 Found 98 hits Enz. Inhib. hit(s) with all data for entry = 50007931
Found 98 hits Enz. Inhib. hit(s) with all data for entry = 50007931 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50514081

(CHEMBL4471466)Show SMILES NCc1cccc(C[C@H](NC(=O)[C@@H](CCCc2ccccc2)NS(=O)(=O)Cc2cccc(c2)C(O)=O)C(=O)NCc2ccc(cc2)C(N)=N)c1 |r| Show InChI InChI=1S/C37H42N6O6S/c38-22-28-11-4-10-27(19-28)21-33(35(44)41-23-26-15-17-30(18-16-26)34(39)40)42-36(45)32(14-6-9-25-7-2-1-3-8-25)43-50(48,49)24-29-12-5-13-31(20-29)37(46)47/h1-5,7-8,10-13,15-20,32-33,43H,6,9,14,21-24,38H2,(H3,39,40)(H,41,44)(H,42,45)(H,46,47)/t32-,33+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein by Michaelis-menten analysis |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50514086
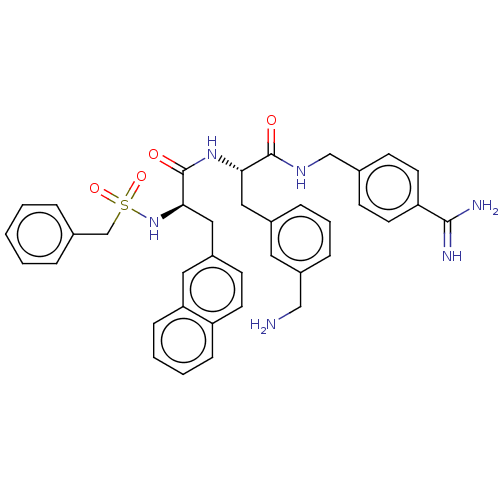
(CHEMBL4524734)Show SMILES NCc1cccc(C[C@H](NC(=O)[C@@H](Cc2ccc3ccccc3c2)NS(=O)(=O)Cc2ccccc2)C(=O)NCc2ccc(cc2)C(N)=N)c1 |r| Show InChI InChI=1S/C38H40N6O4S/c39-23-30-10-6-9-28(19-30)21-34(37(45)42-24-26-13-17-32(18-14-26)36(40)41)43-38(46)35(44-49(47,48)25-27-7-2-1-3-8-27)22-29-15-16-31-11-4-5-12-33(31)20-29/h1-20,34-35,44H,21-25,39H2,(H3,40,41)(H,42,45)(H,43,46)/t34-,35+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein using S2302 as substrate after 10 mins by UV/Vis photometry |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM108110

(US8598206, Table 6, 19)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](CCC2CCNCC2)NC(=O)[C@@H](CCCc2ccccc2)NS(=O)(=O)Cc2ccccc2)cc1 |r| Show InChI InChI=1S/C35H46N6O4S/c36-33(37)30-17-14-28(15-18-30)24-39-34(42)31(19-16-27-20-22-38-23-21-27)40-35(43)32(13-7-12-26-8-3-1-4-9-26)41-46(44,45)25-29-10-5-2-6-11-29/h1-6,8-11,14-15,17-18,27,31-32,38,41H,7,12-13,16,19-25H2,(H3,36,37)(H,39,42)(H,40,43)/t31-,32+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using tosyl-Gly-Pro-Lys-pNA as substrate |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM108098

(US8598206, Table 6, 7)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](CCC2CCNCC2)NC(=O)[C@@H](CCC2CCN(CC2)C(=O)C2CC2)NS(=O)(=O)Cc2ccccc2)cc1 |r| Show InChI InChI=1S/C37H53N7O5S/c38-34(39)30-10-6-28(7-11-30)24-41-35(45)32(14-8-26-16-20-40-21-17-26)42-36(46)33(43-50(48,49)25-29-4-2-1-3-5-29)15-9-27-18-22-44(23-19-27)37(47)31-12-13-31/h1-7,10-11,26-27,31-33,40,43H,8-9,12-25H2,(H3,38,39)(H,41,45)(H,42,46)/t32-,33+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein using S2302 as substrate |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50514081

(CHEMBL4471466)Show SMILES NCc1cccc(C[C@H](NC(=O)[C@@H](CCCc2ccccc2)NS(=O)(=O)Cc2cccc(c2)C(O)=O)C(=O)NCc2ccc(cc2)C(N)=N)c1 |r| Show InChI InChI=1S/C37H42N6O6S/c38-22-28-11-4-10-27(19-28)21-33(35(44)41-23-26-15-17-30(18-16-26)34(39)40)42-36(45)32(14-6-9-25-7-2-1-3-8-25)43-50(48,49)24-29-12-5-13-31(20-29)37(46)47/h1-5,7-8,10-13,15-20,32-33,43H,6,9,14,21-24,38H2,(H3,39,40)(H,41,44)(H,42,45)(H,46,47)/t32-,33+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of activated protein kinase C (unknown origin) |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50514082

(CHEMBL4462811)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](CCc2ccncc2)NC(=O)[C@@H](CCCc2ccccc2)NS(=O)(=O)Cc2ccccc2)cc1 |r| Show InChI InChI=1S/C35H40N6O4S/c36-33(37)30-17-14-28(15-18-30)24-39-34(42)31(19-16-27-20-22-38-23-21-27)40-35(43)32(13-7-12-26-8-3-1-4-9-26)41-46(44,45)25-29-10-5-2-6-11-29/h1-6,8-11,14-15,17-18,20-23,31-32,41H,7,12-13,16,19,24-25H2,(H3,36,37)(H,39,42)(H,40,43)/t31-,32+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using tosyl-Gly-Pro-Lys-pNA as substrate after 10 mins by UV/Vis photometry |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM108098

