 Found 27 hits Enz. Inhib. hit(s) with all data for entry = 50010229
Found 27 hits Enz. Inhib. hit(s) with all data for entry = 50010229 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396023
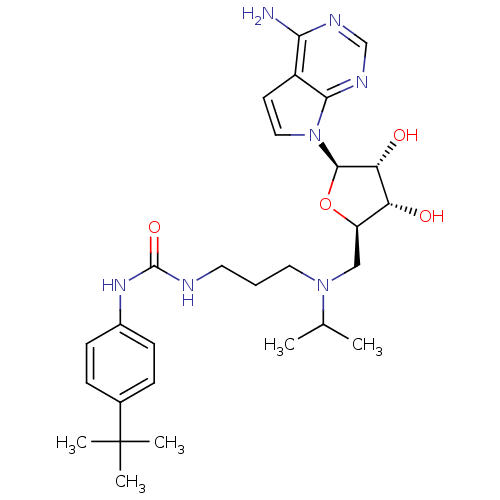
(CHEMBL2169919)Show SMILES CC(C)N(CCCNC(=O)Nc1ccc(cc1)C(C)(C)C)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1ccc2c(N)ncnc12 |r| Show InChI InChI=1S/C28H41N7O4/c1-17(2)34(13-6-12-30-27(38)33-19-9-7-18(8-10-19)28(3,4)5)15-21-22(36)23(37)26(39-21)35-14-11-20-24(29)31-16-32-25(20)35/h7-11,14,16-17,21-23,26,36-37H,6,12-13,15H2,1-5H3,(H2,29,31,32)(H2,30,33,38)/t21-,22-,23-,26-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L (1 to 416 residues) using chicken erythrocyte oligonucleosomes as substrate and [3H]-SAM incubated for 120 mins... |
Bioorg Med Chem Lett 26: 4518-4522 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.041
BindingDB Entry DOI: 10.7270/Q25M697H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396981
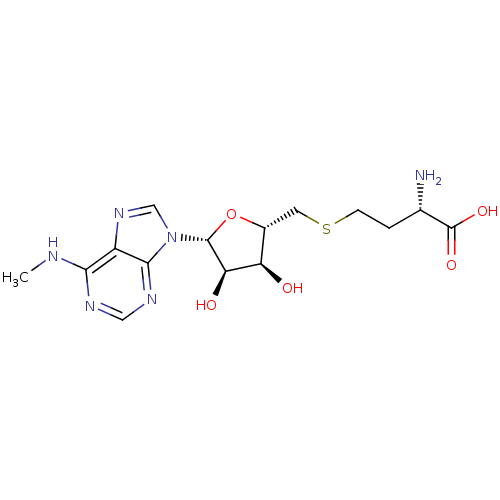
(CHEMBL2171174)Show SMILES CNc1ncnc2n(cnc12)[C@@H]1O[C@H](CSCC[C@H](N)C(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C15H22N6O5S/c1-17-12-9-13(19-5-18-12)21(6-20-9)14-11(23)10(22)8(26-14)4-27-3-2-7(16)15(24)25/h5-8,10-11,14,22-23H,2-4,16H2,1H3,(H,24,25)(H,17,18,19)/t7-,8+,10+,11+,14+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DOT1L (1 to 472 residues) preincubated for 10 mins prior addition of [3H]SAM measured after 30 mins by Beckman LS-650... |
Bioorg Med Chem Lett 26: 4518-4522 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.041
BindingDB Entry DOI: 10.7270/Q25M697H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
DNA (cytosine-5)-methyltransferase 1
(Homo sapiens (Human)) | BDBM50443016
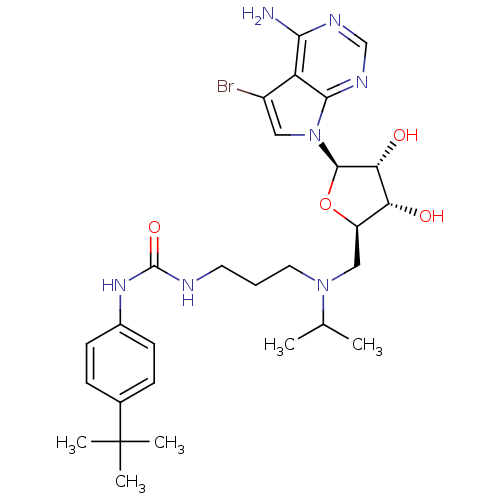
(CHEMBL3087498)Show SMILES CC(C)N(CCCNC(=O)Nc1ccc(cc1)C(C)(C)C)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cc(Br)c2c(N)ncnc12 |r| Show InChI InChI=1S/C28H40BrN7O4/c1-16(2)35(12-6-11-31-27(39)34-18-9-7-17(8-10-18)28(3,4)5)14-20-22(37)23(38)26(40-20)36-13-19(29)21-24(30)32-15-33-25(21)36/h7-10,13,15-16,20,22-23,26,37-38H,6,11-12,14H2,1-5H3,(H2,30,32,33)(H2,31,34,39)/t20-,22-,23-,26-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of DNMT1 (unknown origin) |
Bioorg Med Chem Lett 26: 4518-4522 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.041
BindingDB Entry DOI: 10.7270/Q25M697H |
More data for this
Ligand-Target Pair | |
DNA (cytosine-5)-methyltransferase 1
(Homo sapiens (Human)) | BDBM50396980
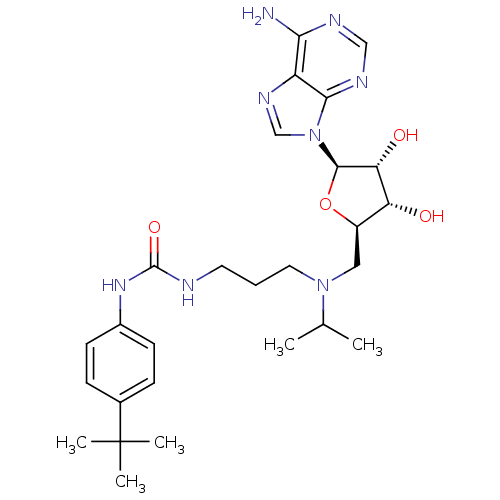
(CHEMBL2171169)Show SMILES CC(C)N(CCCNC(=O)Nc1ccc(cc1)C(C)(C)C)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C27H40N8O4/c1-16(2)34(12-6-11-29-26(38)33-18-9-7-17(8-10-18)27(3,4)5)13-19-21(36)22(37)25(39-19)35-15-32-20-23(28)30-14-31-24(20)35/h7-10,14-16,19,21-22,25,36-37H,6,11-13H2,1-5H3,(H2,28,30,31)(H2,29,33,38)/t19-,21-,22-,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of DNMT1 (unknown origin) |
Bioorg Med Chem Lett 26: 4518-4522 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.041
BindingDB Entry DOI: 10.7270/Q25M697H |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50536666
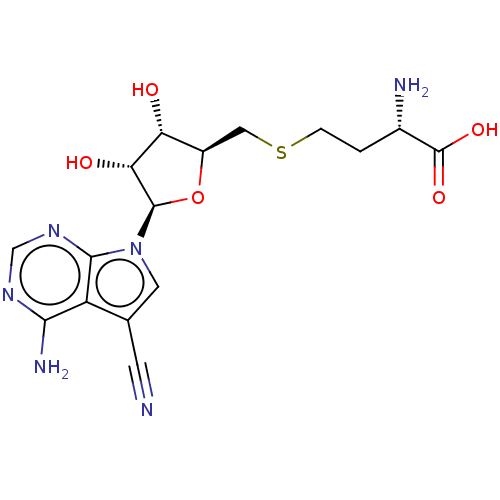
(CHEMBL4588797)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cc(C#N)c2c(N)ncnc12)C(O)=O |r| Show InChI InChI=1S/C16H20N6O5S/c17-3-7-4-22(14-10(7)13(19)20-6-21-14)15-12(24)11(23)9(27-15)5-28-2-1-8(18)16(25)26/h4,6,8-9,11-12,15,23-24H,1-2,5,18H2,(H,25,26)(H2,19,20,21)/t8-,9+,11+,12+,15+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L (1 to 420 amino acids) expressed in Escherichia coli |
Bioorg Med Chem Lett 26: 4518-4522 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.041
BindingDB Entry DOI: 10.7270/Q25M697H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50536666

