

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase Yes (Homo sapiens (Human)) | BDBM50086441 (CHEMBL3426225 | US10266537, Compound 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src [1-530] (Homo sapiens (Human)) | BDBM50086441 (CHEMBL3426225 | US10266537, Compound 3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Breakpoint cluster region protein [1-693] (Homo sapiens (Human)) | BDBM50086441 (CHEMBL3426225 | US10266537, Compound 3) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Yes (Homo sapiens (Human)) | BDBM378885 (US10266537, Compound 93) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50086441 (CHEMBL3426225 | US10266537, Compound 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Yes (Homo sapiens (Human)) | BDBM378888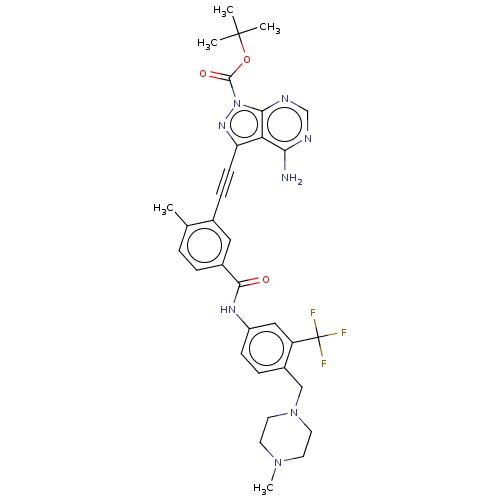 (Preparation of 3-(4-amino-1-(piperidin-4-yl)-1H-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Yes (Homo sapiens (Human)) | BDBM378887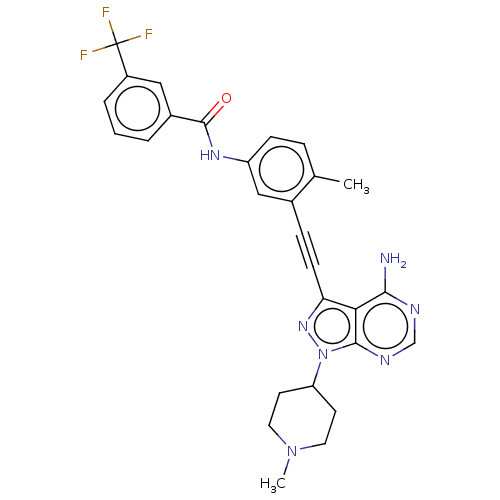 (US10266537, Compound 31) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src [1-530] (Homo sapiens (Human)) | BDBM378890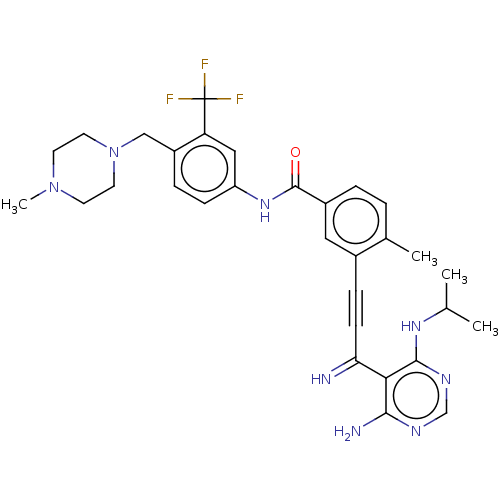 (US10266537, Compound 5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Yes (Homo sapiens (Human)) | BDBM50086442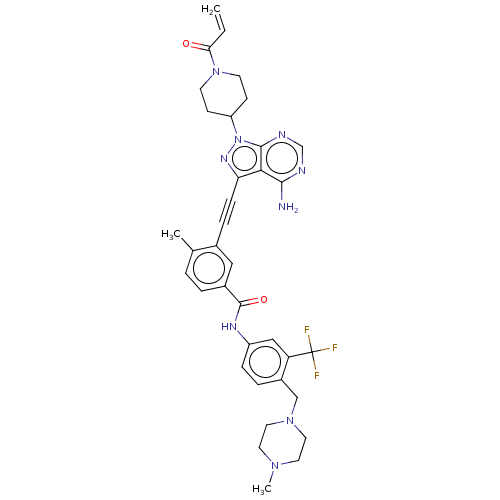 (CHEMBL3426233 | US10266537, Compound 121) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM378885 (US10266537, Compound 93) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src [1-530] (Homo sapiens (Human)) | BDBM378887 (US10266537, Compound 31) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [T315I] (Homo sapiens (Human)) | BDBM378885 (US10266537, Compound 93) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fyn (Homo sapiens (Human)) | BDBM378887 (US10266537, Compound 31) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src [1-530] (Homo sapiens (Human)) | BDBM50086453 (CHEMBL3426220 | US10266537, Compound 14) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM50086441 (CHEMBL3426225 | US10266537, Compound 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src [1-530] (Homo sapiens (Human)) | BDBM378885 (US10266537, Compound 93) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM378885 (US10266537, Compound 93) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src [1-530] (Homo sapiens (Human)) | BDBM378902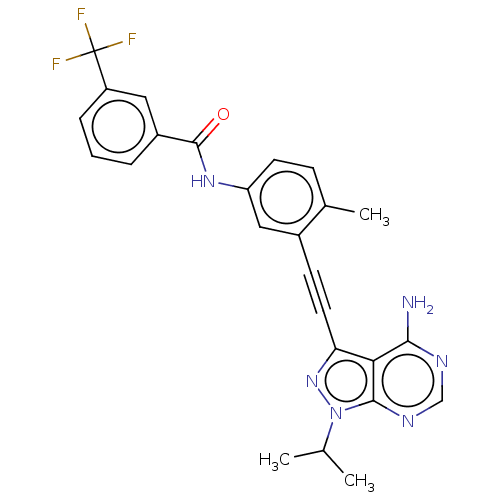 (US10266537, Compound 18) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src [1-530] (Homo sapiens (Human)) | BDBM50086456 (CHEMBL3426218 | US10266537, Compound 17) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src [1-530] (Homo sapiens (Human)) | BDBM378889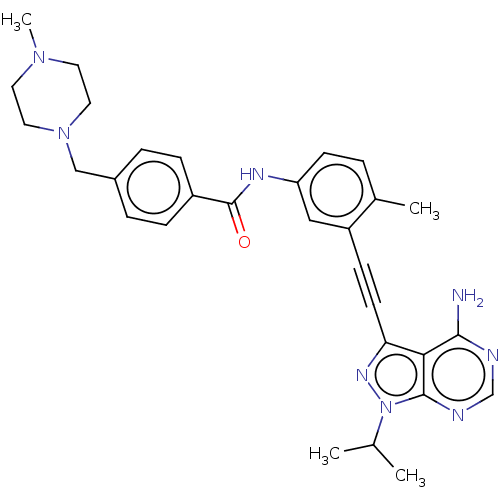 (Preparation of N-(3-((4-amino-1-isopropyl-1H-pyraz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM378885 (US10266537, Compound 93) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL2 (Homo sapiens (Human)) | BDBM378885 (US10266537, Compound 93) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lyn (Homo sapiens (Human)) | BDBM378885 (US10266537, Compound 93) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epithelial discoidin domain-containing receptor 1 (Homo sapiens (Human)) | BDBM378887 (US10266537, Compound 31) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src [1-530] (Homo sapiens (Human)) | BDBM50086586 (CHEMBL3426234 | US10266537, Compound 29) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fyn (Homo sapiens (Human)) | BDBM50086441 (CHEMBL3426225 | US10266537, Compound 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM378887 (US10266537, Compound 31) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytoplasmic tyrosine-protein kinase BMX (Homo sapiens (Human)) | BDBM50086441 (CHEMBL3426225 | US10266537, Compound 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fyn (Homo sapiens (Human)) | BDBM378885 (US10266537, Compound 93) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM378885 (US10266537, Compound 93) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src [1-530] (Homo sapiens (Human)) | BDBM50086449 (CHEMBL3426224 | US10266537, Compound 8) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src [1-530] (Homo sapiens (Human)) | BDBM50086444 (CHEMBL3426230 | US10266537, Compound 20) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src [1-530] (Homo sapiens (Human)) | BDBM50086445 (CHEMBL3426229 | US10266537, Compound 21) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src [1-530] (Homo sapiens (Human)) | BDBM378900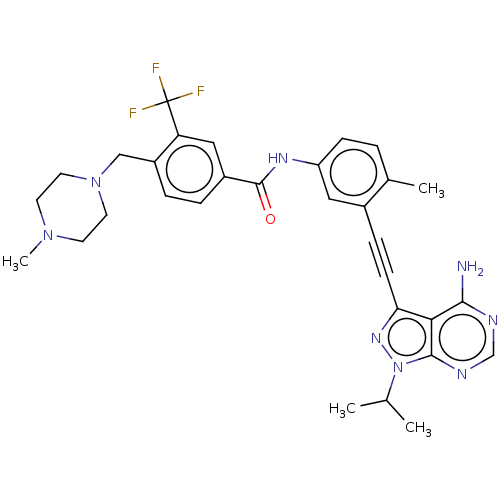 (US10266537, Compound 16) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [T790M,L858R] (Homo