(US8598206, Table 6, 7)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](CCC2CCNCC2)NC(=O)[C@@H](CCC2CCN(CC2)C(=O)C2CC2)NS(=O)(=O)Cc2ccccc2)cc1 |r| Show InChI InChI=1S/C37H53N7O5S/c38-34(39)30-10-6-28(7-11-30)24-41-35(45)32(14-8-26-16-20-40-21-17-26)42-36(46)33(43-50(48,49)25-29-4-2-1-3-5-29)15-9-27-18-22-44(23-19-27)37(47)31-12-13-31/h1-7,10-11,26-27,31-33,40,43H,8-9,12-25H2,(H3,38,39)(H,41,45)(H,42,46)/t32-,33+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using tosyl-Gly-Pro-Lys-pNA as substrate |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50514082

(CHEMBL4462811)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](CCc2ccncc2)NC(=O)[C@@H](CCCc2ccccc2)NS(=O)(=O)Cc2ccccc2)cc1 |r| Show InChI InChI=1S/C35H40N6O4S/c36-33(37)30-17-14-28(15-18-30)24-39-34(42)31(19-16-27-20-22-38-23-21-27)40-35(43)32(13-7-12-26-8-3-1-4-9-26)41-46(44,45)25-29-10-5-2-6-11-29/h1-6,8-11,14-15,17-18,20-23,31-32,41H,7,12-13,16,19,24-25H2,(H3,36,37)(H,39,42)(H,40,43)/t31-,32+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein using S2302 as substrate after 10 mins by UV/Vis photometry |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50514086

(CHEMBL4524734)Show SMILES NCc1cccc(C[C@H](NC(=O)[C@@H](Cc2ccc3ccccc3c2)NS(=O)(=O)Cc2ccccc2)C(=O)NCc2ccc(cc2)C(N)=N)c1 |r| Show InChI InChI=1S/C38H40N6O4S/c39-23-30-10-6-9-28(19-30)21-34(37(45)42-24-26-13-17-32(18-14-26)36(40)41)43-38(46)35(44-49(47,48)25-27-7-2-1-3-8-27)22-29-15-16-31-11-4-5-12-33(31)20-29/h1-20,34-35,44H,21-25,39H2,(H3,40,41)(H,42,45)(H,43,46)/t34-,35+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using tosyl-Gly-Pro-Lys-pNA as substrate after 10 mins by UV/Vis photometry |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM108110

(US8598206, Table 6, 19)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](CCC2CCNCC2)NC(=O)[C@@H](CCCc2ccccc2)NS(=O)(=O)Cc2ccccc2)cc1 |r| Show InChI InChI=1S/C35H46N6O4S/c36-33(37)30-17-14-28(15-18-30)24-39-34(42)31(19-16-27-20-22-38-23-21-27)40-35(43)32(13-7-12-26-8-3-1-4-9-26)41-46(44,45)25-29-10-5-2-6-11-29/h1-6,8-11,14-15,17-18,27,31-32,38,41H,7,12-13,16,19-25H2,(H3,36,37)(H,39,42)(H,40,43)/t31-,32+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein using S2302 as substrate |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM29388
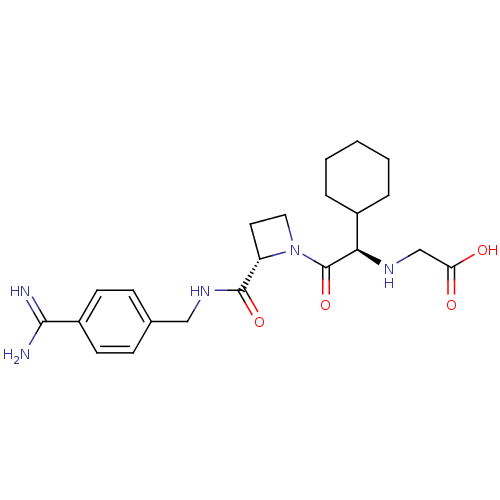
(Exanta | Melagatran | US11584714, Compound 999)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H]2CCN2C(=O)[C@H](NCC(O)=O)C2CCCCC2)cc1 Show InChI InChI=1S/C22H31N5O4/c23-20(24)16-8-6-14(7-9-16)12-26-21(30)17-10-11-27(17)22(31)19(25-13-18(28)29)15-4-2-1-3-5-15/h6-9,15,17,19,25H,1-5,10-13H2,(H3,23,24)(H,26,30)(H,28,29)/t17-,19+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of thrombin (unknown origin) |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Plasminogen
(Homo sapiens (Human)) | BDBM50514081

