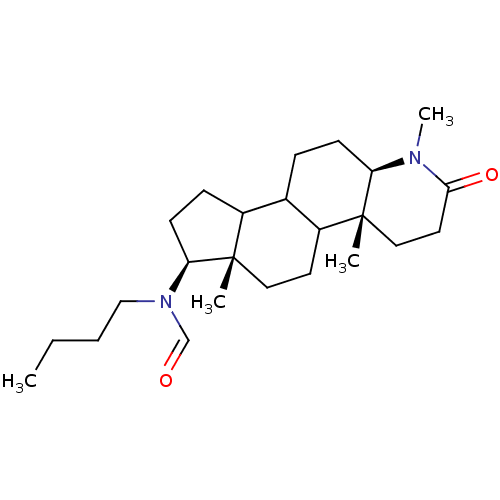 (CHEMBL277053 | N-Butyl-N-((4aR,6aS,7S,11aR)-1,4a,6...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of human steroid 5-alpha-reductase type I in Du-145 cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50034141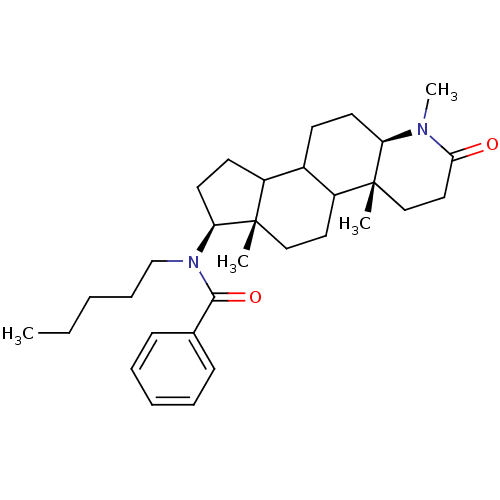 (CHEMBL16784 | N-Pentyl-N-((4aR,6aS,7S,11aR)-1,4a,6...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of human steroid 5-alpha-reductase type I in Du-145 cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50034159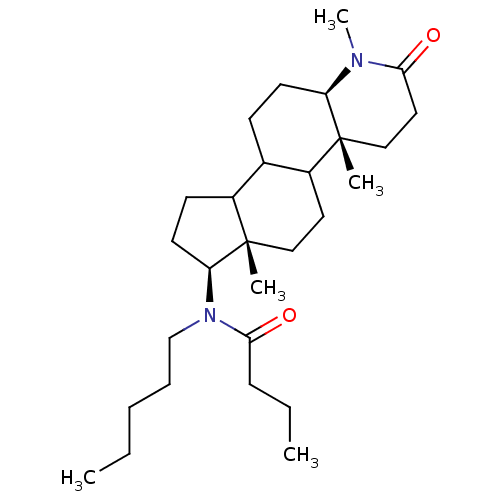 (CHEMBL278490 | N-Pentyl-N-((4aR,6aS,7S,11aR)-1,4a,...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of human steroid 5-alpha-reductase type I in Du-145 cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50034157 (CHEMBL276320 | N-Phenyl-N-((4aR,6aS,7S,11aR)-1,4a,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of human steroid 5-alpha-reductase type 2 in SW-13-transfected cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50034147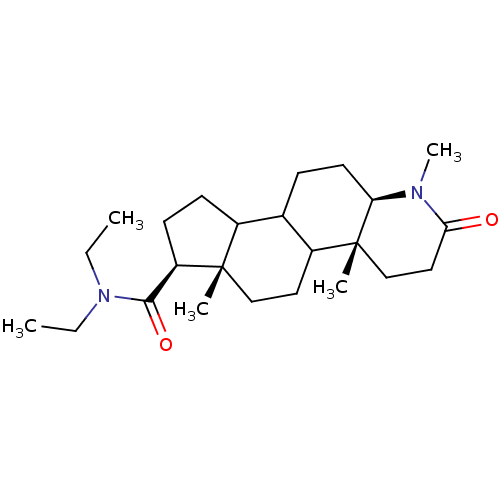 ((4aR,6aS,7S,11aR)-1,4a,6a-Trimethyl-2-oxo-hexadeca...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of human steroid 5-alpha-reductase type I in Du-145 cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50334788 ((17beta-(N-tert-butylcarbamoyl)-4-aza-5alpha-andro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of human steroid 5-alpha-reductase type 2 in SW-13-transfected cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50034130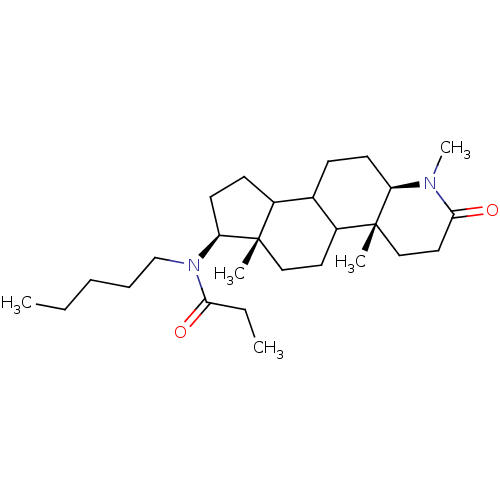 (CHEMBL17194 | N-Pentyl-N-((4aR,6aS,7S,11aR)-1,4a,6...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of human steroid 5-alpha-reductase type I in Du-145 cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50034132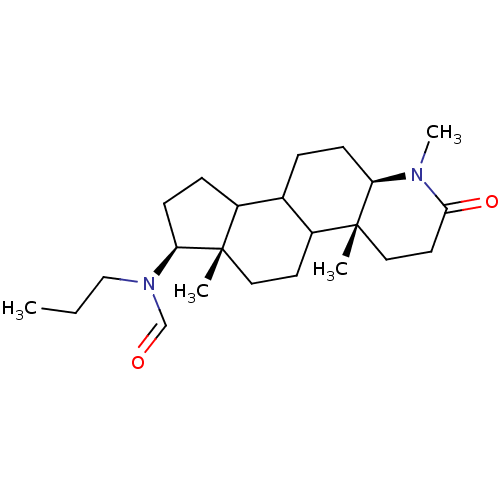 (CHEMBL274276 | N-Propyl-N-((4aR,6aS,7S,11aR)-1,4a,...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of human steroid 5-alpha-reductase type I in Du-145 cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50034150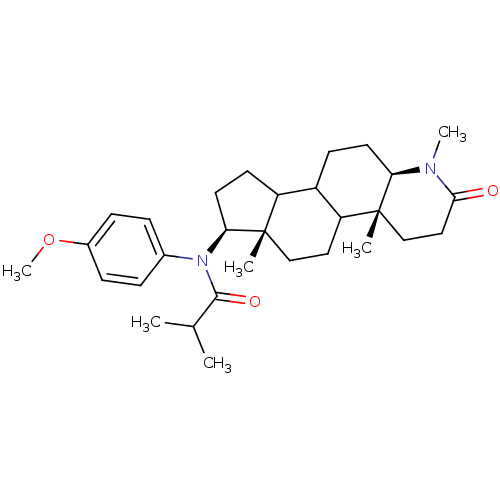 (CHEMBL16700 | N-(4-Methoxy-phenyl)-N-((4aR,6aS,7S,...