(CHEMBL4588797)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cc(C#N)c2c(N)ncnc12)C(O)=O |r| Show InChI InChI=1S/C16H20N6O5S/c17-3-7-4-22(14-10(7)13(19)20-6-21-14)15-12(24)11(23)9(27-15)5-28-2-1-8(18)16(25)26/h4,6,8-9,11-12,15,23-24H,1-2,5,18H2,(H,25,26)(H2,19,20,21)/t8-,9+,11+,12+,15+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of DOT1L (unknown origin) using chicken nucleosome as substrate in presence of [3H]SAM incubated for 1 hr by TopCount method |
Bioorg Med Chem Lett 26: 4518-4522 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.041
BindingDB Entry DOI: 10.7270/Q25M697H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50536667
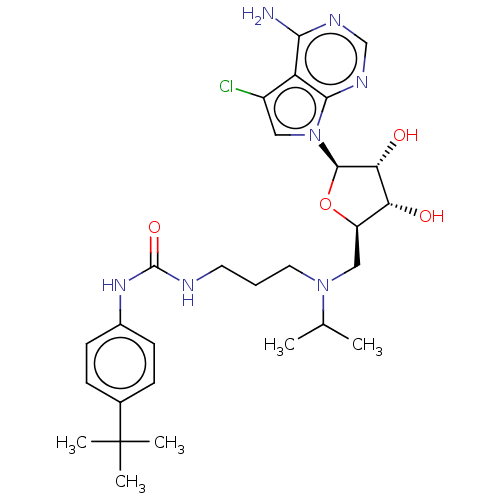
(CHEMBL4559614)Show SMILES CC(C)N(CCCNC(=O)Nc1ccc(cc1)C(C)(C)C)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cc(Cl)c2c(N)ncnc12 |r| Show InChI InChI=1S/C28H40ClN7O4/c1-16(2)35(12-6-11-31-27(39)34-18-9-7-17(8-10-18)28(3,4)5)14-20-22(37)23(38)26(40-20)36-13-19(29)21-24(30)32-15-33-25(21)36/h7-10,13,15-16,20,22-23,26,37-38H,6,11-12,14H2,1-5H3,(H2,30,32,33)(H2,31,34,39)/t20-,22-,23-,26-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of human 6His-tagged DOT1L (1 to 420 residues) measured after 30 mins by AlphaScreen binding assay |
Bioorg Med Chem Lett 26: 4518-4522 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.041
BindingDB Entry DOI: 10.7270/Q25M697H |
More data for this
Ligand-Target Pair | |
DNA (cytosine-5)-methyltransferase 1
(Homo sapiens (Human)) | BDBM50536666