(CHEMBL4471466)Show SMILES NCc1cccc(C[C@H](NC(=O)[C@@H](CCCc2ccccc2)NS(=O)(=O)Cc2cccc(c2)C(O)=O)C(=O)NCc2ccc(cc2)C(N)=N)c1 |r| Show InChI InChI=1S/C37H42N6O6S/c38-22-28-11-4-10-27(19-28)21-33(35(44)41-23-26-15-17-30(18-16-26)34(39)40)42-36(45)32(14-6-9-25-7-2-1-3-8-25)43-50(48,49)24-29-12-5-13-31(20-29)37(46)47/h1-5,7-8,10-13,15-20,32-33,43H,6,9,14,21-24,38H2,(H3,39,40)(H,41,44)(H,42,45)(H,46,47)/t32-,33+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin by Michaelis-menten analysis |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM108101
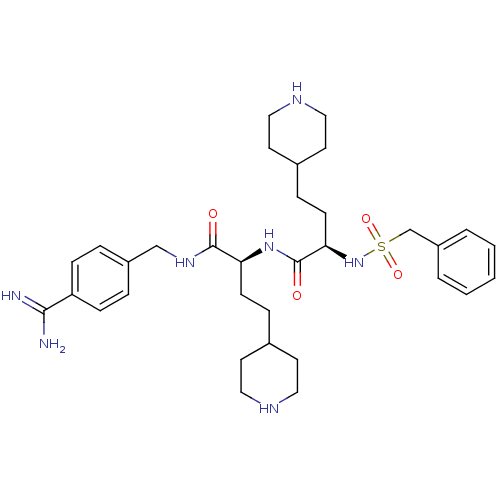
(US8598206, Table 6, 10)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](CCC2CCNCC2)NC(=O)[C@@H](CCC2CCNCC2)NS(=O)(=O)Cc2ccccc2)cc1 |r| Show InChI InChI=1S/C33H49N7O4S/c34-31(35)28-10-6-26(7-11-28)22-38-32(41)29(12-8-24-14-18-36-19-15-24)39-33(42)30(13-9-25-16-20-37-21-17-25)40-45(43,44)23-27-4-2-1-3-5-27/h1-7,10-11,24-25,29-30,36-37,40H,8-9,12-23H2,(H3,34,35)(H,38,41)(H,39,42)/t29-,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using tosyl-Gly-Pro-Lys-pNA as substrate |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50380629
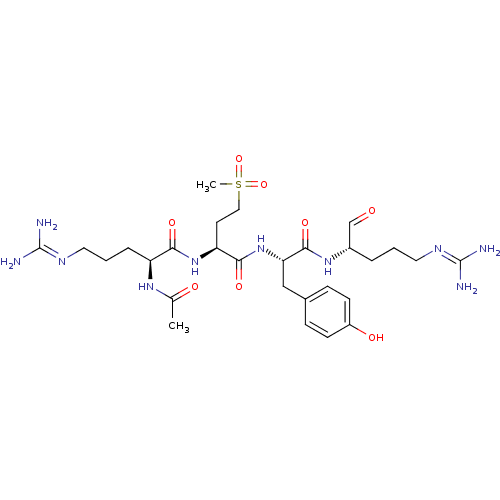
(CHEMBL2016877)Show SMILES [#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]S([#6])(=O)=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6]=O |r| Show InChI InChI=1S/C28H46N10O8S/c1-17(40)35-21(6-4-13-34-28(31)32)24(42)37-22(11-14-47(2,45)46)25(43)38-23(15-18-7-9-20(41)10-8-18)26(44)36-19(16-39)5-3-12-33-27(29)30/h7-10,16,19,21-23,41H,3-6,11-15H2,1-2H3,(H,35,40)(H,36,44)(H,37,42)(H,38,43)(H4,29,30,33)(H4,31,32,34)/t19-,21-,22-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin assessed as reduction in proteolysis activity in presence of fibrinogen after 90 mins by SDS-PAGE analysis |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50514066

(CHEMBL4589217)Show SMILES CC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCS(C)(=O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C=O |r| Show InChI InChI=1S/C28H46N8O8S/c1-18(38)33-22(7-3-4-13-29)25(40)35-23(12-15-45(2,43)44)26(41)36-24(16-19-8-10-21(39)11-9-19)27(42)34-20(17-37)6-5-14-32-28(30)31/h8-11,17,20,22-24,39H,3-7,12-16,29H2,1-2H3,(H,33,38)(H,34,42)(H,35,40)(H,36,41)(H4,30,31,32)/t20-,22-,23-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin assessed as reduction in proteolysis activity in presence of fibrinogen after 90 mins by SDS-PAGE analysis |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM108101

(US8598206, Table 6, 10)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](CCC2CCNCC2)NC(=O)[C@@H](CCC2CCNCC2)NS(=O)(=O)Cc2ccccc2)cc1 |r| Show InChI InChI=1S/C33H49N7O4S/c34-31(35)28-10-6-26(7-11-28)22-38-32(41)29(12-8-24-14-18-36-19-15-24)39-33(42)30(13-9-25-16-20-37-21-17-25)40-45(43,44)23-27-4-2-1-3-5-27/h1-7,10-11,24-25,29-30,36-37,40H,8-9,12-23H2,(H3,34,35)(H,38,41)(H,39,42)/t29-,30+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein using S2302 as substrate |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50514081

(CHEMBL4471466)Show SMILES NCc1cccc(C[C@H](NC(=O)[C@@H](CCCc2ccccc2)NS(=O)(=O)Cc2cccc(c2)C(O)=O)C(=O)NCc2ccc(cc2)C(N)=N)c1 |r| Show InChI InChI=1S/C37H42N6O6S/c38-22-28-11-4-10-27(19-28)21-33(35(44)41-23-26-15-17-30(18-16-26)34(39)40)42-36(45)32(14-6-9-25-7-2-1-3-8-25)43-50(48,49)24-29-12-5-13-31(20-29)37(46)47/h1-5,7-8,10-13,15-20,32-33,43H,6,9,14,21-24,38H2,(H3,39,40)(H,41,44)(H,42,45)(H,46,47)/t32-,33+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human factor 11a |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50514080
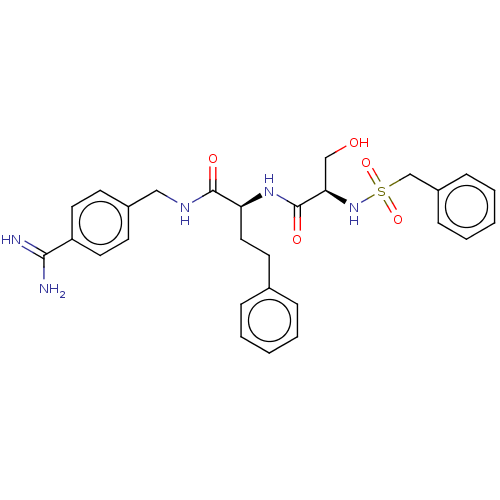
(CHEMBL4520324)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](CCc2ccccc2)NC(=O)[C@@H](CO)NS(=O)(=O)Cc2ccccc2)cc1 |r| Show InChI InChI=1S/C28H33N5O5S/c29-26(30)23-14-11-21(12-15-23)17-31-27(35)24(16-13-20-7-3-1-4-8-20)32-28(36)25(18-34)33-39(37,38)19-22-9-5-2-6-10-22/h1-12,14-15,24-25,33-34H,13,16-19H2,(H3,29,30)(H,31,35)(H,32,36)/t24-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of urokinase-type plasminogen activator (unknown origin) |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50514080

