

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50468698 (CHEMBL4277548) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Displacement of (+)-[3H]pentazocine from guinea pig brain membrane sigma1 receptor by liquid scintillation counting method | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50468689 (CHEMBL4292477) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Displacement of (+)-[3H]pentazocine from guinea pig brain membrane sigma1 receptor by liquid scintillation counting method | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50468682 (CHEMBL4284165) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Displacement of (+)-[3H]pentazocine from guinea pig brain membrane sigma1 receptor by liquid scintillation counting method | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50468700 (CHEMBL4287548) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Displacement of (+)-[3H]pentazocine from guinea pig brain membrane sigma1 receptor by liquid scintillation counting method | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50468699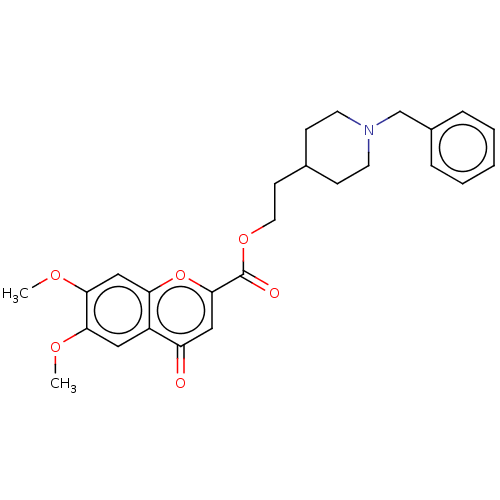 (CHEMBL4284495) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE expressed in HEK293 cells using varying levels of acetylthiocholine iodide as substrate by Lineweaver-burk plot ... | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50468682 (CHEMBL4284165) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE expressed in HEK293 cells using varying levels of acetylthiocholine iodide as substrate by Lineweaver-burk plot ... | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50468698 (CHEMBL4277548) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE expressed in HEK293 cells using varying levels of acetylthiocholine iodide as substrate by Lineweaver-burk plot ... | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50001028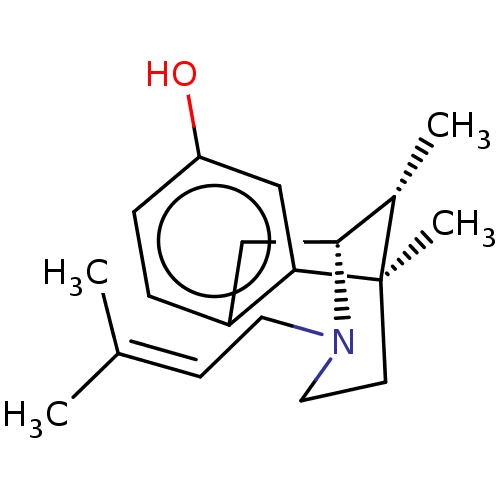 ((+)-PENTAZOCINE | (-)-pentazocine | (2R,6R,11R)-6,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Displacement of (+)-[3H]pentazocine from guinea pig brain membrane sigma1 receptor by liquid scintillation counting method | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50468687 (CHEMBL4294783) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE expressed in HEK293 cells using varying levels of acetylthiocholine iodide as substrate by Lineweaver-burk plot ... | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50468697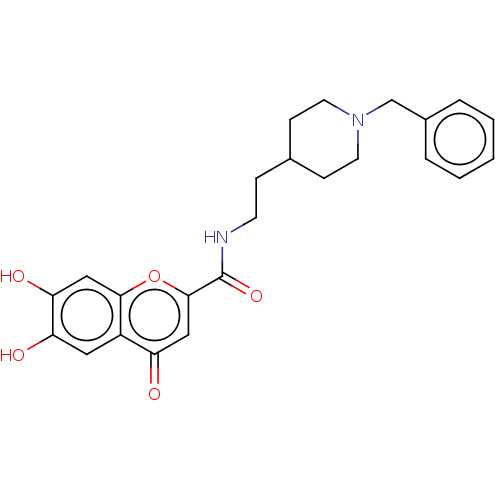 (CHEMBL4280725) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Displacement of (+)-[3H]pentazocine from guinea pig brain membrane sigma1 receptor by liquid scintillation counting method | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50468685 (CHEMBL4293801) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Displacement of (+)-[3H]pentazocine from guinea pig brain membrane sigma1 receptor by liquid scintillation counting method | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50468681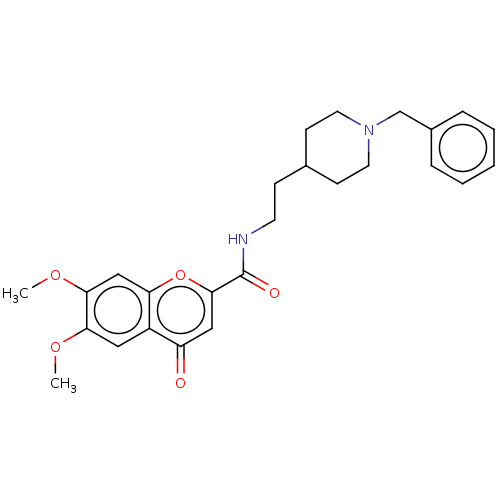 (CHEMBL4287970) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Displacement