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of human steroid 5-alpha-reductase type I in Du-145 cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50034140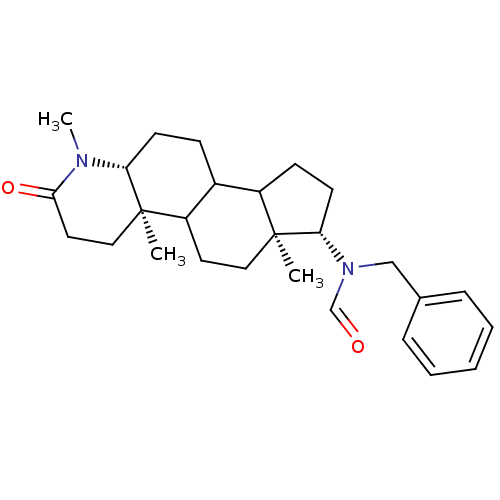 (CHEMBL276527 | N-Benzyl-N-((4aR,6aS,7S,11aR)-1,4a,...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of human steroid 5-alpha-reductase type I in Du-145 cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50034139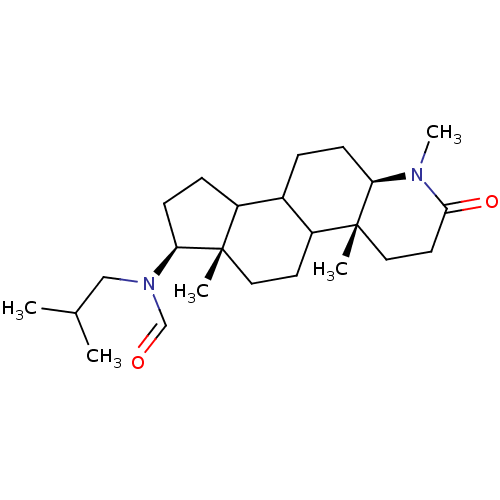 (CHEMBL16690 | N-Isobutyl-N-((4aR,6aS,7S,11aR)-1,4a...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of human steroid 5-alpha-reductase type I in Du-145 cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50034154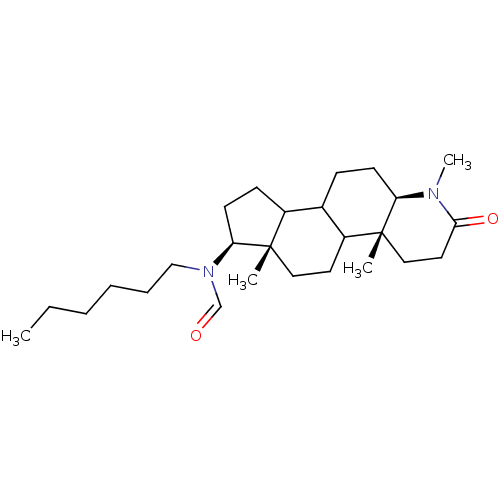 (CHEMBL17506 | N-Hexyl-N-((4aR,6aS,7S,11aR)-1,4a,6a...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of human steroid 5-alpha-reductase type I in Du-145 cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50034147 ((4aR,6aS,7S,11aR)-1,4a,6a-Trimethyl-2-oxo-hexadeca...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of human steroid 5-alpha-reductase type 2 in SW-13-transfected cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50034153 (CHEMBL17268 | N-Isopropyl-N-((4aR,6aS,7S,11aR)-1,4...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of human steroid 5-alpha-reductase type I in Du-145 cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50034133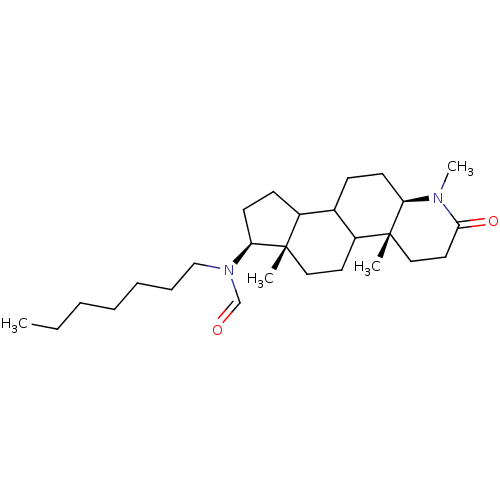 (CHEMBL17206 | N-Heptyl-N-((4aR,6aS,7S,11aR)-1,4a,6...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of human steroid 5-alpha-reductase type I in Du-145 cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50034145 (CHEMBL276355 | N-Cyclopropyl-N-((4aR,6aS,7S,11aR)-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of human steroid 5-alpha-reductase type I in Du-145 cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50034144 (CHEMBL16533 | N-(1-Ethyl-propyl)-N-((4aR,6aS,7S,11...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of human steroid 5-alpha-reductase type I in Du-145 cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50034162 (CHEMBL266519 | N-Phenyl-N-((4aR,6aS,7S,11aR)-1,4a,...