sapiens (Human)) | BDBM378885 (US10266537, Compound 93) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase (Candida dubliniensis CD36) | BDBM378885 (US10266537, Compound 93) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src [1-530] (Homo sapiens (Human)) | BDBM50086446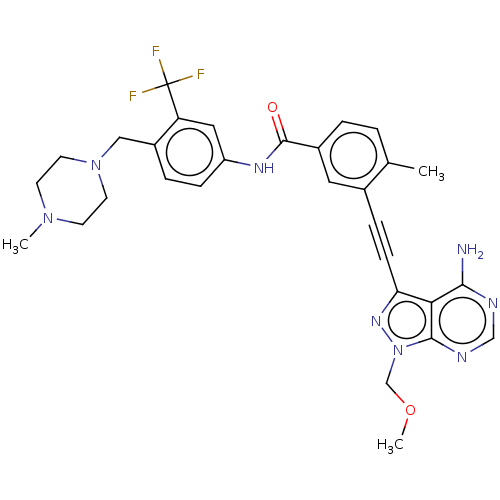 (CHEMBL3426228 | US10266537, Compound 19) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epithelial discoidin domain-containing receptor 1 (Homo sapiens (Human)) | BDBM50086441 (CHEMBL3426225 | US10266537, Compound 3) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src [1-530] (Homo sapiens (Human)) | BDBM50086448 (CHEMBL3426226 | US10266537, Compound 6) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase CSK (Homo sapiens (Human)) | BDBM378885 (US10266537, Compound 93) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src [1-530] (Homo sapiens (Human)) | BDBM378896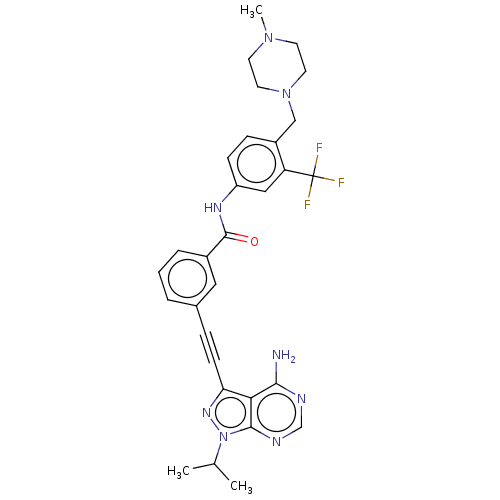 (US10266537, Compound 11) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiopoietin-1 receptor (Homo sapiens (Human)) | BDBM378885 (US10266537, Compound 93) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 2 (Homo sapiens (Human)) | BDBM378887 (US10266537, Compound 31) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src [1-530] (Homo sapiens (Human)) | BDBM378898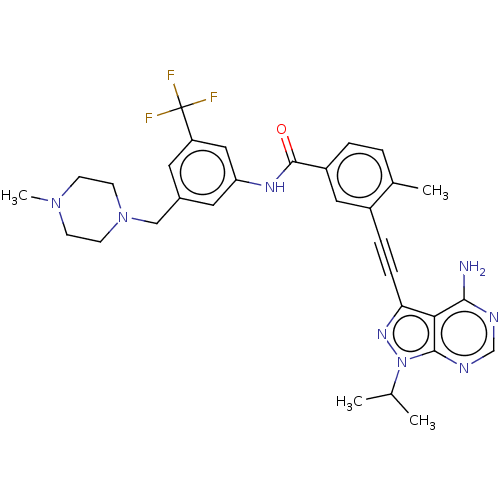 (US10266537, Compound 13) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 4 (Homo sapiens (Human)) | BDBM378887 (US10266537, Compound 31) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytoplasmic tyrosine-protein kinase BMX (Homo sapiens (Human)) | BDBM378888 (Preparation of 3-(4-amino-1-(piperidin-4-yl)-1H-py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src [1-530] (Homo sapiens (Human)) | BDBM50086585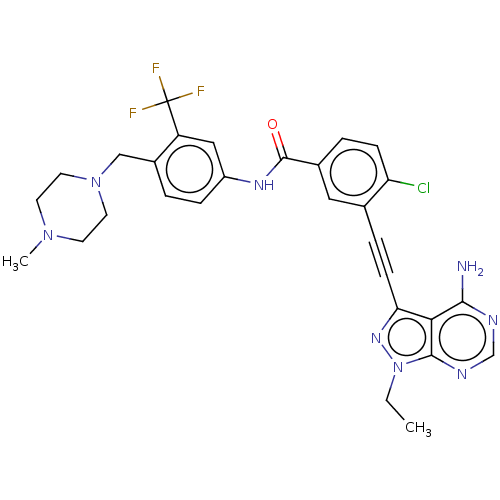 (CHEMBL3426235 | US10266537, Compound 26) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src [1-530] (Homo sapiens (Human)) | BDBM378888 (Preparation of 3-(4-amino-1-(piperidin-4-yl)-1H-py...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Blk (Homo sapiens (Human)) | BDBM378887 (US10266537, Compound 31) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM378887 (US10266537, Compound 31) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 116 total ) | Next | Last >> |