 Found 46 hits Enz. Inhib. hit(s) with all data for entry = 50009383
Found 46 hits Enz. Inhib. hit(s) with all data for entry = 50009383 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
CREB-binding protein
(Homo sapiens (Human)) | BDBM50456444

(CHEMBL3734823)Show SMILES CC1=NN(C(=O)\C1=C\c1ccc(o1)-c1cc(C)c(C)cc1[N+]([O-])=O)c1ccc(cc1)C(O)=O |t:1| Show InChI InChI=1S/C24H19N3O6/c1-13-10-20(21(27(31)32)11-14(13)2)22-9-8-18(33-22)12-19-15(3)25-26(23(19)28)17-6-4-16(5-7-17)24(29)30/h4-12H,1-3H3,(H,29,30)/b19-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of CBP BHC domain (unknown origin) |
Eur J Med Chem 178: 259-286 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.078
BindingDB Entry DOI: 10.7270/Q2ZW1QC8 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase KAT6A [507-778]
(Homo sapiens) | BDBM50518832
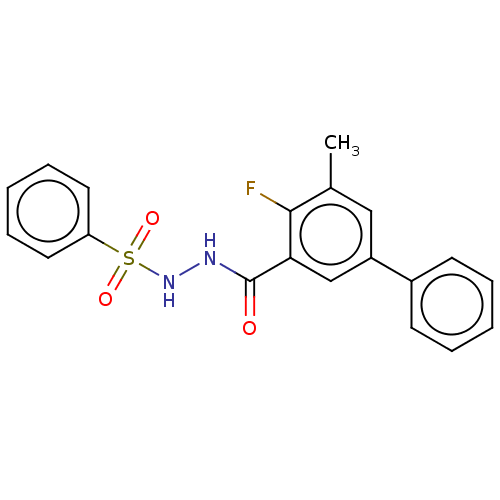
(CHEMBL4455897 | US10829446, Compound 36)Show SMILES Cc1cc(cc(C(=O)NNS(=O)(=O)c2ccccc2)c1F)-c1ccccc1 Show InChI InChI=1S/C20H17FN2O3S/c1-14-12-16(15-8-4-2-5-9-15)13-18(19(14)21)20(24)22-23-27(25,26)17-10-6-3-7-11-17/h2-13,23H,1H3,(H,22,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of KAT6A (unknown origin) |
Eur J Med Chem 178: 259-286 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.078
BindingDB Entry DOI: 10.7270/Q2ZW1QC8 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50456444

(CHEMBL3734823)Show SMILES CC1=NN(C(=O)\C1=C\c1ccc(o1)-c1cc(C)c(C)cc1[N+]([O-])=O)c1ccc(cc1)C(O)=O |t:1| Show InChI InChI=1S/C24H19N3O6/c1-13-10-20(21(27(31)32)11-14(13)2)22-9-8-18(33-22)12-19-15(3)25-26(23(19)28)17-6-4-16(5-7-17)24(29)30/h4-12H,1-3H3,(H,29,30)/b19-12+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of P300 BHC domain (unknown origin) |
Eur J Med Chem 178: 259-286 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.078
BindingDB Entry DOI: 10.7270/Q2ZW1QC8 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50528248
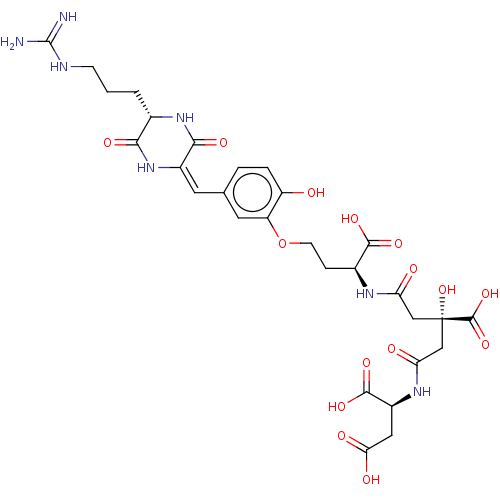
(CHEMBL4524766)Show SMILES NC(=N)NCCC[C@@H]1NC(=O)\C(NC1=O)=C/c1ccc(O)c(OCC[C@H](NC(=O)C[C@](O)(CC(=O)N[C@@H](CC(O)=O)C(O)=O)C(O)=O)C(O)=O)c1 |r| Show InChI InChI=1S/C29H37N7O15/c30-28(31)32-6-1-2-14-23(42)36-16(24(43)35-14)8-13-3-4-18(37)19(9-13)51-7-5-15(25(44)45)33-20(38)11-29(50,27(48)49)12-21(39)34-17(26(46)47)10-22(40)41/h3-4,8-9,14-15,17,37,50H,1-2,5-7,10-12H2,(H,33,38)(H,34,39)(H,35,43)(H,36,42)(H,40,41)(H,44,45)(H,46,47)(H,48,49)(H4,30,31,32)/b16-8+/t14-,15-,17-,29-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-acetyl-CoA from recombinant GST-tagged P300 (unknown origin) by scintillation counting analysis |
Eur J Med Chem 178: 259-286 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.078
BindingDB Entry DOI: 10.7270/Q2ZW1QC8 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50528248

(CHEMBL4524766)Show SMILES NC(=N)NCCC[C@@H]1NC(=O)\C(NC1=O)=C/c1ccc(O)c(OCC[C@H](NC(=O)C[C@](O)(CC(=O)N[C@@H](CC(O)=O)C(O)=O)C(O)=O)C(O)=O)c1 |r| Show InChI InChI=1S/C29H37N7O15/c30-28(31)32-6-1-2-14-23(42)36-16(24(43)35-14)8-13-3-4-18(37)19(9-13)51-7-5-15(25(44)45)33-20(38)11-29(50,27(48)49)12-21(39)34-17(26(46)47)10-22(40)41/h3-4,8-9,14-15,17,37,50H,1-2,5-7,10-12H2,(H,33,38)(H,34,39)(H,35,43)(H,36,42)(H,40,41)(H,44,45)(H,46,47)(H,48,49)(H4,30,31,32)/b16-8+/t14-,15-,17-,29-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-acetyl-CoA from recombinant GST-tagged P300 (unknown origin) after 30 mins by scintillation counting analysis |
Eur J Med Chem 178: 259-286 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.078
BindingDB Entry DOI: 10.7270/Q2ZW1QC8 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50528262
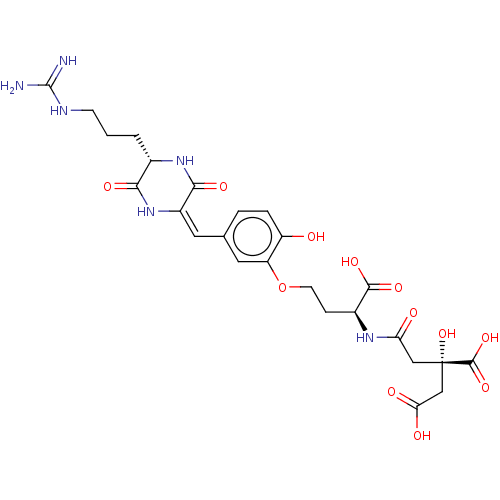
(CHEMBL4447371)Show SMILES NC(=N)NCCC[C@@H]1NC(=O)\C(NC1=O)=C/c1ccc(O)c(OCC[C@H](NC(=O)C[C@](O)(CC(O)=O)C(O)=O)C(O)=O)c1 |r| Show InChI InChI=1S/C25H32N6O12/c26-24(27)28-6-1-2-13-20(36)31-15(21(37)30-13)8-12-3-4-16(32)17(9-12)43-7-5-14(22(38)39)29-18(33)10-25(42,23(40)41)11-19(34)35/h3-4,8-9,13-14,32,42H,1-2,5-7,10-11H2,(H,29,33)(H,30,37)(H,31,36)(H,34,35)(H,38,39)(H,40,41)(H4,26,27,28)/b15-8+/t13-,14-,25-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-acetyl-CoA from recombinant GST-tagged P300 (unknown origin) by scintillation counting analysis |
Eur J Med Chem 178: 259-286 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.078
BindingDB Entry DOI: 10.7270/Q2ZW1QC8 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase KAT6B
(Homo sapiens) | BDBM50518832