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of human steroid 5-alpha-reductase type I in Du-145 cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50034138 (CHEMBL16927 | N-Cyclohexyl-N-((4aR,6aS,7S,11aR)-1,...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of human steroid 5-alpha-reductase type I in Du-145 cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50034155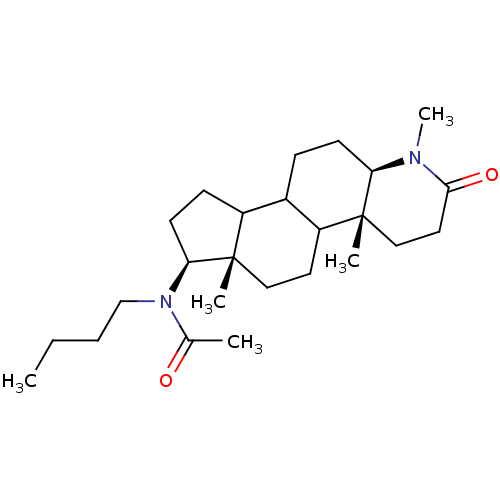 (CHEMBL17514 | N-Butyl-N-((4aR,6aS,7S,11aR)-1,4a,6a...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of human steroid 5-alpha-reductase type I in Du-145 cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50034133 (CHEMBL17206 | N-Heptyl-N-((4aR,6aS,7S,11aR)-1,4a,6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of human steroid 5-alpha-reductase type 2 in SW-13-transfected cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50034146 (CHEMBL417627 | N-Octyl-N-((4aR,6aS,7S,11aR)-1,4a,6...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of human steroid 5-alpha-reductase type I in Du-145 cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50034146 (CHEMBL417627 | N-Octyl-N-((4aR,6aS,7S,11aR)-1,4a,6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of human steroid 5-alpha-reductase type 2 in SW-13-transfected cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50334788 ((17beta-(N-tert-butylcarbamoyl)-4-aza-5alpha-andro...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of human steroid 5-alpha-reductase type I in Du-145 cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50034163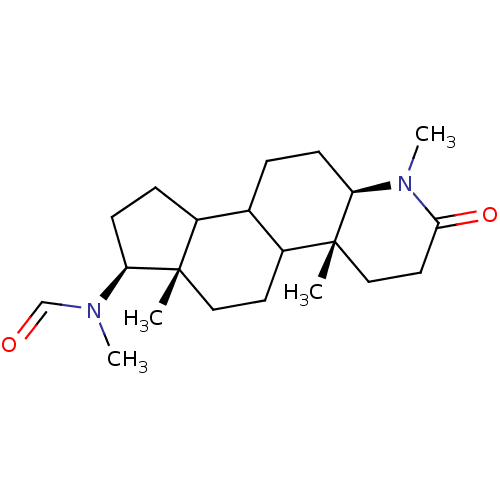 (CHEMBL17193 | N-Methyl-N-((4aR,6aS,7S,11aR)-1,4a,6...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of human steroid 5-alpha-reductase type I in Du-145 cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50034148 (CHEMBL17473 | N-(4-Nitro-phenyl)-N-((4aR,6aS,7S,11...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of human steroid 5-alpha-reductase type I in Du-145 cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Mus musculus) | BDBM50034134 (CHEMBL17222 | N-(3-Methyl-butyl)-N-((4aR,6aS,7S,11...