(CHEMBL4520324)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](CCc2ccccc2)NC(=O)[C@@H](CO)NS(=O)(=O)Cc2ccccc2)cc1 |r| Show InChI InChI=1S/C28H33N5O5S/c29-26(30)23-14-11-21(12-15-23)17-31-27(35)24(16-13-20-7-3-1-4-8-20)32-28(36)25(18-34)33-39(37,38)19-22-9-5-2-6-10-22/h1-12,14-15,24-25,33-34H,13,16-19H2,(H3,29,30)(H,31,35)(H,32,36)/t24-,25+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of plasmin (unknown origin) |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50110015
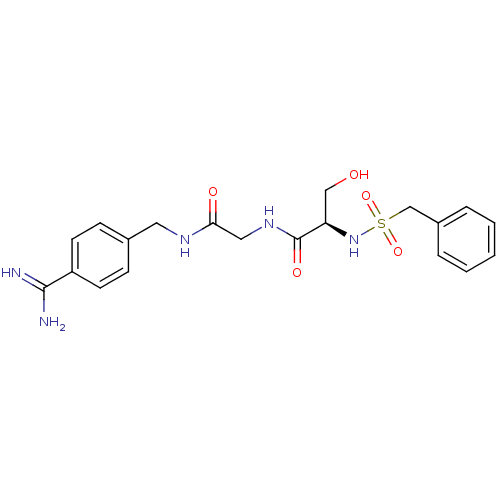
(CHEMBL158936 | N-(BENZYLSULFONYL)SERYL-N~1~-{4-[AM...)Show SMILES NC(=N)c1ccc(CNC(=O)CNC(=O)[C@@H](CO)NS(=O)(=O)Cc2ccccc2)cc1 Show InChI InChI=1S/C20H25N5O5S/c21-19(22)16-8-6-14(7-9-16)10-23-18(27)11-24-20(28)17(12-26)25-31(29,30)13-15-4-2-1-3-5-15/h1-9,17,25-26H,10-13H2,(H3,21,22)(H,23,27)(H,24,28)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of urokinase-type plasminogen activator (unknown origin) |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50514081

(CHEMBL4471466)Show SMILES NCc1cccc(C[C@H](NC(=O)[C@@H](CCCc2ccccc2)NS(=O)(=O)Cc2cccc(c2)C(O)=O)C(=O)NCc2ccc(cc2)C(N)=N)c1 |r| Show InChI InChI=1S/C37H42N6O6S/c38-22-28-11-4-10-27(19-28)21-33(35(44)41-23-26-15-17-30(18-16-26)34(39)40)42-36(45)32(14-6-9-25-7-2-1-3-8-25)43-50(48,49)24-29-12-5-13-31(20-29)37(46)47/h1-5,7-8,10-13,15-20,32-33,43H,6,9,14,21-24,38H2,(H3,39,40)(H,41,44)(H,42,45)(H,46,47)/t32-,33+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM108110

(US8598206, Table 6, 19)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](CCC2CCNCC2)NC(=O)[C@@H](CCCc2ccccc2)NS(=O)(=O)Cc2ccccc2)cc1 |r| Show InChI InChI=1S/C35H46N6O4S/c36-33(37)30-17-14-28(15-18-30)24-39-34(42)31(19-16-27-20-22-38-23-21-27)40-35(43)32(13-7-12-26-8-3-1-4-9-26)41-46(44,45)25-29-10-5-2-6-11-29/h1-6,8-11,14-15,17-18,27,31-32,38,41H,7,12-13,16,19-25H2,(H3,36,37)(H,39,42)(H,40,43)/t31-,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human activated protein kinase C using H-D-Lys(Cbo)-Pro-Arg-pNA as substrate |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM108110

(US8598206, Table 6, 19)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](CCC2CCNCC2)NC(=O)[C@@H](CCCc2ccccc2)NS(=O)(=O)Cc2ccccc2)cc1 |r| Show InChI InChI=1S/C35H46N6O4S/c36-33(37)30-17-14-28(15-18-30)24-39-34(42)31(19-16-27-20-22-38-23-21-27)40-35(43)32(13-7-12-26-8-3-1-4-9-26)41-46(44,45)25-29-10-5-2-6-11-29/h1-6,8-11,14-15,17-18,27,31-32,38,41H,7,12-13,16,19-25H2,(H3,36,37)(H,39,42)(H,40,43)/t31-,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human Complement C1s subcomponent using Val-Ser-Arg-pNA as substrate |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM108110

(US8598206, Table 6, 19)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](CCC2CCNCC2)NC(=O)[C@@H](CCCc2ccccc2)NS(=O)(=O)Cc2ccccc2)cc1 |r| Show InChI InChI=1S/C35H46N6O4S/c36-33(37)30-17-14-28(15-18-30)24-39-34(42)31(19-16-27-20-22-38-23-21-27)40-35(43)32(13-7-12-26-8-3-1-4-9-26)41-46(44,45)25-29-10-5-2-6-11-29/h1-6,8-11,14-15,17-18,27,31-32,38,41H,7,12-13,16,19-25H2,(H3,36,37)(H,39,42)(H,40,43)/t31-,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a using MeOCO-d-Cha-Gly-Arg-pNA as substrate |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM108110