(CHEMBL4455897 | US10829446, Compound 36)Show SMILES Cc1cc(cc(C(=O)NNS(=O)(=O)c2ccccc2)c1F)-c1ccccc1 Show InChI InChI=1S/C20H17FN2O3S/c1-14-12-16(15-8-4-2-5-9-15)13-18(19(14)21)20(24)22-23-27(25,26)17-10-6-3-7-11-17/h2-13,23H,1H3,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of KAT6B (unknown origin) |
Eur J Med Chem 178: 259-286 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.078
BindingDB Entry DOI: 10.7270/Q2ZW1QC8 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50528249
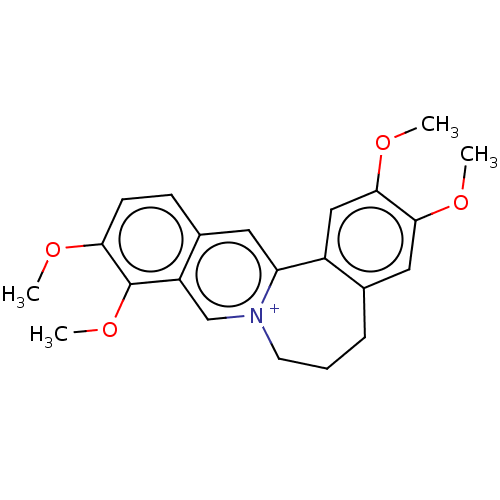
(CHEMBL4573224)Show SMILES CS([O-])(=O)=O.COc1cc2CCC[n+]3cc4c(OC)c(OC)ccc4cc3-c2cc1OC Show InChI InChI=1S/C22H24NO4/c1-24-19-8-7-15-10-18-16-12-21(26-3)20(25-2)11-14(16)6-5-9-23(18)13-17(15)22(19)27-4/h7-8,10-13H,5-6,9H2,1-4H3/q+1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-acetyl-CoA from P300 (unknown origin) using peptide as substrate after 60 mins |
Eur J Med Chem 178: 259-286 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.078
BindingDB Entry DOI: 10.7270/Q2ZW1QC8 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50528253
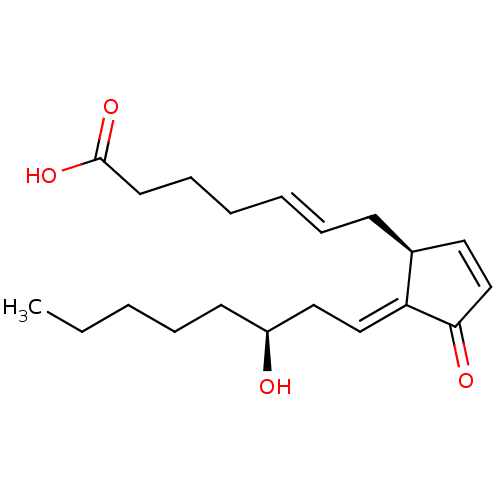
(CHEMBL4579084)Show SMILES CCCCC[C@H](O)C\C=C1/[C@@H](C\C=C\CCCC(O)=O)C=CC1=O |r,c:20| Show InChI InChI=1S/C20H30O4/c1-2-3-6-10-17(21)13-14-18-16(12-15-19(18)22)9-7-4-5-8-11-20(23)24/h4,7,12,14-17,21H,2-3,5-6,8-11,13H2,1H3,(H,23,24)/b7-4+,18-14+/t16-,17-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-acetyl-CoA from P300 in human HeLa cells using peptide as substrate after 3 hrs |
Eur J Med Chem 178: 259-286 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.078
BindingDB Entry DOI: 10.7270/Q2ZW1QC8 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50528253

(CHEMBL4579084)Show SMILES CCCCC[C@H](O)C\C=C1/[C@@H](C\C=C\CCCC(O)=O)C=CC1=O |r,c:20| Show InChI InChI=1S/C20H30O4/c1-2-3-6-10-17(21)13-14-18-16(12-15-19(18)22)9-7-4-5-8-11-20(23)24/h4,7,12,14-17,21H,2-3,5-6,8-11,13H2,1H3,(H,23,24)/b7-4+,18-14+/t16-,17-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of P300 (unknown origin) |
Eur J Med Chem 178: 259-286 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.078
BindingDB Entry DOI: 10.7270/Q2ZW1QC8 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50528247
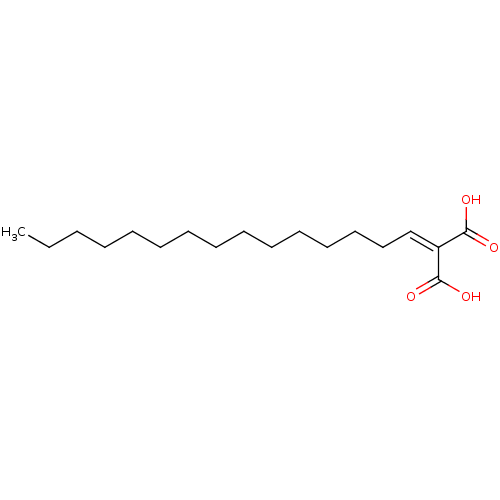
(CHEMBL4465296)Show SMILES [#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]\[#6]=[#6](\[#6](-[#8])=O)-[#6](-[#8])=O Show InChI InChI=1S/C18H32O4/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16(17(19)20)18(21)22/h15H,2-14H2,1H3,(H,19,20)(H,21,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human P300 (1284 to1673 residues) catalytic domain expressed in Escherichia coli by SPR analysis |
Eur J Med Chem 178: 259-286 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.078
BindingDB Entry DOI: 10.7270/Q2ZW1QC8 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50456444