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of DHT (dihydrotestosterone) on proliferation of androgen-sensitive cancer Schionogi (SC-3) cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Mus musculus) | BDBM50034146 (CHEMBL417627 | N-Octyl-N-((4aR,6aS,7S,11aR)-1,4a,6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of DHT (dihydrotestosterone) on proliferation of androgen-sensitive cancer Schionogi (SC-3) cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Mus musculus) | BDBM35909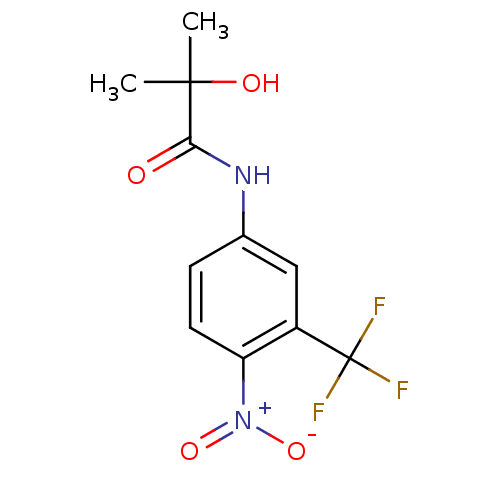 (2-Hydroxy-2-methyl-N-(4-nitro-3-trifluoromethyl-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of DHT (dihydrotestosterone) on proliferation of androgen-sensitive cancer Schionogi (SC-3) cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Mus musculus) | BDBM50034133 (CHEMBL17206 | N-Heptyl-N-((4aR,6aS,7S,11aR)-1,4a,6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of DHT (dihydrotestosterone) on proliferation of androgen-sensitive cancer Schionogi (SC-3) cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50034149 (CHEMBL17422 | N-(4-Nitro-3-trifluoromethyl-phenyl)...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of human steroid 5-alpha-reductase type I in Du-145 cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Mus musculus) | BDBM50034154 (CHEMBL17506 | N-Hexyl-N-((4aR,6aS,7S,11aR)-1,4a,6a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of DHT (dihydrotestosterone) on proliferation of androgen-sensitive cancer Schionogi (SC-3) cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Mus musculus) | BDBM50034144 (CHEMBL16533 | N-(1-Ethyl-propyl)-N-((4aR,6aS,7S,11...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of DHT (dihydrotestosterone) on proliferation of androgen-sensitive cancer Schionogi (SC-3) cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Mus musculus) | BDBM50034137 (CHEMBL275153 | N-Pentyl-N-((4aR,6aS,7S,11aR)-1,4a,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of DHT (dihydrotestosterone) on proliferation of androgen-sensitive cancer Schionogi (SC-3) cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50034144 (CHEMBL16533 | N-(1-Ethyl-propyl)-N-((4aR,6aS,7S,11...