(US8598206, Table 6, 19)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](CCC2CCNCC2)NC(=O)[C@@H](CCCc2ccccc2)NS(=O)(=O)Cc2ccccc2)cc1 |r| Show InChI InChI=1S/C35H46N6O4S/c36-33(37)30-17-14-28(15-18-30)24-39-34(42)31(19-16-27-20-22-38-23-21-27)40-35(43)32(13-7-12-26-8-3-1-4-9-26)41-46(44,45)25-29-10-5-2-6-11-29/h1-6,8-11,14-15,17-18,27,31-32,38,41H,7,12-13,16,19-25H2,(H3,36,37)(H,39,42)(H,40,43)/t31-,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human factor 11a using H-D-Lys(Cbo)-Pro-Arg-pNA as substrate |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM108110

(US8598206, Table 6, 19)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](CCC2CCNCC2)NC(=O)[C@@H](CCCc2ccccc2)NS(=O)(=O)Cc2ccccc2)cc1 |r| Show InChI InChI=1S/C35H46N6O4S/c36-33(37)30-17-14-28(15-18-30)24-39-34(42)31(19-16-27-20-22-38-23-21-27)40-35(43)32(13-7-12-26-8-3-1-4-9-26)41-46(44,45)25-29-10-5-2-6-11-29/h1-6,8-11,14-15,17-18,27,31-32,38,41H,7,12-13,16,19-25H2,(H3,36,37)(H,39,42)(H,40,43)/t31-,32+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human tissue-type plasminogen activator using Mes-d-Cha- Gly-Arg-pNA as substrate |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM108110

(US8598206, Table 6, 19)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](CCC2CCNCC2)NC(=O)[C@@H](CCCc2ccccc2)NS(=O)(=O)Cc2ccccc2)cc1 |r| Show InChI InChI=1S/C35H46N6O4S/c36-33(37)30-17-14-28(15-18-30)24-39-34(42)31(19-16-27-20-22-38-23-21-27)40-35(43)32(13-7-12-26-8-3-1-4-9-26)41-46(44,45)25-29-10-5-2-6-11-29/h1-6,8-11,14-15,17-18,27,31-32,38,41H,7,12-13,16,19-25H2,(H3,36,37)(H,39,42)(H,40,43)/t31-,32+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human factor 2a using Mes-d-Cha-Gly-Arg-pNA as substrate |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Complement C1r subcomponent
(Homo sapiens (Human)) | BDBM108110

(US8598206, Table 6, 19)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](CCC2CCNCC2)NC(=O)[C@@H](CCCc2ccccc2)NS(=O)(=O)Cc2ccccc2)cc1 |r| Show InChI InChI=1S/C35H46N6O4S/c36-33(37)30-17-14-28(15-18-30)24-39-34(42)31(19-16-27-20-22-38-23-21-27)40-35(43)32(13-7-12-26-8-3-1-4-9-26)41-46(44,45)25-29-10-5-2-6-11-29/h1-6,8-11,14-15,17-18,27,31-32,38,41H,7,12-13,16,19-25H2,(H3,36,37)(H,39,42)(H,40,43)/t31-,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human Complement C1r subcomponent using Val-Ser-Arg-pNA as substrate |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Coagulation factor XII
(Homo sapiens (Human)) | BDBM108110

(US8598206, Table 6, 19)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](CCC2CCNCC2)NC(=O)[C@@H](CCCc2ccccc2)NS(=O)(=O)Cc2ccccc2)cc1 |r| Show InChI InChI=1S/C35H46N6O4S/c36-33(37)30-17-14-28(15-18-30)24-39-34(42)31(19-16-27-20-22-38-23-21-27)40-35(43)32(13-7-12-26-8-3-1-4-9-26)41-46(44,45)25-29-10-5-2-6-11-29/h1-6,8-11,14-15,17-18,27,31-32,38,41H,7,12-13,16,19-25H2,(H3,36,37)(H,39,42)(H,40,43)/t31-,32+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human factor 12a using CHA-Gly-Arg- pNA as substrate |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50272222
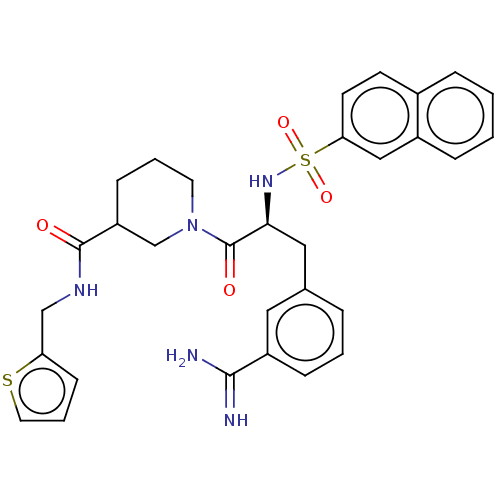
(CHEMBL4128356)Show SMILES NC(=N)c1cccc(C[C@H](NS(=O)(=O)c2ccc3ccccc3c2)C(=O)N2CCCC(C2)C(=O)NCc2cccs2)c1 |r| Show InChI InChI=1S/C31H33N5O4S2/c32-29(33)24-9-3-6-21(16-24)17-28(35-42(39,40)27-13-12-22-7-1-2-8-23(22)18-27)31(38)36-14-4-10-25(20-36)30(37)34-19-26-11-5-15-41-26/h1-3,5-9,11-13,15-16,18,25,28,35H,4,10,14,17,19-20H2,(H3,32,33)(H,34,37)/t25?,28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50514064
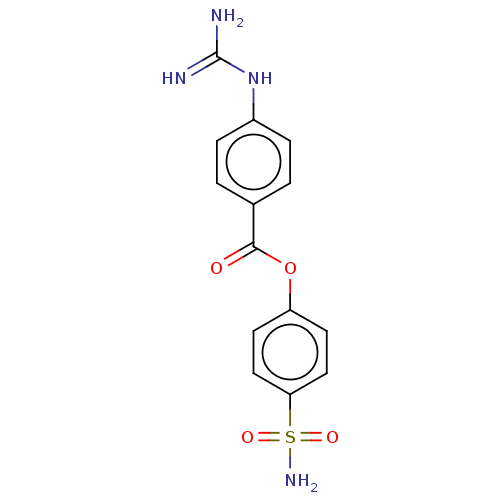
(CHEMBL4471405)Show SMILES NC(=N)Nc1ccc(cc1)C(=O)Oc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C14H14N4O4S/c15-14(16)18-10-3-1-9(2-4-10)13(19)22-11-5-7-12(8-6-11)23(17,20)21/h1-8H,(H4,15,16,18)(H2,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using S-2251 as substrate after 30 mins by spectrophotometric method |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM23891
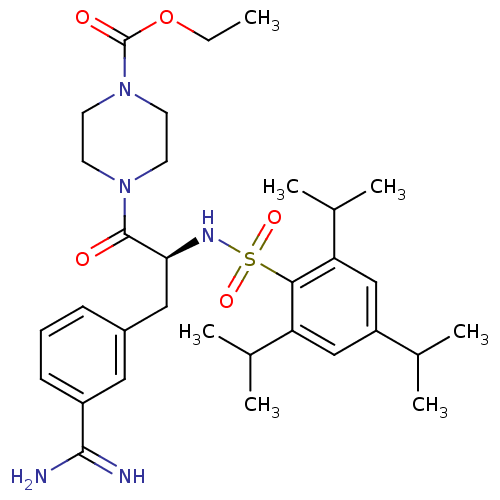
(3-amidinophenylalanine deriv., 35 | CHEMBL107955 |...)Show SMILES CCOC(=O)N1CCN(CC1)C(=O)[C@H](Cc1cccc(c1)C(N)=N)NS(=O)(=O)c1c(cc(cc1C(C)C)C(C)C)C(C)C |r| Show InChI InChI=1S/C32H47N5O5S/c1-8-42-32(39)37-14-12-36(13-15-37)31(38)28(17-23-10-9-11-24(16-23)30(33)34)35-43(40,41)29-26(21(4)5)18-25(20(2)3)19-27(29)22(6)7/h9-11,16,18-22,28,35H,8,12-15,17H2,1-7H3,(H3,33,34)/t28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50063698