of (+)-[3H]pentazocine from guinea pig brain membrane sigma1 receptor by liquid scintillation counting method | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50468681 (CHEMBL4287970) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE expressed in HEK293 cells using varying levels of acetylthiocholine iodide as substrate by Lineweaver-burk plot ... | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50468680 (CHEMBL4291296) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Displacement of (+)-[3H]pentazocine from guinea pig brain membrane sigma1 receptor by liquid scintillation counting method | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50468677 (CHEMBL4282527) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Displacement of (+)-[3H]pentazocine from guinea pig brain membrane sigma1 receptor by liquid scintillation counting method | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50468679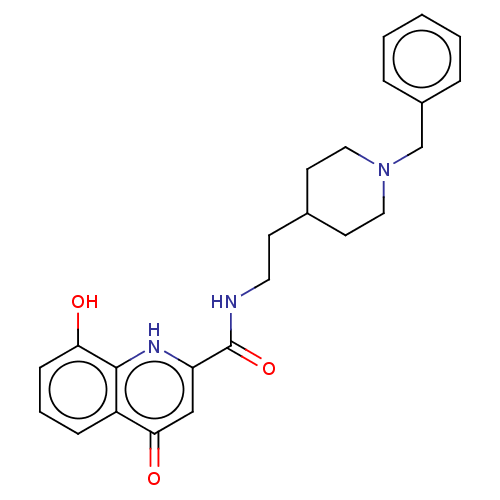 (CHEMBL4285912) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Displacement of (+)-[3H]pentazocine from guinea pig brain membrane sigma1 receptor by liquid scintillation counting method | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Rattus norvegicus (Rat)) | BDBM50009307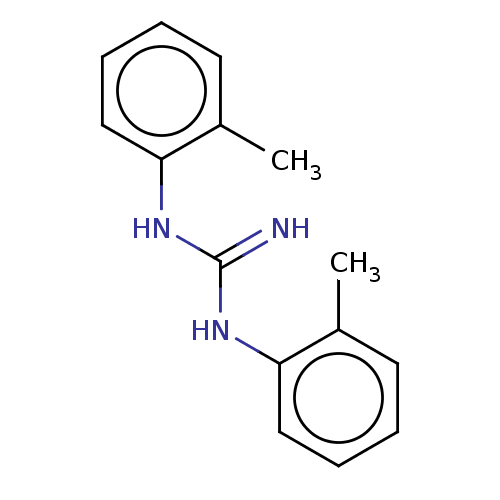 (DITOLYLGUANIDINE | Di-o-tolylguanidine) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Displacement of [3H]DTG from sigma2 receptor in rat liver membranes by liquid scintillation counting method | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50468702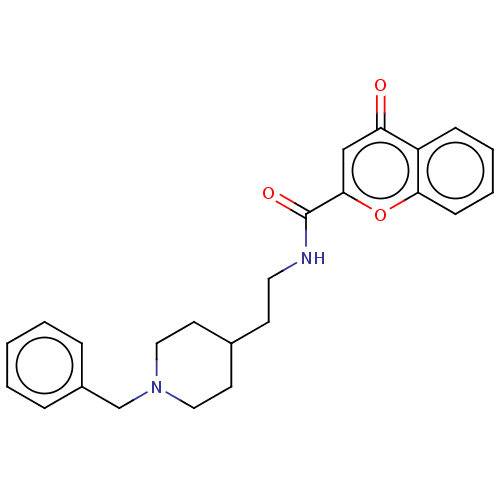 (CHEMBL4286799) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE expressed in HEK293 cells using varying levels of acetylthiocholine iodide as substrate by Lineweaver-burk plot ... | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50468685 (CHEMBL4293801) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE expressed in HEK293 cells using varying levels of acetylthiocholine iodide as substrate by Lineweaver-burk plot ... | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50468689 (CHEMBL4292477) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE expressed in HEK293 cells using varying levels of acetylthiocholine iodide as substrate by Lineweaver-burk plot ... | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Rattus norvegicus (Rat)) | BDBM50468681 (CHEMBL4287970) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 239 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Displacement of [3H]DTG from sigma2 receptor in rat liver membranes by liquid scintillation counting method | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Rattus norvegicus (Rat)) | BDBM50468697 (CHEMBL4280725) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 278 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Displacement of [3H]DTG from sigma2 receptor in rat liver membranes by liquid scintillation counting method | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Rattus norvegicus (Rat)) | BDBM50468698 (CHEMBL4277548) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 