

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Probable maltase-glucoamylase 2 (Homo sapiens) | BDBM50241453 (1,3,6,7-tetrahydroxy-2,8-bis(3-methylbut-2-enyl)-9...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of alpha-glucosidase (unknown origin) using PNP-G as substrate by Dixon plot analysis | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50454316 (CHEMBL4211682) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using P-NPP as substrate after 10 mins by Dixon plot analysis | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50454316 (CHEMBL4211682) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Time dependent inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using P-NPP as substrate preincubated with enz... | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50241453 (1,3,6,7-tetrahydroxy-2,8-bis(3-methylbut-2-enyl)-9...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using P-NPP as substrate after 10 mins by Dixon plot analysis | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50346335 (CHEMBL1782241 | Cochinchinone A) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using P-NPP as substrate after 10 mins by Dixon plot analysis | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50214969 (1,3,6-Trihydroxy-7-methoxy-2,8-bis-(3-methyl-but-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using P-NPP as substrate after 10 mins by Dixon plot analysis | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50454321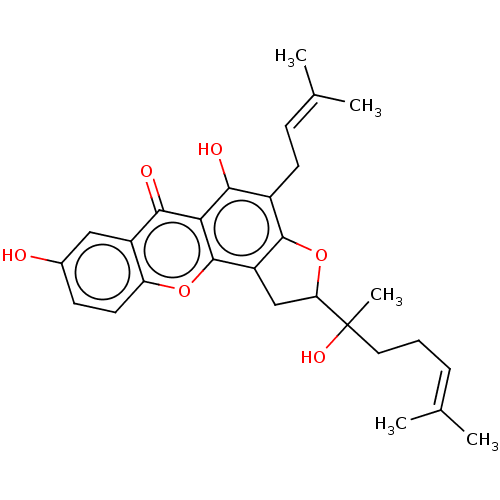 (CHEMBL4208135) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using P-NPP as substrate after 10 mins by Dixon plot analysis | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable maltase-glucoamylase 2 (Homo sapiens) | BDBM50454316 (CHEMBL4211682) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of alpha-glucosidase (unknown origin) using PNP-G as substrate by Dixon plot analysis | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50454319 (CHEMBL4214309) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using P-NPP as substrate after 10 mins by Dixon plot analysis | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable maltase-glucoamylase 2 (Homo sapiens) | BDBM50454316 (CHEMBL4211682) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Mixed type inhibition of alpha-glucosidase (unknown origin) assessed as enzyme-substrate-inhibitor complex formation using PNP-G as substrate by Line... | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable maltase-glucoamylase 2 (Homo sapiens) | BDBM50214969 (1,3,6-Trihydroxy-7-methoxy-2,8-bis-(3-methyl-but-2...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of alpha-glucosidase (unknown origin) using PNP-G as substrate by Dixon plot analysis | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable maltase-glucoamylase 2 (Homo sapiens) | BDBM50454317 (CHEMBL4203265) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of alpha-glucosidase (unknown origin) using PNP-G as substrate by Dixon plot analysis | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50454317 (CHEMBL4203265) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using P-NPP as substrate after 10 mins by Dixon plot analysis | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable maltase-glucoamylase 2 (Homo sapiens) | BDBM50454321 (CHEMBL4208135) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of alpha-glucosidase (unknown origin) using PNP-G as substrate by Dixon plot analysis | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50346334 (1,3,7-trihydroxy-2,4-diisoprenylxanthone | CHEMBL1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using P-NPP as substrate after 10 mins by Dixon plot analysis | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable maltase-glucoamylase 2 (Homo sapiens) | BDBM50454318 (CHEMBL4202640) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of alpha-glucosidase (unknown origin) using PNP-G as substrate by Dixon plot analysis | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable maltase-glucoamylase 2 (Homo sapiens) | BDBM50346335 (CHEMBL1782241 | Cochinchinone A) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of alpha-glucosidase (unknown origin) using PNP-G as substrate by Dixon plot analysis | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable maltase-glucoamylase 2 (Homo sapiens) | BDBM50454323 (CHEMBL4209191) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of alpha-glucosidase (unknown origin) using PNP-G as substrate by Dixon plot analysis | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable maltase-glucoamylase 2 (Homo sapiens) | BDBM50346334 (1,3,7-trihydroxy-2,4-diisoprenylxanthone | CHEMBL1...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of alpha-glucosidase (unknown origin) using PNP-G as substrate by Dixon plot analysis | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50454323 (CHEMBL4209191) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using P-NPP as substrate after 10 mins by Dixon plot analysis | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50454320 (CHEMBL4217516) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 2.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using P-NPP as substrate after 10 mins by Dixon plot analysis | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable maltase-glucoamylase 2 (Homo sapiens) | BDBM50454320 (CHEMBL4217516) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of alpha-glucosidase (unknown origin) using PNP-G as substrate by Dixon plot analysis | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable maltase-glucoamylase 2 (Homo sapiens) | BDBM50454319 (CHEMBL4214309) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of alpha-glucosidase (unknown origin) using PNP-G as substrate by Dixon plot analysis | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50454318 (CHEMBL4202640) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using P-NPP as substrate after 10 mins by Dixon plot analysis | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50454322 (CHEMBL4218731) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using P-NPP as substrate after 10 mins by Dixon plot analysis | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable maltase-glucoamylase 2 (Homo sapiens) | BDBM50454322 (CHEMBL4218731) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of alpha-glucosidase (unknown origin) using PNP-G as substrate by Dixon plot analysis | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable maltase-glucoamylase 2 (Homo sapiens) | BDBM50241453 (1,3,6,7-tetrahydroxy-2,8-bis(3-methylbut-2-enyl)-9...