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of human steroid 5-alpha-reductase type 2 in SW-13-transfected cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50034139 (CHEMBL16690 | N-Isobutyl-N-((4aR,6aS,7S,11aR)-1,4a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of human steroid 5-alpha-reductase type 2 in SW-13-transfected cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50034154 (CHEMBL17506 | N-Hexyl-N-((4aR,6aS,7S,11aR)-1,4a,6a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of human steroid 5-alpha-reductase type 2 in SW-13-transfected cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50034140 (CHEMBL276527 | N-Benzyl-N-((4aR,6aS,7S,11aR)-1,4a,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of human steroid 5-alpha-reductase type 2 in SW-13-transfected cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50034134 (CHEMBL17222 | N-(3-Methyl-butyl)-N-((4aR,6aS,7S,11...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of human steroid 5-alpha-reductase type 2 in SW-13-transfected cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50034143 (CHEMBL277053 | N-Butyl-N-((4aR,6aS,7S,11aR)-1,4a,6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of human steroid 5-alpha-reductase type 2 in SW-13-transfected cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50034153 (CHEMBL17268 | N-Isopropyl-N-((4aR,6aS,7S,11aR)-1,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of human steroid 5-alpha-reductase type 2 in SW-13-transfected cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50034145 (CHEMBL276355 | N-Cyclopropyl-N-((4aR,6aS,7S,11aR)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of human steroid 5-alpha-reductase type 2 in SW-13-transfected cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50034142 (CHEMBL16863 | N-(3,3-Dimethyl-butyl)-N-((4aR,6aS,7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of human steroid 5-alpha-reductase type 2 in SW-13-transfected cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 81 total ) | Next | Last >> |