287 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Displacement of [3H]DTG from sigma2 receptor in rat liver membranes by liquid scintillation counting method | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Rattus norvegicus (Rat)) | BDBM50468679 (CHEMBL4285912) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Displacement of [3H]DTG from sigma2 receptor in rat liver membranes by liquid scintillation counting method | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Rattus norvegicus (Rat)) | BDBM50468682 (CHEMBL4284165) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 301 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Displacement of [3H]DTG from sigma2 receptor in rat liver membranes by liquid scintillation counting method | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Rattus norvegicus (Rat)) | BDBM50468680 (CHEMBL4291296) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 345 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Displacement of [3H]DTG from sigma2 receptor in rat liver membranes by liquid scintillation counting method | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Rattus norvegicus (Rat)) | BDBM50468677 (CHEMBL4282527) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 376 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Displacement of [3H]DTG from sigma2 receptor in rat liver membranes by liquid scintillation counting method | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Rattus norvegicus (Rat)) | BDBM50468689 (CHEMBL4292477) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 421 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Displacement of [3H]DTG from sigma2 receptor in rat liver membranes by liquid scintillation counting method | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Rattus norvegicus (Rat)) | BDBM50468700 (CHEMBL4287548) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 459 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Displacement of [3H]DTG from sigma2 receptor in rat liver membranes by liquid scintillation counting method | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Rattus norvegicus (Rat)) | BDBM50468685 (CHEMBL4293801) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 487 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Displacement of [3H]DTG from sigma2 receptor in rat liver membranes by liquid scintillation counting method | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrat... | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM15579 (CHEMBL972 | DEPRENYL | L-Deprenyl | N-methyl-N-[(2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B expressed in baculovirus infected BTI insect cells using p-tyramine as substrate pretreated for 15 mins followe... | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50468698 (CHEMBL4277548) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrat... | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50468682 (CHEMBL4284165) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrat... | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50468690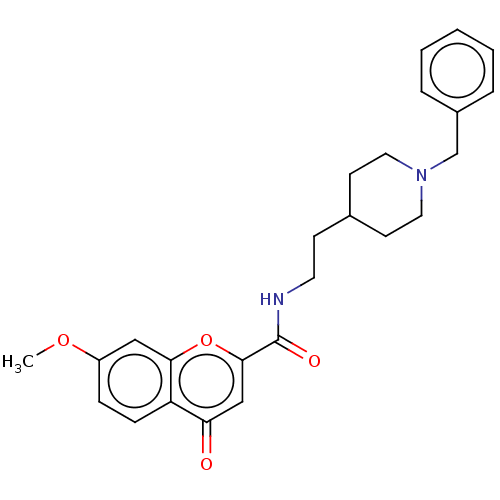 (CHEMBL4285779) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrat... | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50468689 (CHEMBL4292477) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrat... | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50468681 (CHEMBL4287970) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrat... | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50468699 (CHEMBL4284495) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrat... | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50468680 (CHEMBL4291296) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrat... | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM32020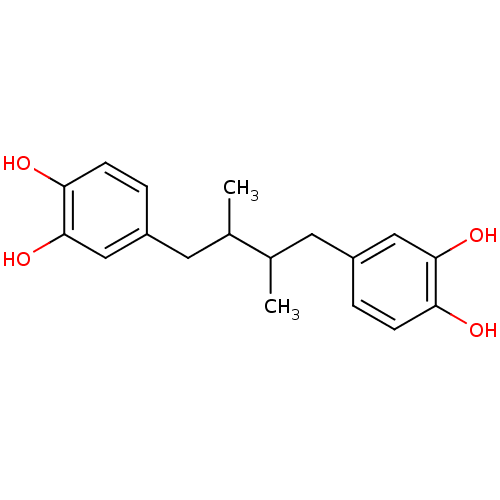 (4-[4-(3,4-dihydroxyphenyl)-2,3-dimethyl-butyl]pyro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Inhibition of human 5-lipoxygenase using arachidonic acid as substrate pretreated for 10 mins followed by substrate and ATP addition and measured aft... | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50468687 (CHEMBL4294783) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrat... | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50468702 (CHEMBL4286799) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrat... | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50000541 ((+-)-1-(1-Benzo[b]thien-2-ylethyl)-1-hydroxyurea |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Inhibition of human 5-lipoxygenase using arachidonic acid as substrate pretreated for 10 mins followed by substrate and ATP addition and measured aft... | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50468685 (CHEMBL4293801) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrat... | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50468691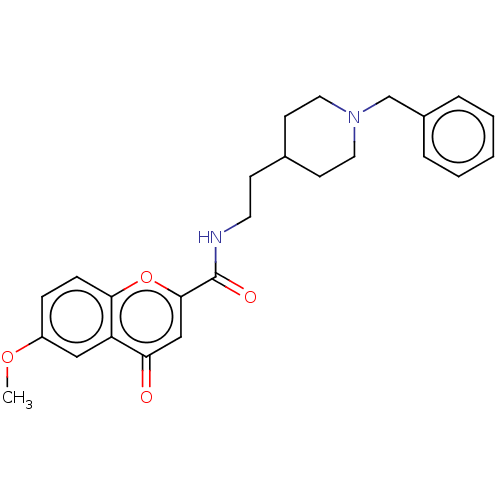 (CHEMBL4283450) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrat... | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50468686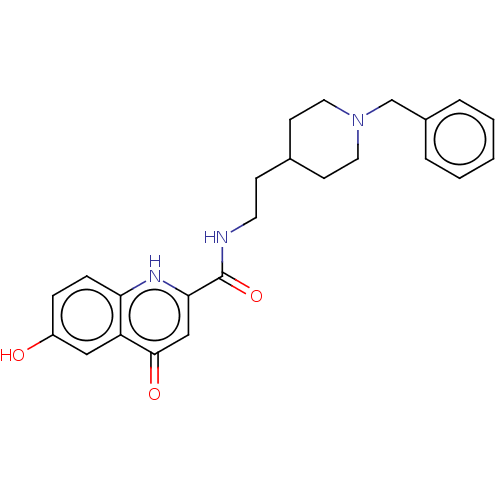 (CHEMBL4294609) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrat... | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50468694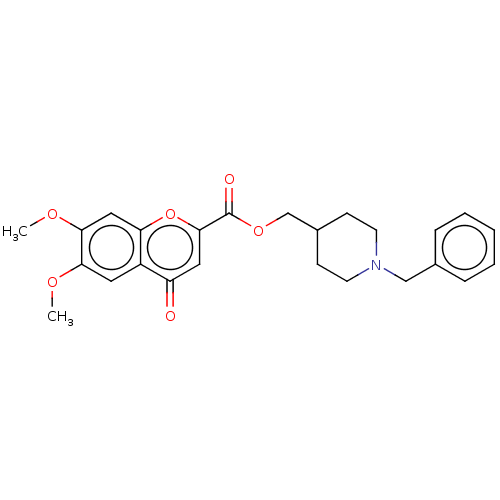 (CHEMBL4285601) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrat... | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50468705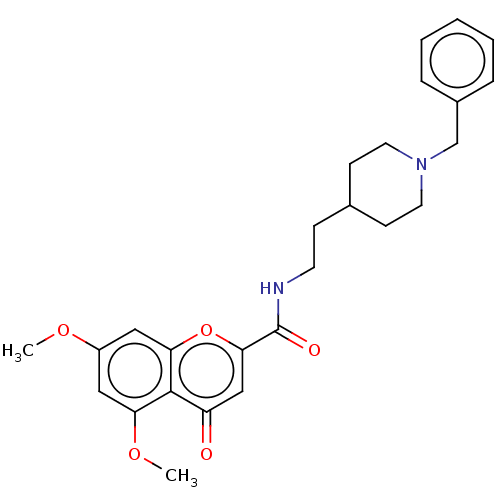 (CHEMBL4284591) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrat... | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50468684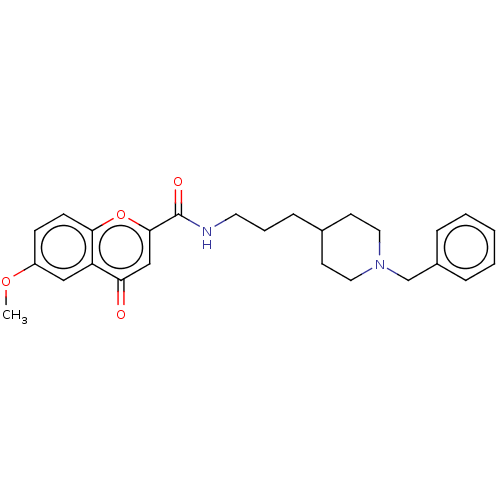 (CHEMBL4291372) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrat... | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50468677 (CHEMBL4282527) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Consejo Superior de Investigaciones Cient�ficas (IQM-CSIC) Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrat... | Eur J Med Chem 156: 534-553 (2018) Article DOI: 10.1016/j.ejmech.2018.07.026 BindingDB Entry DOI: 10.7270/Q2N300PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 149 total ) | Next | Last >> |