(4-Guanidino-benzoic acid 6-carbamimidoyl-naphthale...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(cc1)-[#6](=O)-[#8]-c1ccc2cc(ccc2c1)-[#6](-[#7])=[#7] Show InChI InChI=1S/C19H17N5O2/c20-17(21)14-2-1-13-10-16(8-5-12(13)9-14)26-18(25)11-3-6-15(7-4-11)24-19(22)23/h1-10H,(H3,20,21)(H4,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin assessed as reduction in hydrolytic activity using TAME as substrate after 5 mins by spectrophotometric method |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM29388

(Exanta | Melagatran | US11584714, Compound 999)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H]2CCN2C(=O)[C@H](NCC(O)=O)C2CCCCC2)cc1 Show InChI InChI=1S/C22H31N5O4/c23-20(24)16-8-6-14(7-9-16)12-26-21(30)17-10-11-27(17)22(31)19(25-13-18(28)29)15-4-2-1-3-5-15/h6-9,15,17,19,25H,1-5,10-13H2,(H3,23,24)(H,26,30)(H,28,29)/t17-,19+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of plasmin (unknown origin) |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM29388

(Exanta | Melagatran | US11584714, Compound 999)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H]2CCN2C(=O)[C@H](NCC(O)=O)C2CCCCC2)cc1 Show InChI InChI=1S/C22H31N5O4/c23-20(24)16-8-6-14(7-9-16)12-26-21(30)17-10-11-27(17)22(31)19(25-13-18(28)29)15-4-2-1-3-5-15/h6-9,15,17,19,25H,1-5,10-13H2,(H3,23,24)(H,26,30)(H,28,29)/t17-,19+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of tissue-type plasminogen activator (unknown origin) |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM108098

(US8598206, Table 6, 7)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](CCC2CCNCC2)NC(=O)[C@@H](CCC2CCN(CC2)C(=O)C2CC2)NS(=O)(=O)Cc2ccccc2)cc1 |r| Show InChI InChI=1S/C37H53N7O5S/c38-34(39)30-10-6-28(7-11-30)24-41-35(45)32(14-8-26-16-20-40-21-17-26)42-36(46)33(43-50(48,49)25-29-4-2-1-3-5-29)15-9-27-18-22-44(23-19-27)37(47)31-12-13-31/h1-7,10-11,26-27,31-33,40,43H,8-9,12-25H2,(H3,38,39)(H,41,45)(H,42,46)/t32-,33+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human factor 2a using Mes-d-Cha-Gly-Arg-pNA as substrate |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM108098

(US8598206, Table 6, 7)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](CCC2CCNCC2)NC(=O)[C@@H](CCC2CCN(CC2)C(=O)C2CC2)NS(=O)(=O)Cc2ccccc2)cc1 |r| Show InChI InChI=1S/C37H53N7O5S/c38-34(39)30-10-6-28(7-11-30)24-41-35(45)32(14-8-26-16-20-40-21-17-26)42-36(46)33(43-50(48,49)25-29-4-2-1-3-5-29)15-9-27-18-22-44(23-19-27)37(47)31-12-13-31/h1-7,10-11,26-27,31-33,40,43H,8-9,12-25H2,(H3,38,39)(H,41,45)(H,42,46)/t32-,33+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human tissue-type plasminogen activator using Mes-d-Cha- Gly-Arg-pNA as substrate |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM108098