(CHEMBL3734823)Show SMILES CC1=NN(C(=O)\C1=C\c1ccc(o1)-c1cc(C)c(C)cc1[N+]([O-])=O)c1ccc(cc1)C(O)=O |t:1| Show InChI InChI=1S/C24H19N3O6/c1-13-10-20(21(27(31)32)11-14(13)2)22-9-8-18(33-22)12-19-15(3)25-26(23(19)28)17-6-4-16(5-7-17)24(29)30/h4-12H,1-3H3,(H,29,30)/b19-12+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of P300 (unknown origin) (1287 to 1652 residues) expressed in Escherichia coli BL21(RIL)-DE3 cells using [12C]-acetyl-CoA/[14C]-acetyl-CoA... |
Eur J Med Chem 178: 259-286 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.078
BindingDB Entry DOI: 10.7270/Q2ZW1QC8 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50456444

(CHEMBL3734823)Show SMILES CC1=NN(C(=O)\C1=C\c1ccc(o1)-c1cc(C)c(C)cc1[N+]([O-])=O)c1ccc(cc1)C(O)=O |t:1| Show InChI InChI=1S/C24H19N3O6/c1-13-10-20(21(27(31)32)11-14(13)2)22-9-8-18(33-22)12-19-15(3)25-26(23(19)28)17-6-4-16(5-7-17)24(29)30/h4-12H,1-3H3,(H,29,30)/b19-12+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of P300 (1287 to 1652 residues) VMA intein chitin binding domain (unknown origin) expressed in Escherichia coli BL21(RIL)-D... |
Eur J Med Chem 178: 259-286 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.078
BindingDB Entry DOI: 10.7270/Q2ZW1QC8 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50528243
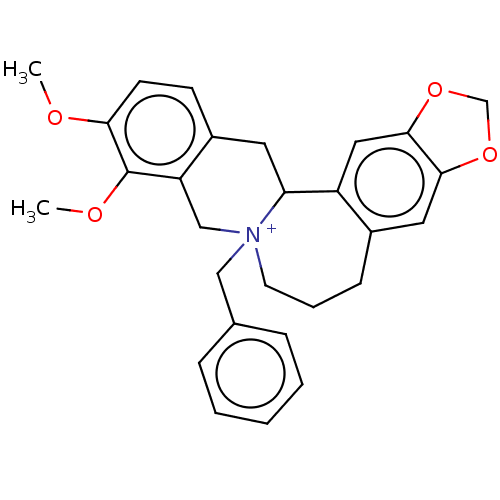
(CHEMBL4564898)Show SMILES [Br-].COc1ccc2CC3c4cc5OCOc5cc4CCC[N+]3(Cc3ccccc3)Cc2c1OC Show InChI InChI=1S/C28H30NO4.BrH/c1-30-25-11-10-21-13-24-22-15-27-26(32-18-33-27)14-20(22)9-6-12-29(24,17-23(21)28(25)31-2)16-19-7-4-3-5-8-19;/h3-5,7-8,10-11,14-15,24H,6,9,12-13,16-18H2,1-2H3;1H/q+1;/p-1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-acetyl-CoA from P300 (unknown origin) using peptide as substrate after 60 mins |
Eur J Med Chem 178: 259-286 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.078
BindingDB Entry DOI: 10.7270/Q2ZW1QC8 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50528261
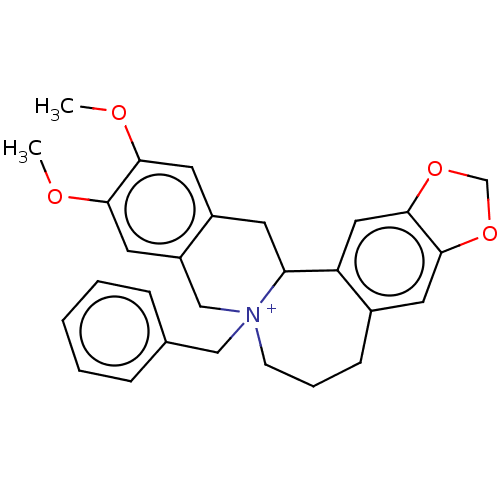
(CHEMBL4591290)Show SMILES [Br-].COc1cc2CC3c4cc5OCOc5cc4CCC[N+]3(Cc3ccccc3)Cc2cc1OC Show InChI InChI=1S/C28H30NO4.BrH/c1-30-25-13-21-11-24-23-15-28-27(32-18-33-28)12-20(23)9-6-10-29(24,16-19-7-4-3-5-8-19)17-22(21)14-26(25)31-2;/h3-5,7-8,12-15,24H,6,9-11,16-18H2,1-2H3;1H/q+1;/p-1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-acetyl-CoA from P300 (unknown origin) using peptide as substrate after 60 mins |
Eur J Med Chem 178: 259-286 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.078
BindingDB Entry DOI: 10.7270/Q2ZW1QC8 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50528252
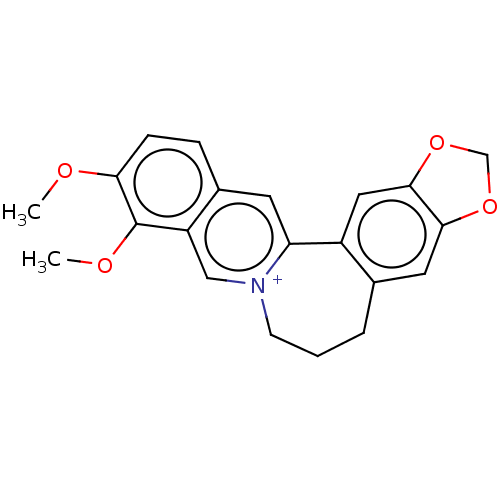
(CHEMBL4461297)Show SMILES CS([O-])(=O)=O.COc1ccc2cc3-c4cc5OCOc5cc4CCC[n+]3cc2c1OC Show InChI InChI=1S/C21H20NO4/c1-23-18-6-5-14-8-17-15-10-20-19(25-12-26-20)9-13(15)4-3-7-22(17)11-16(14)21(18)24-2/h5-6,8-11H,3-4,7,12H2,1-2H3/q+1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-acetyl-CoA from P300 (unknown origin) using peptide as substrate after 60 mins |
Eur J Med Chem 178: 259-286 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.078
BindingDB Entry DOI: 10.7270/Q2ZW1QC8 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50151658
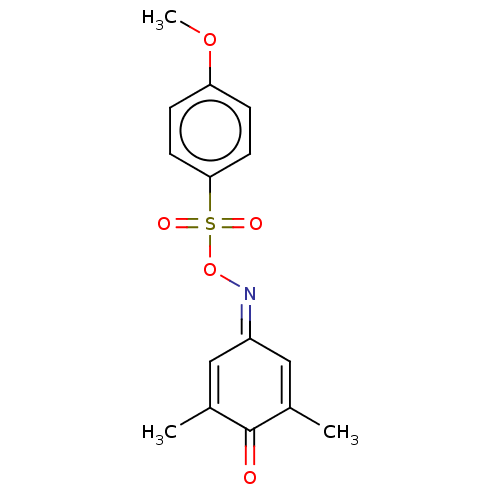
(CHEMBL3775671)Show SMILES [#6]-[#8]-c1ccc(cc1)S(=O)(=O)[#8]\[#7]=[#6]-1/[#6]=[#6](-[#6])-[#6](=O)-[#6](-[#6])=[#6]-1 |c:21,t:15| Show InChI InChI=1S/C15H15NO5S/c1-10-8-12(9-11(2)15(10)17)16-21-22(18,19)14-6-4-13(20-3)5-7-14/h4-9H,1-3H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of P300 (unknown origin) using histone H3 as substrate by fluorescence assay |
Eur J Med Chem 178: 259-286 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.078
BindingDB Entry DOI: 10.7270/Q2ZW1QC8 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase KAT5
(Homo sapiens (Human)) | BDBM50151660
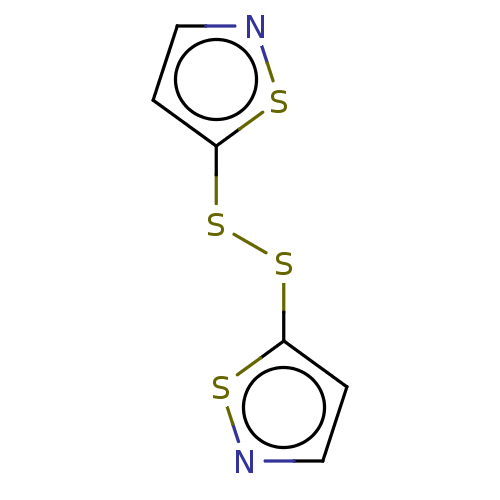
(CHEMBL3774630)Show InChI InChI=1S/C6H4N2S4/c1-3-7-9-5(1)11-12-6-2-4-8-10-6/h1-4H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of Tip60 (unknown origin) using [3H]-acetyl-CoA as substrate preincubated for 10 mins followed by substrate addition measured after 10 min... |
Eur J Med Chem 178: 259-286 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.078
BindingDB Entry DOI: 10.7270/Q2ZW1QC8 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase KAT5
(Homo sapiens (Human)) | BDBM50151660

