

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nociceptin receptor (Homo sapiens (Human)) | BDBM29988 (benzimidazole analogue, 7h | benzimidazole derivat...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.810 | n/a | 1.30 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM30012 (benzimidazole analogue, 7k) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | 1.10 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM30017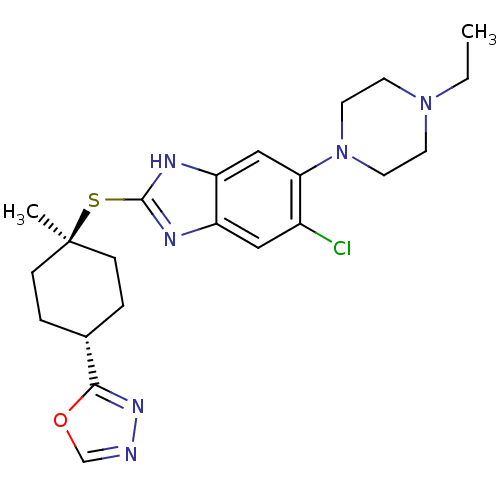 (benzimidazole analogue, 7p) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | 0.590 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM30011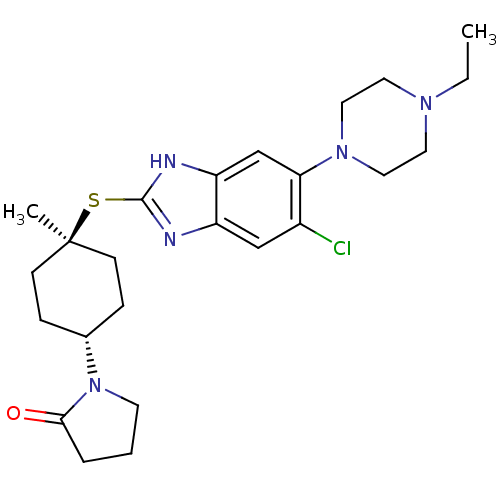 (benzimidazole analogue, 7j) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | 0.550 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM30016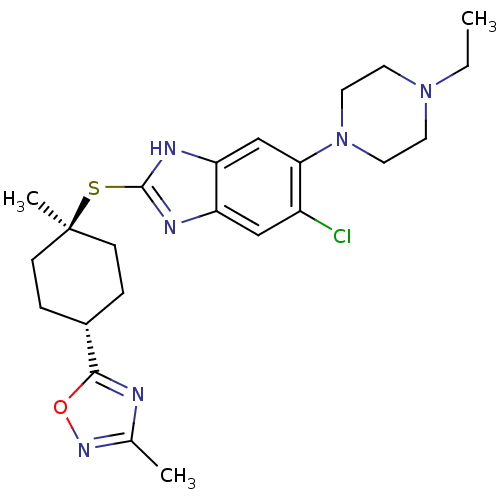 (benzimidazole analogue, 7o) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | 0.910 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM30018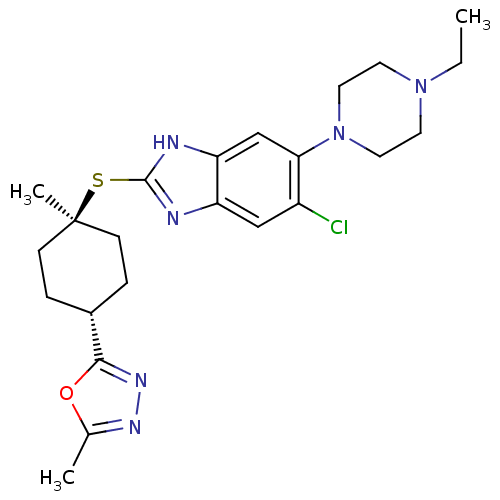 (benzimidazole analogue, 7q) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | 7.10 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM30008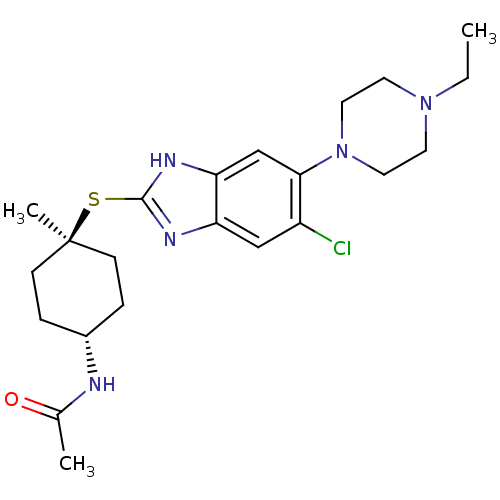 (benzimidazole analogue, 7g) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | 1.10 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM30010 (benzimidazole analogue, 7i) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | 1.80 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM30014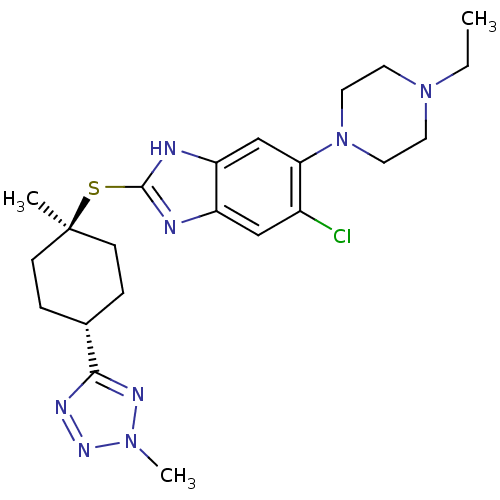 (benzimidazole analogue, 7m) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | 0.900 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM29987 (benzimidazole analogue, 7e | benzimidazole derivat...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | 0.720 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM29995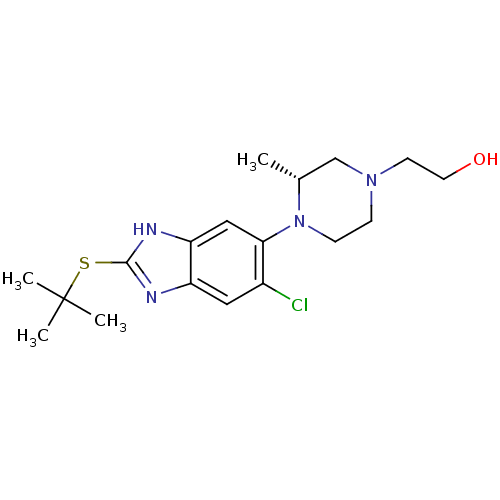 (CHEMBL494350 | benzimidazole-based antagonist, 1) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.60 | n/a | 0.650 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM30004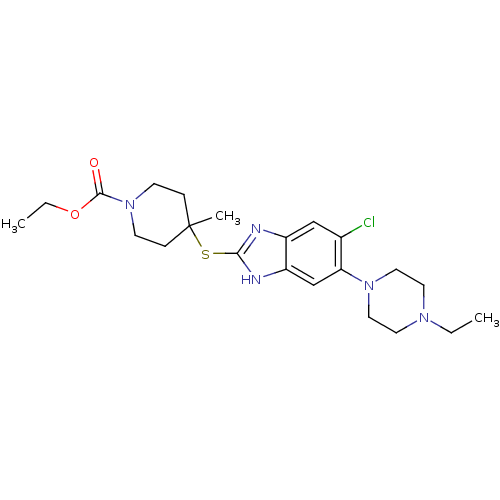 (benzimidazole analogue, 7c) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30 | n/a | 1.10 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM30013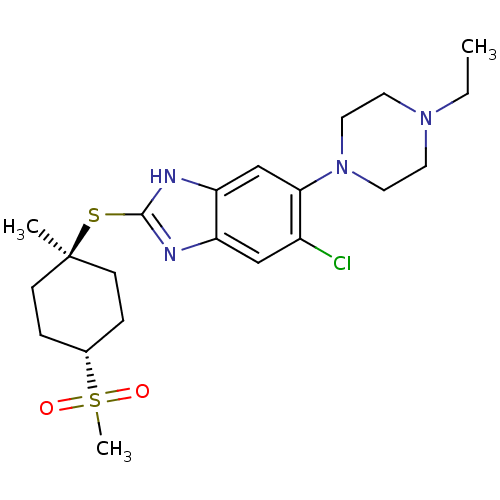 (benzimidazole analogue, 7l) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.90 | n/a | 4 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM30015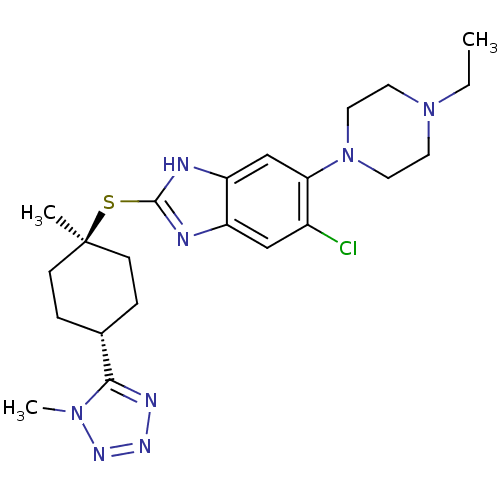 (benzimidazole analogue, 7n) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.40 | n/a | 3.70 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM29996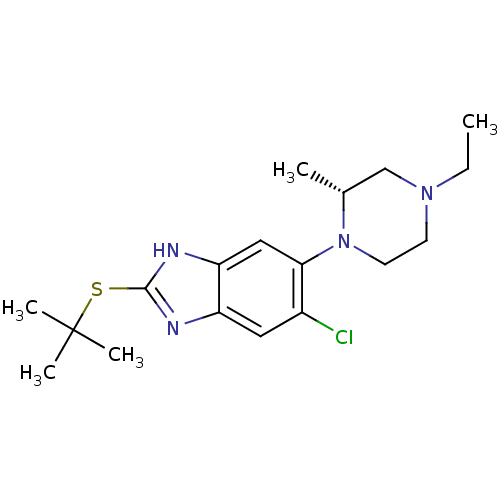 (benzimidazole analogue, 5a) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.20 | n/a | 2.90 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM30005 (benzimidazole analogue, 7d) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.5 | n/a | 2.10 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM30007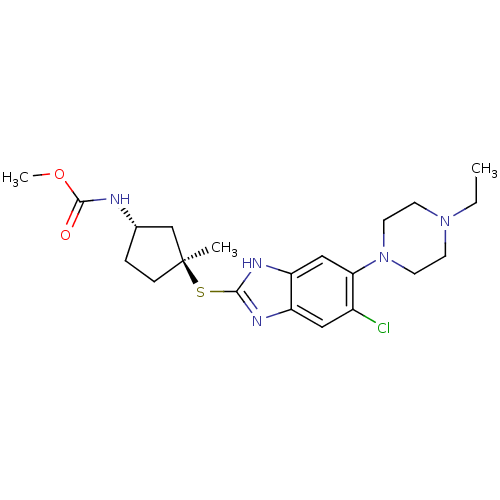 (Racemate | benzimidazole analogue, 7f) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | 46 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM30003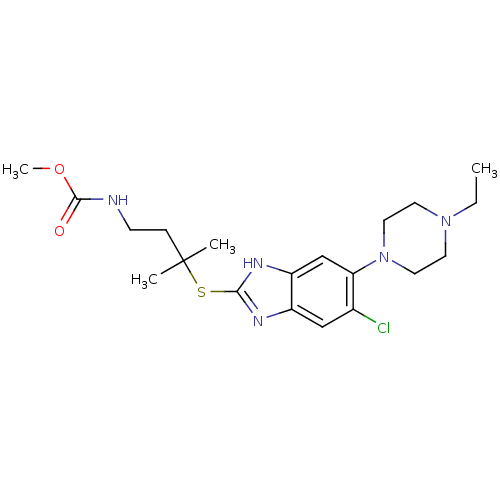 (benzimidazole analogue, 7b) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | 8.60 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM30002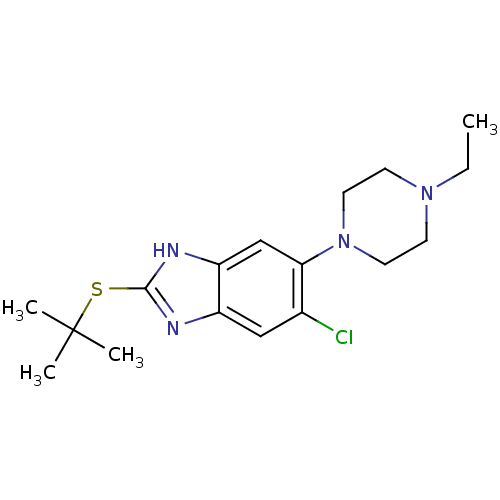 (benzimidazole analogue, 7a) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM29997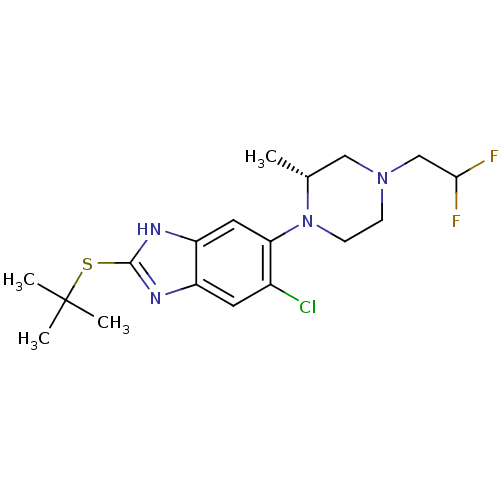 (benzimidazole analogue, 5b) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM30000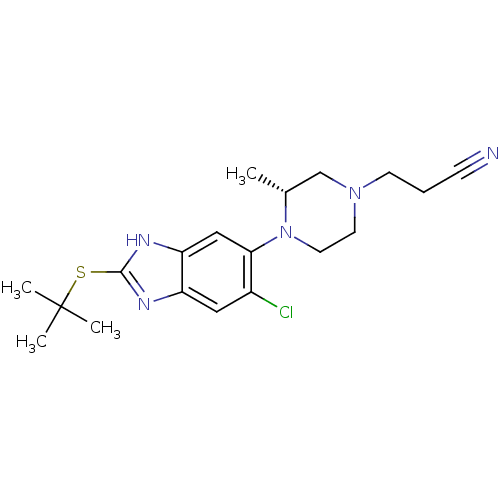 (benzimidazole analogue, 5e) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | 6.40 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM30001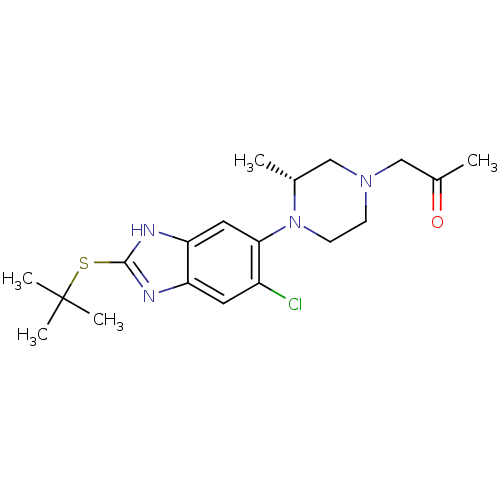 (benzimidazole analogue, 5f) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | 41 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM29999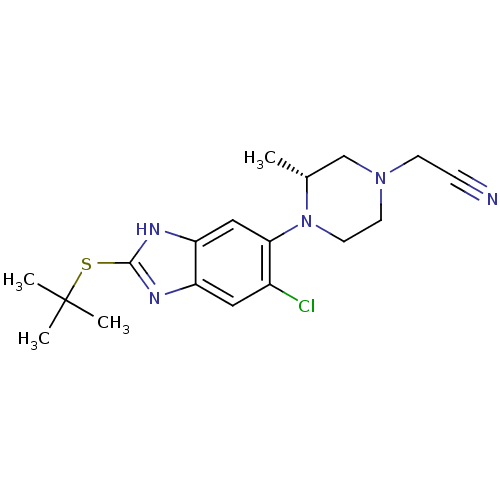 (benzimidazole analogue, 5d) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM30016 (benzimidazole analogue, 7o) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co. | Assay Description Binding affinity to the hERG K+ channel was measured by displacement of [35S]-radiolabeled MK499 in membranes derived from HEK 293 cells stably trans... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM29998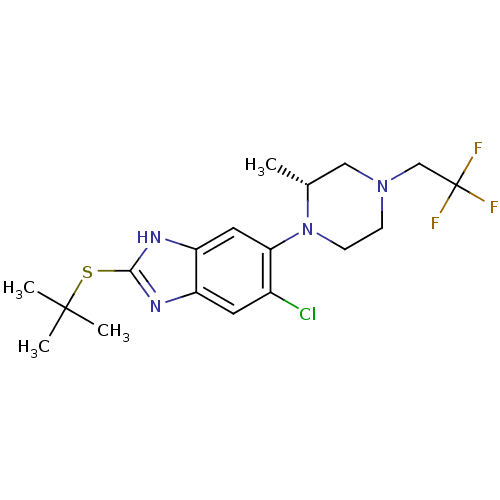 (benzimidazole analogue, 5c) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM29996 (benzimidazole analogue, 5a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 990 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co. | Assay Description Binding affinity to the hERG K+ channel was measured by displacement of [35S]-radiolabeled MK499 in membranes derived from HEK 293 cells stably trans... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM30005 (benzimidazole analogue, 7d) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co. | Assay Description Binding affinity to the hERG K+ channel was measured by displacement of [35S]-radiolabeled MK499 in membranes derived from HEK 293 cells stably trans... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM29995 (CHEMBL494350 | benzimidazole-based antagonist, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co. | Assay Description Binding affinity to the hERG K+ channel was measured by displacement of [35S]-radiolabeled MK499 in membranes derived from HEK 293 cells stably trans... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM30007 (Racemate | benzimidazole analogue, 7f) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co. | Assay Description Binding affinity to the hERG K+ channel was measured by displacement of [35S]-radiolabeled MK499 in membranes derived from HEK 293 cells stably trans... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM30017 (benzimidazole analogue, 7p) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co. | Assay Description Binding affinity to the hERG K+ channel was measured by displacement of [35S]-radiolabeled MK499 in membranes derived from HEK 293 cells stably trans... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM29987 (benzimidazole analogue, 7e | benzimidazole derivat...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co. | Assay Description Binding affinity to the hERG K+ channel was measured by displacement of [35S]-radiolabeled MK499 in membranes derived from HEK 293 cells stably trans... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM30004 (benzimidazole analogue, 7c) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co. | Assay Description Binding affinity to the hERG K+ channel was measured by displacement of [35S]-radiolabeled MK499 in membranes derived from HEK 293 cells stably trans... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM30014 (benzimidazole analogue, 7m) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co. | Assay Description Binding affinity to the hERG K+ channel was measured by displacement of [35S]-radiolabeled MK499 in membranes derived from HEK 293 cells stably trans... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM30015 (benzimidazole analogue, 7n) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co. | Assay Description Binding affinity to the hERG K+ channel was measured by displacement of [35S]-radiolabeled MK499 in membranes derived from HEK 293 cells stably trans... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM29988 (benzimidazole analogue, 7h | benzimidazole derivat...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co. | Assay Description Binding affinity to the hERG K+ channel was measured by displacement of [35S]-radiolabeled MK499 in membranes derived from HEK 293 cells stably trans... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM30010 (benzimidazole analogue, 7i) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co. | Assay Description Binding affinity to the hERG K+ channel was measured by displacement of [35S]-radiolabeled MK499 in membranes derived from HEK 293 cells stably trans... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM30011 (benzimidazole analogue, 7j) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co. | Assay Description Binding affinity to the hERG K+ channel was measured by displacement of [35S]-radiolabeled MK499 in membranes derived from HEK 293 cells stably trans... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM30012 (benzimidazole analogue, 7k) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co. | Assay Description Binding affinity to the hERG K+ channel was measured by displacement of [35S]-radiolabeled MK499 in membranes derived from HEK 293 cells stably trans... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM30001 (benzimidazole analogue, 5f) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co. | Assay Description Binding affinity to the hERG K+ channel was measured by displacement of [35S]-radiolabeled MK499 in membranes derived from HEK 293 cells stably trans... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM29999 (benzimidazole analogue, 5d) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co. | Assay Description Binding affinity to the hERG K+ channel was measured by displacement of [35S]-radiolabeled MK499 in membranes derived from HEK 293 cells stably trans... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM29997 (benzimidazole analogue, 5b) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co. | Assay Description Binding affinity to the hERG K+ channel was measured by displacement of [35S]-radiolabeled MK499 in membranes derived from HEK 293 cells stably trans... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM30018 (benzimidazole analogue, 7q) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co. | Assay Description Binding affinity to the hERG K+ channel was measured by displacement of [35S]-radiolabeled MK499 in membranes derived from HEK 293 cells stably trans... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM30008 (benzimidazole analogue, 7g) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co. | Assay Description Binding affinity to the hERG K+ channel was measured by displacement of [35S]-radiolabeled MK499 in membranes derived from HEK 293 cells stably trans... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM30000 (benzimidazole analogue, 5e) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co. | Assay Description Binding affinity to the hERG K+ channel was measured by displacement of [35S]-radiolabeled MK499 in membranes derived from HEK 293 cells stably trans... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM30013 (benzimidazole analogue, 7l) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co. | Assay Description Binding affinity to the hERG K+ channel was measured by displacement of [35S]-radiolabeled MK499 in membranes derived from HEK 293 cells stably trans... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||