(CHEMBL4588797)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cc(C#N)c2c(N)ncnc12)C(O)=O |r| Show InChI InChI=1S/C16H20N6O5S/c17-3-7-4-22(14-10(7)13(19)20-6-21-14)15-12(24)11(23)9(27-15)5-28-2-1-8(18)16(25)26/h4,6,8-9,11-12,15,23-24H,1-2,5,18H2,(H,25,26)(H2,19,20,21)/t8-,9+,11+,12+,15+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of DNMT1 (unknown origin) |
Bioorg Med Chem Lett 26: 4518-4522 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.041
BindingDB Entry DOI: 10.7270/Q25M697H |
More data for this
Ligand-Target Pair | |
DNA (cytosine-5)-methyltransferase 1
(Homo sapiens (Human)) | BDBM50009672

(AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O Show InChI InChI=1S/C14H20N6O5S/c15-6(14(23)24)1-2-26-3-7-9(21)10(22)13(25-7)20-5-19-8-11(16)17-4-18-12(8)20/h4-7,9-10,13,21-22H,1-3,15H2,(H,23,24)(H2,16,17,18)/t6-,7+,9+,10+,13+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of DNMT1 (unknown origin) |
Bioorg Med Chem Lett 26: 4518-4522 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.041
BindingDB Entry DOI: 10.7270/Q25M697H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50009672

(AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O Show InChI InChI=1S/C14H20N6O5S/c15-6(14(23)24)1-2-26-3-7-9(21)10(22)13(25-7)20-5-19-8-11(16)17-4-18-12(8)20/h4-7,9-10,13,21-22H,1-3,15H2,(H,23,24)(H2,16,17,18)/t6-,7+,9+,10+,13+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of PRMT5 (unknown origin) |
Bioorg Med Chem Lett 26: 4518-4522 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.041
BindingDB Entry DOI: 10.7270/Q25M697H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50009672

(AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O Show InChI InChI=1S/C14H20N6O5S/c15-6(14(23)24)1-2-26-3-7-9(21)10(22)13(25-7)20-5-19-8-11(16)17-4-18-12(8)20/h4-7,9-10,13,21-22H,1-3,15H2,(H,23,24)(H2,16,17,18)/t6-,7+,9+,10+,13+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L (1 to 420 amino acids) expressed in Escherichia coli |
Bioorg Med Chem Lett 26: 4518-4522 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.041
BindingDB Entry DOI: 10.7270/Q25M697H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein arginine N-methyltransferase 3
(Homo sapiens) | BDBM50536666

(CHEMBL4588797)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cc(C#N)c2c(N)ncnc12)C(O)=O |r| Show InChI InChI=1S/C16H20N6O5S/c17-3-7-4-22(14-10(7)13(19)20-6-21-14)15-12(24)11(23)9(27-15)5-28-2-1-8(18)16(25)26/h4,6,8-9,11-12,15,23-24H,1-2,5,18H2,(H,25,26)(H2,19,20,21)/t8-,9+,11+,12+,15+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of PRMT3 (unknown origin) |
Bioorg Med Chem Lett 26: 4518-4522 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.041
BindingDB Entry DOI: 10.7270/Q25M697H |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 3
(Homo sapiens) | BDBM50009672

(AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O Show InChI InChI=1S/C14H20N6O5S/c15-6(14(23)24)1-2-26-3-7-9(21)10(22)13(25-7)20-5-19-8-11(16)17-4-18-12(8)20/h4-7,9-10,13,21-22H,1-3,15H2,(H,23,24)(H2,16,17,18)/t6-,7+,9+,10+,13+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of PRMT3 (unknown origin) |
Bioorg Med Chem Lett 26: 4518-4522 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.041
BindingDB Entry DOI: 10.7270/Q25M697H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50536666

(CHEMBL4588797)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cc(C#N)c2c(N)ncnc12)C(O)=O |r| Show InChI InChI=1S/C16H20N6O5S/c17-3-7-4-22(14-10(7)13(19)20-6-21-14)15-12(24)11(23)9(27-15)5-28-2-1-8(18)16(25)26/h4,6,8-9,11-12,15,23-24H,1-2,5,18H2,(H,25,26)(H2,19,20,21)/t8-,9+,11+,12+,15+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of PRMT5 (unknown origin) |
Bioorg Med Chem Lett 26: 4518-4522 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.041
BindingDB Entry DOI: 10.7270/Q25M697H |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50009672

(AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O Show InChI InChI=1S/C14H20N6O5S/c15-6(14(23)24)1-2-26-3-7-9(21)10(22)13(25-7)20-5-19-8-11(16)17-4-18-12(8)20/h4-7,9-10,13,21-22H,1-3,15H2,(H,23,24)(H2,16,17,18)/t6-,7+,9+,10+,13+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of G9a (unknown origin) |
Bioorg Med Chem Lett 26: 4518-4522 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.041
BindingDB Entry DOI: 10.7270/Q25M697H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50375654

(CHEMBL99203 | US11633415, Compound 5-iodotubercidi...)Show SMILES Nc1ncnc2n(cc(I)c12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H13IN4O4/c12-4-1-16(10-6(4)9(13)14-3-15-10)11-8(19)7(18)5(2-17)20-11/h1,3,5,7-8,11,17-19H,2H2,(H2,13,14,15)/t5-,7-,8-,11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of DOT1L (unknown origin) using chicken nucleosome as substrate in presence of [3H]SAM incubated for 1 hr by TopCount method |
Bioorg Med Chem Lett 26: 4518-4522 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.041
BindingDB Entry DOI: 10.7270/Q25M697H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase SUV39H2
(Homo sapiens (Human)) | BDBM50009672

(AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O Show InChI InChI=1S/C14H20N6O5S/c15-6(14(23)24)1-2-26-3-7-9(21)10(22)13(25-7)20-5-19-8-11(16)17-4-18-12(8)20/h4-7,9-10,13,21-22H,1-3,15H2,(H,23,24)(H2,16,17,18)/t6-,7+,9+,10+,13+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of SUV39H2 (unknown origin) |
Bioorg Med Chem Lett 26: 4518-4522 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.041
BindingDB Entry DOI: 10.7270/Q25M697H |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50533467
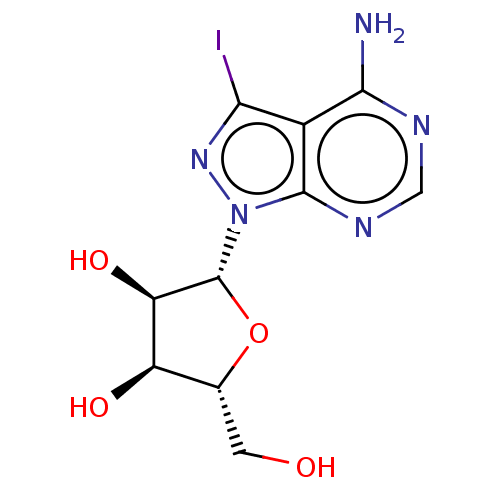
(CHEMBL4441282)Show SMILES Nc1ncnc2n(nc(I)c12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H12IN5O4/c11-7-4-8(12)13-2-14-9(4)16(15-7)10-6(19)5(18)3(1-17)20-10/h2-3,5-6,10,17-19H,1H2,(H2,12,13,14)/t3-,5-,6-,10-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of DOT1L (unknown origin) using chicken nucleosome as substrate in presence of [3H]SAM incubated for 1 hr by TopCount method |
Bioorg Med Chem Lett 26: 4518-4522 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.041
BindingDB Entry DOI: 10.7270/Q25M697H |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50049820
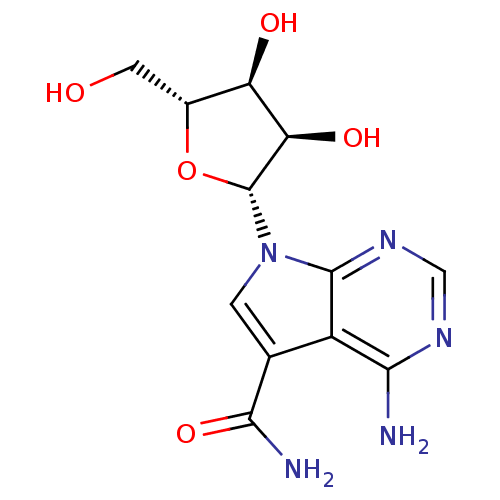
(4-Amino-5-carboxamide-7-(D-ribofuranosyl)pyrrolo[2...)Show SMILES NC(=O)c1cn([C@@H]2O[C@H](CO)[C@@H](O)[C@H]2O)c2ncnc(N)c12 |r| Show InChI InChI=1S/C12H15N5O5/c13-9-6-4(10(14)21)1-17(11(6)16-3-15-9)12-8(20)7(19)5(2-18)22-12/h1,3,5,7-8,12,18-20H,2H2,(H2,14,21)(H2,13,15,16)/t5-,7-,8-,12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of DOT1L (unknown origin) using chicken nucleosome as substrate in presence of [3H]SAM incubated for 1 hr by TopCount method |
Bioorg Med Chem Lett 26: 4518-4522 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.041
BindingDB Entry DOI: 10.7270/Q25M697H |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50350207