(CHEMBL3774630)Show InChI InChI=1S/C6H4N2S4/c1-3-7-9-5(1)11-12-6-2-4-8-10-6/h1-4H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-acetyl-CoA from recombinant Tip60 (unknown origin) by scintillation counting analysis |
Eur J Med Chem 178: 259-286 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.078
BindingDB Entry DOI: 10.7270/Q2ZW1QC8 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50528246
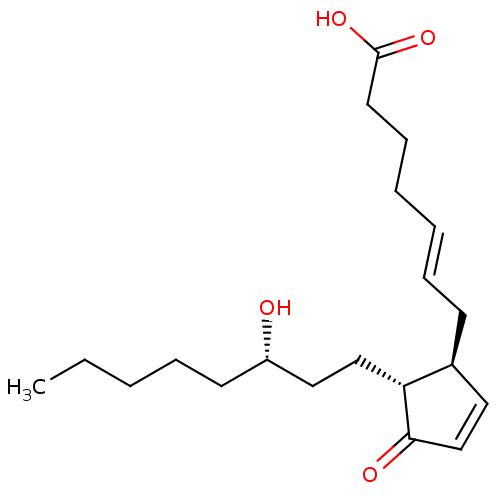
(CHEMBL4468817)Show SMILES CCCCC[C@H](O)CC[C@@H]1[C@@H](C\C=C\CCCC(O)=O)C=CC1=O |r,c:20| Show InChI InChI=1S/C20H32O4/c1-2-3-6-10-17(21)13-14-18-16(12-15-19(18)22)9-7-4-5-8-11-20(23)24/h4,7,12,15-18,21H,2-3,5-6,8-11,13-14H2,1H3,(H,23,24)/b7-4+/t16-,17-,18+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of P300 (unknown origin) |
Eur J Med Chem 178: 259-286 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.078
BindingDB Entry DOI: 10.7270/Q2ZW1QC8 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase KAT2B
(Homo sapiens (Human)) | BDBM50528257
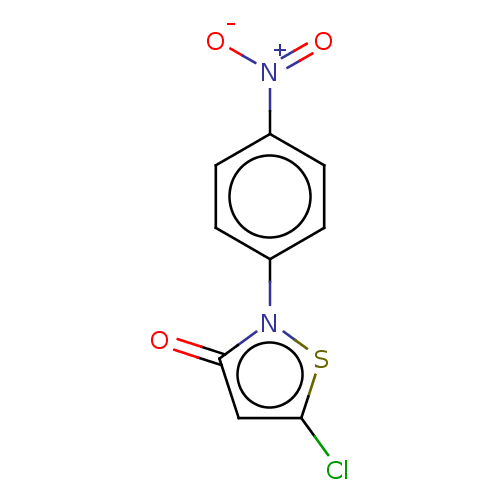
(CHEMBL4590729)Show InChI InChI=1S/C9H5ClN2O3S/c10-8-5-9(13)11(16-8)6-1-3-7(4-2-6)12(14)15/h1-5H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PCAF (32 residues) (unknown origin) expressed in Escherichia coli using radiolabelled acetyl-CoA as substrate af... |
Eur J Med Chem 178: 259-286 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.078
BindingDB Entry DOI: 10.7270/Q2ZW1QC8 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50528259
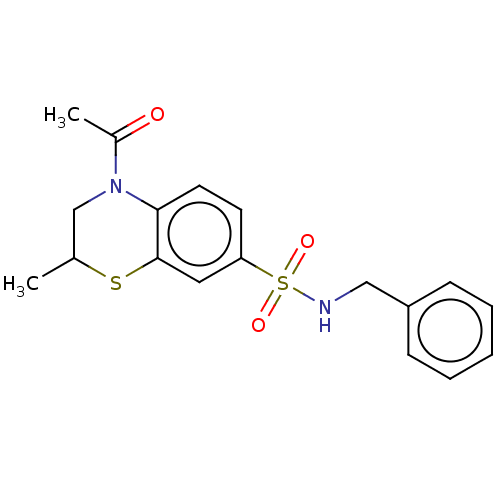
(CHEMBL4446385)Show SMILES CC1CN(C(C)=O)c2ccc(cc2S1)S(=O)(=O)NCc1ccccc1 Show InChI InChI=1S/C18H20N2O3S2/c1-13-12-20(14(2)21)17-9-8-16(10-18(17)24-13)25(22,23)19-11-15-6-4-3-5-7-15/h3-10,13,19H,11-12H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of P300 (unknown origin) |
Eur J Med Chem 178: 259-286 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.078
BindingDB Entry DOI: 10.7270/Q2ZW1QC8 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50528245
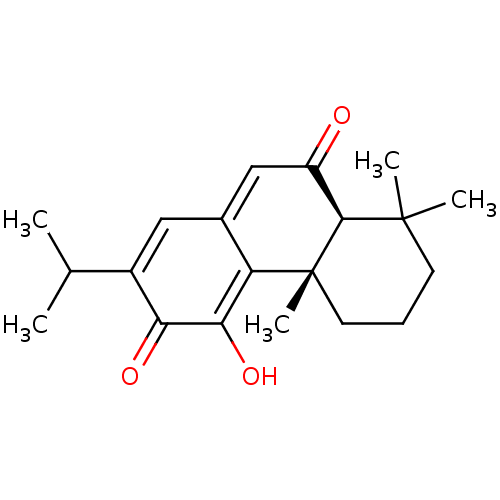
(CHEBI:9419 | taxodione)Show SMILES [H][C@@]12C(=O)C=C3C=C(C(C)C)C(=O)C(O)=C3[C@@]1(C)CCCC2(C)C |c:14,t:4,6| Show InChI InChI=1S/C20H26O3/c1-11(2)13-9-12-10-14(21)18-19(3,4)7-6-8-20(18,5)15(12)17(23)16(13)22/h9-11,18,23H,6-8H2,1-5H3/t18-,20+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 4.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of P300 (unknown origin) |
Eur J Med Chem 178: 259-286 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.078