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of alpha-glucosidase (unknown origin) using PNP-G as substrate measured for 15 mins by spectrophotometry | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50454316 (CHEMBL4211682) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using P-NPP as substrate after 10 mins by spectrophotometry | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50241453 (1,3,6,7-tetrahydroxy-2,8-bis(3-methylbut-2-enyl)-9...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using P-NPP as substrate after 10 mins by spectrophotometry | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable maltase-glucoamylase 2 (Homo sapiens) | BDBM50454316 (CHEMBL4211682) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of alpha-glucosidase (unknown origin) using PNP-G as substrate measured for 15 mins by spectrophotometry | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50346335 (CHEMBL1782241 | Cochinchinone A) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using P-NPP as substrate after 10 mins by spectrophotometry | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50214969 (1,3,6-Trihydroxy-7-methoxy-2,8-bis-(3-methyl-but-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using P-NPP as substrate after 10 mins by spectrophotometry | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable maltase-glucoamylase 2 (Homo sapiens) | BDBM50214969 (1,3,6-Trihydroxy-7-methoxy-2,8-bis-(3-methyl-but-2...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of alpha-glucosidase (unknown origin) using PNP-G as substrate measured for 15 mins by spectrophotometry | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50454319 (CHEMBL4214309) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using P-NPP as substrate after 10 mins by spectrophotometry | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50454321 (CHEMBL4208135) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using P-NPP as substrate after 10 mins by spectrophotometry | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable maltase-glucoamylase 2 (Homo sapiens) | BDBM50454317 (CHEMBL4203265) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of alpha-glucosidase (unknown origin) using PNP-G as substrate measured for 15 mins by spectrophotometry | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50346334 (1,3,7-trihydroxy-2,4-diisoprenylxanthone | CHEMBL1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using P-NPP as substrate after 10 mins by spectrophotometry | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50454317 (CHEMBL4203265) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using P-NPP as substrate after 10 mins by spectrophotometry | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable maltase-glucoamylase 2 (Homo sapiens) | BDBM50454318 (CHEMBL4202640) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of alpha-glucosidase (unknown origin) using PNP-G as substrate measured for 15 mins by spectrophotometry | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50148911 ((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using P-NPP as substrate after 10 mins by spectrophotometry | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable maltase-glucoamylase 2 (Homo sapiens) | BDBM50454321 (CHEMBL4208135) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of alpha-glucosidase (unknown origin) using PNP-G as substrate measured for 15 mins by spectrophotometry | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable maltase-glucoamylase 2 (Homo sapiens) | BDBM50346335 (CHEMBL1782241 | Cochinchinone A) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of alpha-glucosidase (unknown origin) using PNP-G as substrate measured for 15 mins by spectrophotometry | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable maltase-glucoamylase 2 (Homo sapiens) | BDBM50454323 (CHEMBL4209191) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of alpha-glucosidase (unknown origin) using PNP-G as substrate measured for 15 mins by spectrophotometry | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50454320 (CHEMBL4217516) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using P-NPP as substrate after 10 mins by spectrophotometry | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50454318 (CHEMBL4202640) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using P-NPP as substrate after 10 mins by spectrophotometry | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable maltase-glucoamylase 2 (Homo sapiens) | BDBM50346334 (1,3,7-trihydroxy-2,4-diisoprenylxanthone | CHEMBL1...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of alpha-glucosidase (unknown origin) using PNP-G as substrate measured for 15 mins by spectrophotometry | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable maltase-glucoamylase 2 (Homo sapiens) | BDBM50454319 (CHEMBL4214309) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of alpha-glucosidase (unknown origin) using PNP-G as substrate measured for 15 mins by spectrophotometry | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable maltase-glucoamylase 2 (Homo sapiens) | BDBM50454320 (CHEMBL4217516) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of alpha-glucosidase (unknown origin) using PNP-G as substrate measured for 15 mins by spectrophotometry | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50454323 (CHEMBL4209191) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using P-NPP as substrate after 10 mins by spectrophotometry | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable maltase-glucoamylase 2 (Homo sapiens) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of alpha-glucosidase (unknown origin) using PNP-G as substrate measured for 15 mins by spectrophotometry | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 52 total ) | Next | Last >> |