(US8598206, Table 6, 7)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](CCC2CCNCC2)NC(=O)[C@@H](CCC2CCN(CC2)C(=O)C2CC2)NS(=O)(=O)Cc2ccccc2)cc1 |r| Show InChI InChI=1S/C37H53N7O5S/c38-34(39)30-10-6-28(7-11-30)24-41-35(45)32(14-8-26-16-20-40-21-17-26)42-36(46)33(43-50(48,49)25-29-4-2-1-3-5-29)15-9-27-18-22-44(23-19-27)37(47)31-12-13-31/h1-7,10-11,26-27,31-33,40,43H,8-9,12-25H2,(H3,38,39)(H,41,45)(H,42,46)/t32-,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a using MeOCO-d-Cha-Gly-Arg-pNA as substrate |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Coagulation factor XII
(Homo sapiens (Human)) | BDBM108098

(US8598206, Table 6, 7)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](CCC2CCNCC2)NC(=O)[C@@H](CCC2CCN(CC2)C(=O)C2CC2)NS(=O)(=O)Cc2ccccc2)cc1 |r| Show InChI InChI=1S/C37H53N7O5S/c38-34(39)30-10-6-28(7-11-30)24-41-35(45)32(14-8-26-16-20-40-21-17-26)42-36(46)33(43-50(48,49)25-29-4-2-1-3-5-29)15-9-27-18-22-44(23-19-27)37(47)31-12-13-31/h1-7,10-11,26-27,31-33,40,43H,8-9,12-25H2,(H3,38,39)(H,41,45)(H,42,46)/t32-,33+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human factor 12a using CHA-Gly-Arg- pNA as substrate |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Complement C1r subcomponent
(Homo sapiens (Human)) | BDBM108098

(US8598206, Table 6, 7)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](CCC2CCNCC2)NC(=O)[C@@H](CCC2CCN(CC2)C(=O)C2CC2)NS(=O)(=O)Cc2ccccc2)cc1 |r| Show InChI InChI=1S/C37H53N7O5S/c38-34(39)30-10-6-28(7-11-30)24-41-35(45)32(14-8-26-16-20-40-21-17-26)42-36(46)33(43-50(48,49)25-29-4-2-1-3-5-29)15-9-27-18-22-44(23-19-27)37(47)31-12-13-31/h1-7,10-11,26-27,31-33,40,43H,8-9,12-25H2,(H3,38,39)(H,41,45)(H,42,46)/t32-,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human Complement C1r subcomponent using Val-Ser-Arg-pNA as substrate |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM108098

(US8598206, Table 6, 7)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](CCC2CCNCC2)NC(=O)[C@@H](CCC2CCN(CC2)C(=O)C2CC2)NS(=O)(=O)Cc2ccccc2)cc1 |r| Show InChI InChI=1S/C37H53N7O5S/c38-34(39)30-10-6-28(7-11-30)24-41-35(45)32(14-8-26-16-20-40-21-17-26)42-36(46)33(43-50(48,49)25-29-4-2-1-3-5-29)15-9-27-18-22-44(23-19-27)37(47)31-12-13-31/h1-7,10-11,26-27,31-33,40,43H,8-9,12-25H2,(H3,38,39)(H,41,45)(H,42,46)/t32-,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human Complement C1s subcomponent using Val-Ser-Arg-pNA as substrate |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM108098

(US8598206, Table 6, 7)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](CCC2CCNCC2)NC(=O)[C@@H](CCC2CCN(CC2)C(=O)C2CC2)NS(=O)(=O)Cc2ccccc2)cc1 |r| Show InChI InChI=1S/C37H53N7O5S/c38-34(39)30-10-6-28(7-11-30)24-41-35(45)32(14-8-26-16-20-40-21-17-26)42-36(46)33(43-50(48,49)25-29-4-2-1-3-5-29)15-9-27-18-22-44(23-19-27)37(47)31-12-13-31/h1-7,10-11,26-27,31-33,40,43H,8-9,12-25H2,(H3,38,39)(H,41,45)(H,42,46)/t32-,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human activated protein kinase C using H-D-Lys(Cbo)-Pro-Arg-pNA as substrate |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50104435
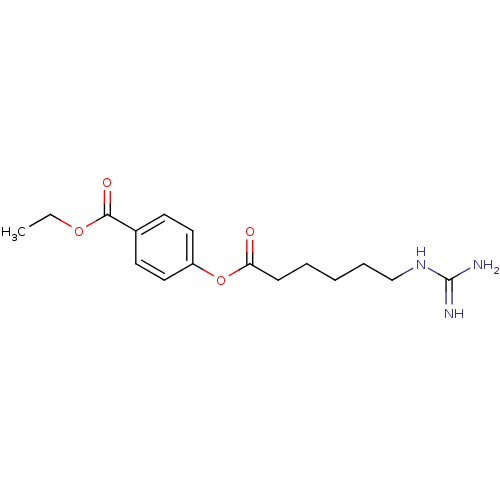
(4-(6-Guanidino-hexanoyloxy)-benzoic acid ethyl est...)Show InChI InChI=1S/C16H23N3O4/c1-2-22-15(21)12-7-9-13(10-8-12)23-14(20)6-4-3-5-11-19-16(17)18/h7-10H,2-6,11H2,1H3,(H4,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Competitive inhibition of human plasmin assessed as reduction in hydrolytic activity using S-2251 as substrate by spectrophotometric method |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50514084
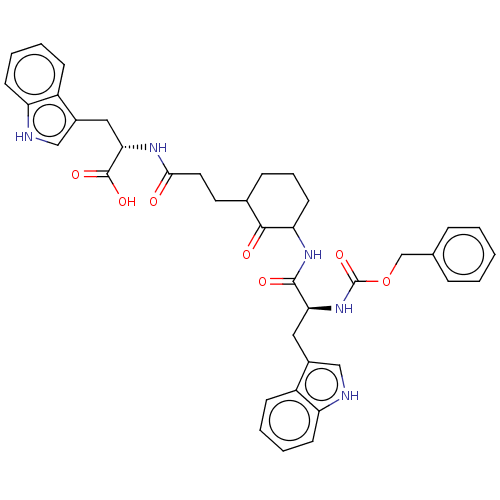
(CHEMBL4526664)Show SMILES OC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CCC1CCCC(NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)OCc2ccccc2)C1=O |r| Show InChI InChI=1S/C39H41N5O7/c45-35(42-34(38(48)49)20-27-22-41-31-15-7-5-13-29(27)31)18-17-25-11-8-16-32(36(25)46)43-37(47)33(19-26-21-40-30-14-6-4-12-28(26)30)44-39(50)51-23-24-9-2-1-3-10-24/h1-7,9-10,12-15,21-22,25,32-34,40-41H,8,11,16-20,23H2,(H,42,45)(H,43,47)(H,44,50)(H,48,49)/t25?,32?,33-,34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of plasmin (unknown origin) using D-Val-Leu-Lys-p-nitroanilide as substrate |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM29388