BindingDB Entry DOI: 10.7270/Q2ZW1QC8 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50528256
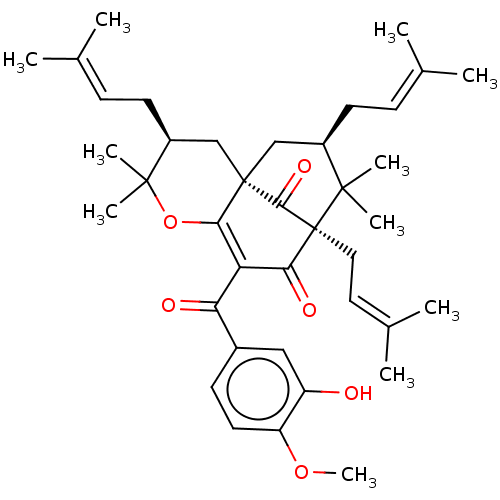
(CHEMBL4446656)Show SMILES [#6]-[#8]-c1ccc(cc1-[#8])-[#6](=O)-[#6]-1=[#6]2-[#8]C([#6])([#6])[#6@@H](-[#6]\[#6]=[#6](\[#6])-[#6])-[#6][C@@]22[#6]-[#6@@H](-[#6]\[#6]=[#6](/[#6])-[#6])C([#6])([#6])[C@@]([#6]\[#6]=[#6](\[#6])-[#6])([#6]-1=O)[#6]2=O |r,c:12,THB:27:26:43:11.41.12| Show InChI InChI=1S/C39H52O6/c1-23(2)12-15-27-21-38-22-28(16-13-24(3)4)37(9,10)45-34(38)31(32(41)26-14-17-30(44-11)29(40)20-26)33(42)39(35(38)43,36(27,7)8)19-18-25(5)6/h12-14,17-18,20,27-28,40H,15-16,19,21-22H2,1-11H3/t27-,28+,38+,39+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of P300 (unknown origin) |
Eur J Med Chem 178: 259-286 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.078
BindingDB Entry DOI: 10.7270/Q2ZW1QC8 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50528244
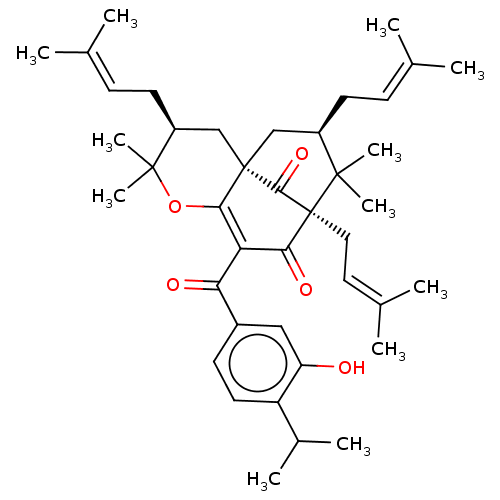
(CHEMBL4456463)Show SMILES [#6]-[#6](-[#6])-c1ccc(cc1-[#8])-[#6](=O)-[#6]-1=[#6]2-[#8]C([#6])([#6])[#6@@H](-[#6]\[#6]=[#6](\[#6])-[#6])-[#6][C@@]22[#6]-[#6@@H](-[#6]\[#6]=[#6](\[#6])-[#6])C([#6])([#6])[C@@]([#6]\[#6]=[#6](\[#6])-[#6])([#6]-1=O)[#6]2=O |r,c:13,THB:28:27:44:12.42.13| Show InChI InChI=1S/C41H56O5/c1-24(2)13-16-29-22-40-23-30(17-14-25(3)4)39(11,12)46-36(40)33(34(43)28-15-18-31(27(7)8)32(42)21-28)35(44)41(37(40)45,38(29,9)10)20-19-26(5)6/h13-15,18-19,21,27,29-30,42H,16-17,20,22-23H2,1-12H3/t29-,30+,40+,41+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of P300 (unknown origin) |
Eur J Med Chem 178: 259-286 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.078
BindingDB Entry DOI: 10.7270/Q2ZW1QC8 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50528242
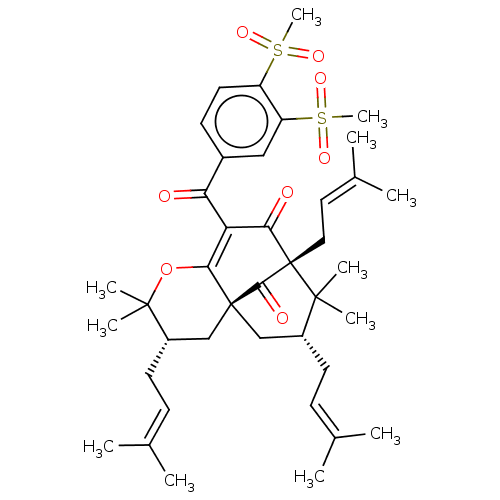
(CHEMBL4516760)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6@H]1-[#6][C@@]23[#6]-[#6@@H](-[#6]\[#6]=[#6](\[#6])-[#6])C([#6])([#6])[C@@]([#6]\[#6]=[#6](\[#6])-[#6])([#6](=O)-[#6](-[#6](=O)-c4ccc(c(c4)S([#6])(=O)=O)S([#6])(=O)=O)=[#6]2-[#8]C1([#6])[#6])[#6]3=O |r,c:43,THB:10:9:48:26.24.43| Show InChI InChI=1S/C40H54O8S2/c1-24(2)13-16-28-22-39-23-29(17-14-25(3)4)38(9,10)48-35(39)32(34(42)40(36(39)43,37(28,7)8)20-19-26(5)6)33(41)27-15-18-30(49(11,44)45)31(21-27)50(12,46)47/h13-15,18-19,21,28-29H,16-17,20,22-23H2,1-12H3/t28-,29+,39+,40+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of P300 (unknown origin) |
Eur J Med Chem 178: 259-286 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.078
BindingDB Entry DOI: 10.7270/Q2ZW1QC8 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50528256