(CHEMBL1814776)Show SMILES Nc1ncnc2n(cc(C#C)c12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C13H14N4O4/c1-2-6-3-17(12-8(6)11(14)15-5-16-12)13-10(20)9(19)7(4-18)21-13/h1,3,5,7,9-10,13,18-20H,4H2,(H2,14,15,16)/t7-,9-,10-,13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of DOT1L (unknown origin) using chicken nucleosome as substrate in presence of [3H]SAM incubated for 1 hr by TopCount method |
Bioorg Med Chem Lett 26: 4518-4522 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.041
BindingDB Entry DOI: 10.7270/Q25M697H |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50536668
(CHEMBL4525316)Show SMILES Nc1ncnc2n(cc(-c3ncco3)c12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C14H15N5O5/c15-11-8-6(13-16-1-2-23-13)3-19(12(8)18-5-17-11)14-10(22)9(21)7(4-20)24-14/h1-3,5,7,9-10,14,20-22H,4H2,(H2,15,17,18)/t7-,9-,10-,14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of DOT1L (unknown origin) using chicken nucleosome as substrate in presence of [3H]SAM incubated for 1 hr by TopCount method |
Bioorg Med Chem Lett 26: 4518-4522 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.041
BindingDB Entry DOI: 10.7270/Q25M697H |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50049823
(4-Amino-7-((2R,3R,4S,5R)-3,4-dihydroxy-5-hydroxyme...)Show SMILES Nc1ncnc2n(cc(C#N)c12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C12H13N5O4/c13-1-5-2-17(11-7(5)10(14)15-4-16-11)12-9(20)8(19)6(3-18)21-12/h2,4,6,8-9,12,18-20H,3H2,(H2,14,15,16)/t6-,8-,9-,12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of DOT1L (unknown origin) using chicken nucleosome as substrate in presence of [3H]SAM incubated for 1 hr by TopCount method |
Bioorg Med Chem Lett 26: 4518-4522 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.041
BindingDB Entry DOI: 10.7270/Q25M697H |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase SETD7
(Homo sapiens (Human)) | BDBM50009672

(AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O Show InChI InChI=1S/C14H20N6O5S/c15-6(14(23)24)1-2-26-3-7-9(21)10(22)13(25-7)20-5-19-8-11(16)17-4-18-12(8)20/h4-7,9-10,13,21-22H,1-3,15H2,(H,23,24)(H2,16,17,18)/t6-,7+,9+,10+,13+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of SETD7 (unknown origin) |
Bioorg Med Chem Lett 26: 4518-4522 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.041
BindingDB Entry DOI: 10.7270/Q25M697H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50009672

(AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O Show InChI InChI=1S/C14H20N6O5S/c15-6(14(23)24)1-2-26-3-7-9(21)10(22)13(25-7)20-5-19-8-11(16)17-4-18-12(8)20/h4-7,9-10,13,21-22H,1-3,15H2,(H,23,24)(H2,16,17,18)/t6-,7+,9+,10+,13+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 (unknown origin) |
Bioorg Med Chem Lett 26: 4518-4522 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.041
BindingDB Entry DOI: 10.7270/Q25M697H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50536666

(CHEMBL4588797)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cc(C#N)c2c(N)ncnc12)C(O)=O |r| Show InChI InChI=1S/C16H20N6O5S/c17-3-7-4-22(14-10(7)13(19)20-6-21-14)15-12(24)11(23)9(27-15)5-28-2-1-8(18)16(25)26/h4,6,8-9,11-12,15,23-24H,1-2,5,18H2,(H,25,26)(H2,19,20,21)/t8-,9+,11+,12+,15+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 9.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of G9a (unknown origin) |
Bioorg Med Chem Lett 26: 4518-4522 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.041
BindingDB Entry DOI: 10.7270/Q25M697H |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50536664
(CHEMBL4553185)Show SMILES Nc1ncnc2n([C@@H]3O[C@H](CO)[C@@H](O)[C@H]3O)c(Br)c(C#N)c12 |r| Show InChI InChI=1S/C12H12BrN5O4/c13-9-4(1-14)6-10(15)16-3-17-11(6)18(9)12-8(21)7(20)5(2-19)22-12/h3,5,7-8,12,19-21H,2H2,(H2,15,16,17)/t5-,7-,8-,12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of DOT1L (unknown origin) using chicken nucleosome as substrate in presence of [3H]SAM incubated for 1 hr by TopCount method |
Bioorg Med Chem Lett 26: 4518-4522 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.041
BindingDB Entry DOI: 10.7270/Q25M697H |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50536665
(CHEMBL4587788)Show SMILES Nc1ncnc2n(cc(F)c12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H13FN4O4/c12-4-1-16(10-6(4)9(13)14-3-15-10)11-8(19)7(18)5(2-17)20-11/h1,3,5,7-8,11,17-19H,2H2,(H2,13,14,15)/t5-,7-,8-,11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of DOT1L (unknown origin) using chicken nucleosome as substrate in presence of [3H]SAM incubated for 1 hr by TopCount method |
Bioorg Med Chem Lett 26: 4518-4522 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.041
BindingDB Entry DOI: 10.7270/Q25M697H |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data