(Exanta | Melagatran | US11584714, Compound 999)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H]2CCN2C(=O)[C@H](NCC(O)=O)C2CCCCC2)cc1 Show InChI InChI=1S/C22H31N5O4/c23-20(24)16-8-6-14(7-9-16)12-26-21(30)17-10-11-27(17)22(31)19(25-13-18(28)29)15-4-2-1-3-5-15/h6-9,15,17,19,25H,1-5,10-13H2,(H3,23,24)(H,26,30)(H,28,29)/t17-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of urokinase-type plasminogen activator (unknown origin) |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50110015

(CHEMBL158936 | N-(BENZYLSULFONYL)SERYL-N~1~-{4-[AM...)Show SMILES NC(=N)c1ccc(CNC(=O)CNC(=O)[C@@H](CO)NS(=O)(=O)Cc2ccccc2)cc1 Show InChI InChI=1S/C20H25N5O5S/c21-19(22)16-8-6-14(7-9-16)10-23-18(27)11-24-20(28)17(12-26)25-31(29,30)13-15-4-2-1-3-5-15/h1-9,17,25-26H,10-13H2,(H3,21,22)(H,23,27)(H,24,28)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of plasmin (unknown origin) |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50079381
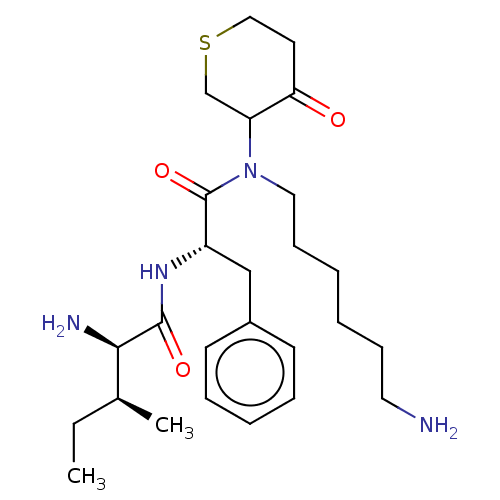
((R)-2-Amino-3-methyl-pentanoic acid {(S)-1-[(6-ami...)Show SMILES CC[C@H](C)[C@@H](N)C(=O)N[C@@H](Cc1ccccc1)C(=O)N(CCCCCCN)C1CSCCC1=O Show InChI InChI=1S/C26H42N4O3S/c1-3-19(2)24(28)25(32)29-21(17-20-11-7-6-8-12-20)26(33)30(15-10-5-4-9-14-27)22-18-34-16-13-23(22)31/h6-8,11-12,19,21-22,24H,3-5,9-10,13-18,27-28H2,1-2H3,(H,29,32)/t19?,21-,22?,24+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of plasmin (unknown origin) using D-Val-Leu-Lys-pNA as substrate |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50079380
(3-[Bis-(6-amino-hexyl)-amino]-tetrahydro-thiopyran...)Show InChI InChI=1S/C17H35N3OS/c18-10-5-1-3-7-12-20(13-8-4-2-6-11-19)16-15-22-14-9-17(16)21/h16H,1-15,18-19H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of plasmin (unknown origin) using D-Val-Leu-Lys-pNA as substrate |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50428067

(CL-65336 | Cyklokapron | Lysteda | TRANEXAMIC ACID...)Show SMILES NC[C@H]1CC[C@@H](CC1)C(O)=O |r,wU:5.8,wD:2.1,(1.2,4.21,;-.14,3.44,;-.14,1.9,;-1.47,1.13,;-1.47,-.41,;-.14,-1.18,;1.2,-.41,;1.2,1.13,;-.14,-2.72,;-1.47,-3.49,;1.2,-3.49,)| Show InChI InChI=1S/C8H15NO2/c9-5-6-1-3-7(4-2-6)8(10)11/h6-7H,1-5,9H2,(H,10,11)/t6-,7- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human urokinase-type plasminogen activator using S-2444 as substrate |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Plasminogen
(Homo sapiens (Human)) | BDBM50428067

(CL-65336 | Cyklokapron | Lysteda | TRANEXAMIC ACID...)Show SMILES NC[C@H]1CC[C@@H](CC1)C(O)=O |r,wU:5.8,wD:2.1,(1.2,4.21,;-.14,3.44,;-.14,1.9,;-1.47,1.13,;-1.47,-.41,;-.14,-1.18,;1.2,-.41,;1.2,1.13,;-.14,-2.72,;-1.47,-3.49,;1.2,-3.49,)| Show InChI InChI=1S/C8H15NO2/c9-5-6-1-3-7(4-2-6)8(10)11/h6-7H,1-5,9H2,(H,10,11)/t6-,7- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
| DrugBank
MMDB
PDB
Article
PubMed
| 2.50E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of plasmin (unknown origin) assessed as reduction in amidolytic activity |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data