(CHEMBL4446656)Show SMILES [#6]-[#8]-c1ccc(cc1-[#8])-[#6](=O)-[#6]-1=[#6]2-[#8]C([#6])([#6])[#6@@H](-[#6]\[#6]=[#6](\[#6])-[#6])-[#6][C@@]22[#6]-[#6@@H](-[#6]\[#6]=[#6](/[#6])-[#6])C([#6])([#6])[C@@]([#6]\[#6]=[#6](\[#6])-[#6])([#6]-1=O)[#6]2=O |r,c:12,THB:27:26:43:11.41.12| Show InChI InChI=1S/C39H52O6/c1-23(2)12-15-27-21-38-22-28(16-13-24(3)4)37(9,10)45-34(38)31(32(41)26-14-17-30(44-11)29(40)20-26)33(42)39(35(38)43,36(27,7)8)19-18-25(5)6/h12-14,17-18,20,27-28,40H,15-16,19,21-22H2,1-11H3/t27-,28+,38+,39+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of P300 (unknown origin) assessed as reduction in p53 acetylation |
Eur J Med Chem 178: 259-286 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.078
BindingDB Entry DOI: 10.7270/Q2ZW1QC8 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50528258
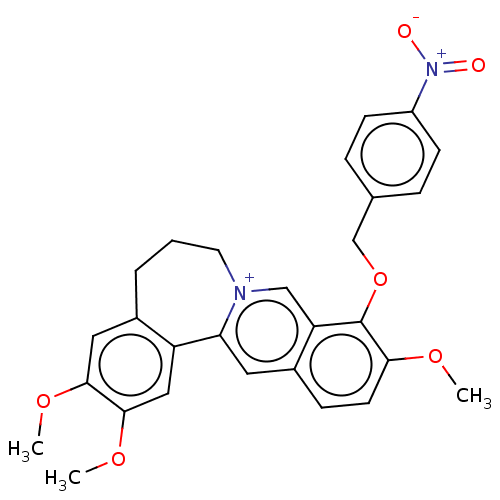
(CHEMBL4466490)Show SMILES [Br-].COc1cc2CCC[n+]3cc4c(OCc5ccc(cc5)[N+]([O-])=O)c(OC)ccc4cc3-c2cc1OC Show InChI InChI=1S/C28H27N2O6/c1-33-25-11-8-20-13-24-22-15-27(35-3)26(34-2)14-19(22)5-4-12-29(24)16-23(20)28(25)36-17-18-6-9-21(10-7-18)30(31)32/h6-11,13-16H,4-5,12,17H2,1-3H3/q+1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-acetyl-CoA from P300 (unknown origin) using peptide as substrate after 60 mins |
Eur J Med Chem 178: 259-286 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.078
BindingDB Entry DOI: 10.7270/Q2ZW1QC8 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50528251
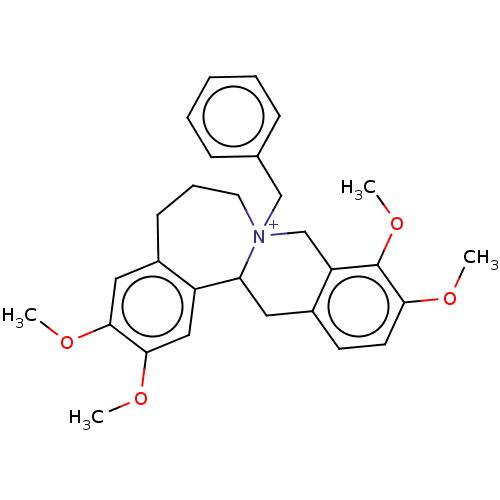
(CHEMBL4536287)Show SMILES [Br-].COc1cc2CCC[N+]3(Cc4ccccc4)Cc4c(CC3c2cc1OC)ccc(OC)c4OC Show InChI InChI=1S/C29H34NO4.BrH/c1-31-26-13-12-22-15-25-23-17-28(33-3)27(32-2)16-21(23)11-8-14-30(25,19-24(22)29(26)34-4)18-20-9-6-5-7-10-20;/h5-7,9-10,12-13,16-17,25H,8,11,14-15,18-19H2,1-4H3;1H/q+1;/p-1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-acetyl-CoA from P300 (unknown origin) using peptide as substrate after 60 mins |
Eur J Med Chem 178: 259-286 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.078
BindingDB Entry DOI: 10.7270/Q2ZW1QC8 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50528250
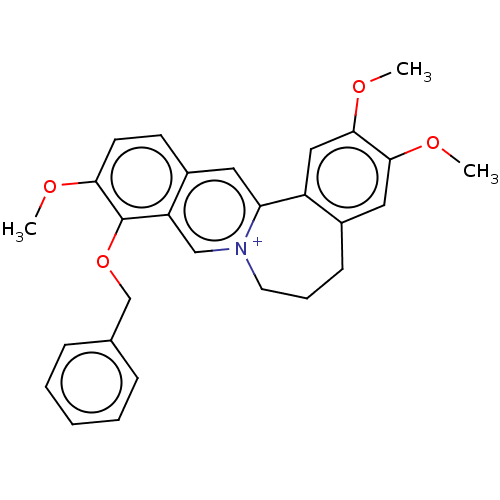
(CHEMBL4538233)Show SMILES [Cl-].COc1ccc2cc3-c4cc(OC)c(OC)cc4CCC[n+]3cc2c1OCc1ccccc1 Show InChI InChI=1S/C28H28NO4/c1-30-25-12-11-21-14-24-22-16-27(32-3)26(31-2)15-20(22)10-7-13-29(24)17-23(21)28(25)33-18-19-8-5-4-6-9-19/h4-6,8-9,11-12,14-17H,7,10,13,18H2,1-3H3/q+1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-acetyl-CoA from P300 (unknown origin) using peptide as substrate after 60 mins |
Eur J Med Chem 178: 259-286 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.078
BindingDB Entry DOI: 10.7270/Q2ZW1QC8 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50528255
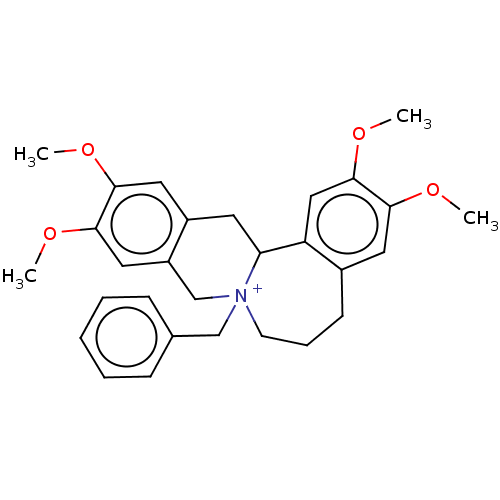
(CHEMBL4455088)Show SMILES [Br-].COc1cc2CC3c4cc(OC)c(OC)cc4CCC[N+]3(Cc3ccccc3)Cc2cc1OC Show InChI InChI=1S/C29H34NO4.BrH/c1-31-26-14-21-11-8-12-30(18-20-9-6-5-7-10-20)19-23-16-28(33-3)27(32-2)15-22(23)13-25(30)24(21)17-29(26)34-4;/h5-7,9-10,14-17,25H,8,11-13,18-19H2,1-4H3;1H/q+1;/p-1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-acetyl-CoA from P300 (unknown origin) using peptide as substrate after 60 mins |
Eur J Med Chem 178: 259-286 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.078
BindingDB Entry DOI: 10.7270/Q2ZW1QC8 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50528260
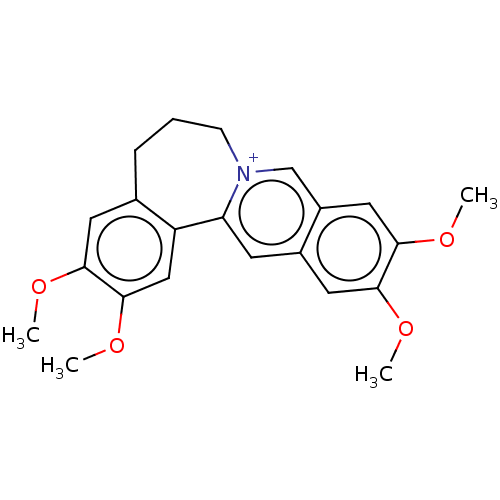
(CHEMBL4457203)Show SMILES CS([O-])(=O)=O.COc1cc2CCC[n+]3cc4cc(OC)c(OC)cc4cc3-c2cc1OC Show InChI InChI=1S/C22H24NO4/c1-24-19-9-14-6-5-7-23-13-16-11-21(26-3)20(25-2)10-15(16)8-18(23)17(14)12-22(19)27-4/h8-13H,5-7H2,1-4H3/q+1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-acetyl-CoA from P300 (unknown origin) using peptide as substrate after 60 mins |
Eur J Med Chem 178: 259-286 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.078
BindingDB Entry DOI: 10.7270/Q2ZW1QC8 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50012070

(5-Hydroxy-2-methyl-[1,4]naphthoquinone | 5-hydroxy...)Show InChI InChI=1S/C11H8O3/c1-6-5-9(13)10-7(11(6)14)3-2-4-8(10)12/h2-5,12H,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of P300 in human HeLa cells using [3H]-acetyl-CoA as substrate preincubated for 10 mins followed by substrate addition measured after 10 m... |
Eur J Med Chem 178: 259-286 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.078
BindingDB Entry DOI: 10.7270/Q2ZW1QC8 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50012070

(5-Hydroxy-2-methyl-[1,4]naphthoquinone | 5-hydroxy...)Show InChI InChI=1S/C11H8O3/c1-6-5-9(13)10-7(11(6)14)3-2-4-8(10)12/h2-5,12H,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-acetyl-CoA from recombinant P300 (unknown origin) expressed in baculovirus expression system preincubated for 10 mins followed b... |
Eur J Med Chem 178: 259-286 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.078
BindingDB Entry DOI: 10.7270/Q2ZW1QC8 |
More data for this
Ligand-Target Pair | |
CREB-binding protein/Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50140172

(CHEBI:3962 | CHEMBL140 | Curcumin | US9409845, Tab...)Show SMILES COc1cc(\C=C\C(=O)CC(=O)\C=C\c2ccc(O)c(OC)c2)ccc1O Show InChI InChI=1S/C21H20O6/c1-26-20-11-14(5-9-18(20)24)3-7-16(22)13-17(23)8-4-15-6-10-19(25)21(12-15)27-2/h3-12,24-25H,13H2,1-2H3/b7-3+,8-4+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of p300 (unknown origin)/CBP (unknown origin) |
Eur J Med Chem 178: 259-286 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.078
BindingDB Entry DOI: 10.7270/Q2ZW1QC8 |
More data for this
Ligand-Target Pair | |
CREB-binding protein/Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50326997
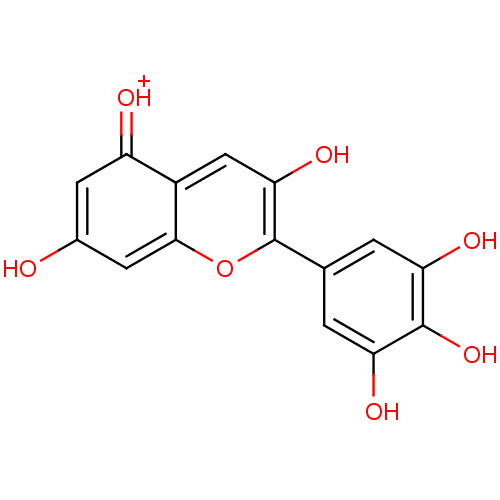
(CHEMBL590878 | Delphinidin)Show SMILES Oc1cc2oc(c(O)cc2c(=[OH+])c1)-c1cc(O)c(O)c(O)c1 Show InChI InChI=1S/C15H10O7/c16-7-3-9(17)8-5-12(20)15(22-13(8)4-7)6-1-10(18)14(21)11(19)2-6/h1-5,16,18-21H/p+1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of p300 (unknown origin)/CBP (unknown origin) |
Eur J Med Chem 178: 259-286 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.078
BindingDB Entry DOI: 10.7270/Q2ZW1QC8 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50070942

((-)-Epigallocatechin gallate | (-)-Epigallocatechi...)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@H](Oc2c1)c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C22H18O11/c23-10-5-12(24)11-7-18(33-22(31)9-3-15(27)20(30)16(28)4-9)21(32-17(11)6-10)8-1-13(25)19(29)14(26)2-8/h1-6,18,21,23-30H,7H2/t18-,21-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of P300 (unknown origin) |
Eur J Med Chem 178: 259-286 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.078
BindingDB Entry DOI: 10.7270/Q2ZW1QC8 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50151658

(CHEMBL3775671)Show SMILES [#6]-[#8]-c1ccc(cc1)S(=O)(=O)[#8]\[#7]=[#6]-1/[#6]=[#6](-[#6])-[#6](=O)-[#6](-[#6])=[#6]-1 |c:21,t:15| Show InChI InChI=1S/C15H15NO5S/c1-10-8-12(9-11(2)15(10)17)16-21-22(18,19)14-6-4-13(20-3)5-7-14/h4-9H,1-3H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of P300 (unknown origin) |
Eur J Med Chem 178: 259-286 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.078
BindingDB Entry DOI: 10.7270/Q2ZW1QC8 |
More data for this
Ligand-Target Pair | |
CREB-binding protein
(Homo sapiens (Human)) | BDBM50070942

((-)-Epigallocatechin gallate | (-)-Epigallocatechi...)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@H](Oc2c1)c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C22H18O11/c23-10-5-12(24)11-7-18(33-22(31)9-3-15(27)20(30)16(28)4-9)21(32-17(11)6-10)8-1-13(25)19(29)14(26)2-8/h1-6,18,21,23-30H,7H2/t18-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of CBP (unknown origin) |
Eur J Med Chem 178: 259-286 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.078
BindingDB Entry DOI: 10.7270/Q2ZW1QC8 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase KAT2B
(Homo sapiens (Human)) | BDBM50012070

(5-Hydroxy-2-methyl-[1,4]naphthoquinone | 5-hydroxy...)Show InChI InChI=1S/C11H8O3/c1-6-5-9(13)10-7(11(6)14)3-2-4-8(10)12/h2-5,12H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of PCAF in human HeLa cells using [3H]-acetyl-CoA as substrate preincubated for 10 mins followed by substrate addition measured after 10 m... |
Eur J Med Chem 178: 259-286 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.078
BindingDB Entry DOI: 10.7270/Q2ZW1QC8 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase KAT2B
(Homo sapiens (Human)) | BDBM50012070

(5-Hydroxy-2-methyl-[1,4]naphthoquinone | 5-hydroxy...)Show InChI InChI=1S/C11H8O3/c1-6-5-9(13)10-7(11(6)14)3-2-4-8(10)12/h2-5,12H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-acetyl-CoA from recombinant PCAF (unknown origin) expressed in baculovirus expression system preincubated for 10 mins followed b... |
Eur J Med Chem 178: 259-286 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.078
BindingDB Entry DOI: 10.7270/Q2ZW1QC8 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50528254
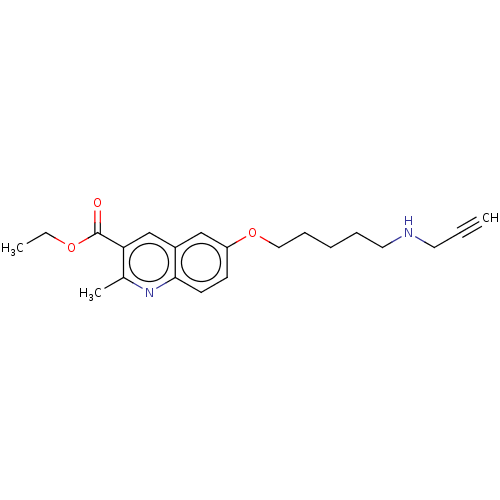
(CHEMBL4515219)Show InChI InChI=1S/C21H26N2O3/c1-4-11-22-12-7-6-8-13-26-18-9-10-20-17(14-18)15-19(16(3)23-20)21(24)25-5-2/h1,9-10,14-15,22H,5-8,11-13H2,2-3H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of P300 (unknown origin) |
Eur J Med Chem 178: 259-286 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.078
BindingDB Entry DOI: 10.7270/Q2ZW1QC8 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase KAT2B
(Homo sapiens (Human)) | BDBM50070942

((-)-Epigallocatechin gallate | (-)-Epigallocatechi...)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@H](Oc2c1)c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C22H18O11/c23-10-5-12(24)11-7-18(33-22(31)9-3-15(27)20(30)16(28)4-9)21(32-17(11)6-10)8-1-13(25)19(29)14(26)2-8/h1-6,18,21,23-30H,7H2/t18-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of PCAF (unknown origin) |
Eur J Med Chem 178: 259-286 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.078
BindingDB Entry DOI: 10.7270/Q2ZW1QC8 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase KAT5
(Homo sapiens (Human)) | BDBM50070942

((-)-Epigallocatechin gallate | (-)-Epigallocatechi...)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@H](Oc2c1)c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C22H18O11/c23-10-5-12(24)11-7-18(33-22(31)9-3-15(27)20(30)16(28)4-9)21(32-17(11)6-10)8-1-13(25)19(29)14(26)2-8/h1-6,18,21,23-30H,7H2/t18-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of Tip60 (unknown origin) |
Eur J Med Chem 178: 259-286 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.078
BindingDB Entry DOI: 10.7270/Q2ZW1QC8 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase KAT7
(Homo sapiens) | BDBM50518801
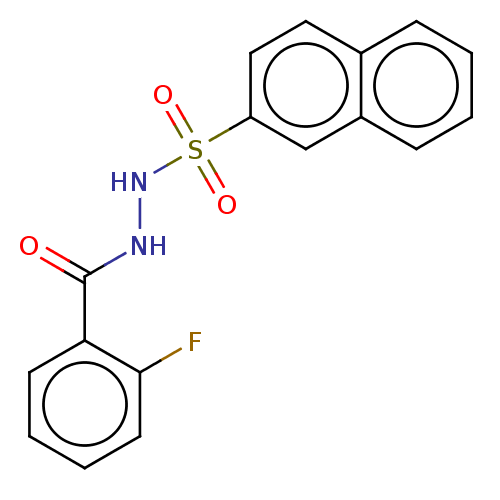
(CHEMBL4470091)Show InChI InChI=1S/C17H13FN2O3S/c18-16-8-4-3-7-15(16)17(21)19-20-24(22,23)14-10-9-12-5-1-2-6-13(12)11-14/h1-11,20H,(H,19,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of KAT7 (unknown origin) |
Eur J Med Chem 178: 259-286 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.078
BindingDB Entry DOI: 10.7270/Q2ZW1QC8 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase KAT5
(Homo sapiens (Human)) | BDBM50518801

(CHEMBL4470091)Show InChI InChI=1S/C17H13FN2O3S/c18-16-8-4-3-7-15(16)17(21)19-20-24(22,23)14-10-9-12-5-1-2-6-13(12)11-14/h1-11,20H,(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of KAT5 (unknown origin) |
Eur J Med Chem 178: 259-286 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.078
BindingDB Entry DOI: 10.7270/Q2ZW1QC8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data