

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21010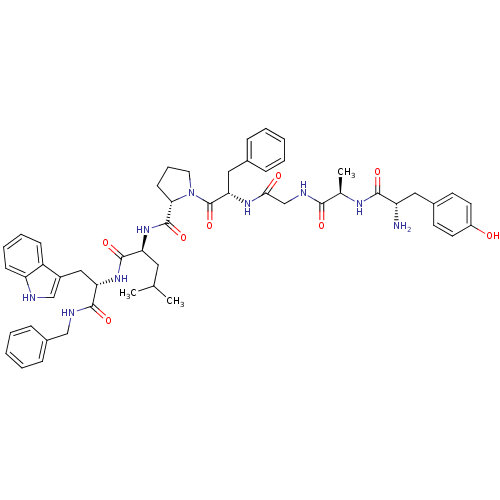 ((2S)-2-{[(2S)-1-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.650 | -52.4 | n/a | n/a | 0.710 | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 51: 1369-76 (2008) Article DOI: 10.1021/jm070332f BindingDB Entry DOI: 10.7270/Q2X63K7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21014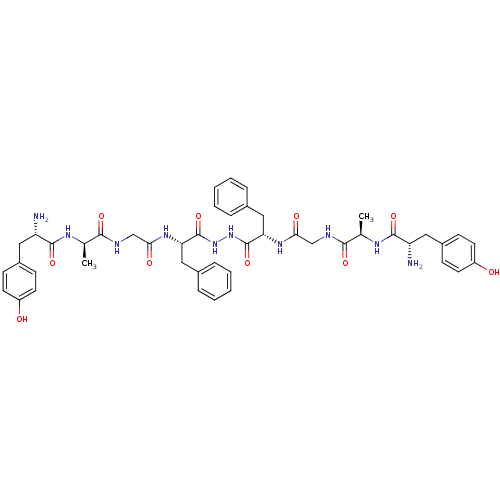 ((2S)-2-amino-N-[(1R)-1-[({[(1S)-1-{N'-[(2S)-2-{2-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.60 | -49.0 | n/a | n/a | 1.10 | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 51: 1369-76 (2008) Article DOI: 10.1021/jm070332f BindingDB Entry DOI: 10.7270/Q2X63K7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21013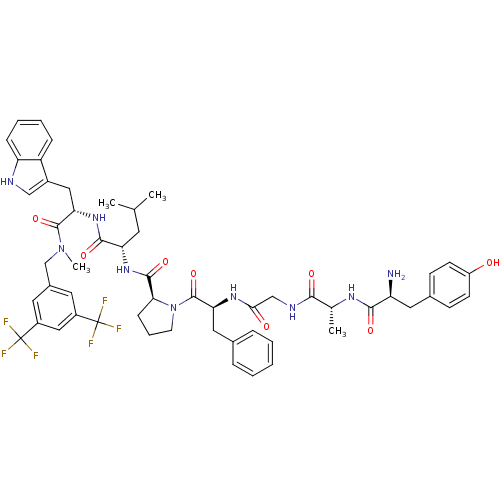 ((2S)-2-{[(2S)-1-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.80 | -46.6 | n/a | n/a | 72 | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 51: 1369-76 (2008) Article DOI: 10.1021/jm070332f BindingDB Entry DOI: 10.7270/Q2X63K7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21012 ((2S)-2-{[(2S)-1-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9.5 | -45.8 | n/a | n/a | 57 | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 51: 1369-76 (2008) Article DOI: 10.1021/jm070332f BindingDB Entry DOI: 10.7270/Q2X63K7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21010 ((2S)-2-{[(2S)-1-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 10 | -45.7 | n/a | n/a | 17 | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 51: 1369-76 (2008) Article DOI: 10.1021/jm070332f BindingDB Entry DOI: 10.7270/Q2X63K7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21009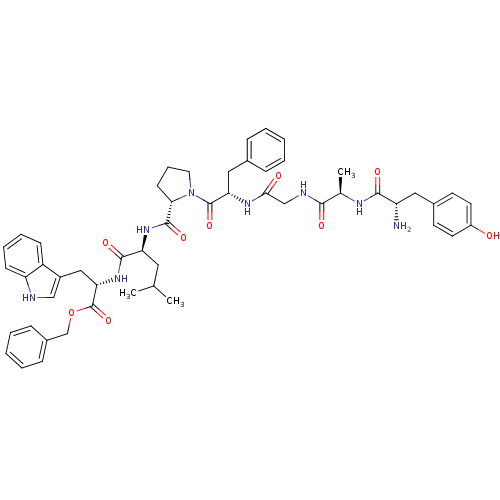 (C-terminal modified bifunctional peptide, 2 | H-Ty...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 29 | -43.0 | n/a | n/a | 36 | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 51: 1369-76 (2008) Article DOI: 10.1021/jm070332f BindingDB Entry DOI: 10.7270/Q2X63K7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21013 ((2S)-2-{[(2S)-1-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 31 | -42.9 | n/a | n/a | 120 | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 51: 1369-76 (2008) Article DOI: 10.1021/jm070332f BindingDB Entry DOI: 10.7270/Q2X63K7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21009 (C-terminal modified bifunctional peptide, 2 | H-Ty...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 31 | -42.9 | n/a | n/a | 85 | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 51: 1369-76 (2008) Article DOI: 10.1021/jm070332f BindingDB Entry DOI: 10.7270/Q2X63K7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21011 ((2S)-2-{[(2S)-1-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 46 | -41.9 | n/a | n/a | 29 | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 51: 1369-76 (2008) Article DOI: 10.1021/jm070332f BindingDB Entry DOI: 10.7270/Q2X63K7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21007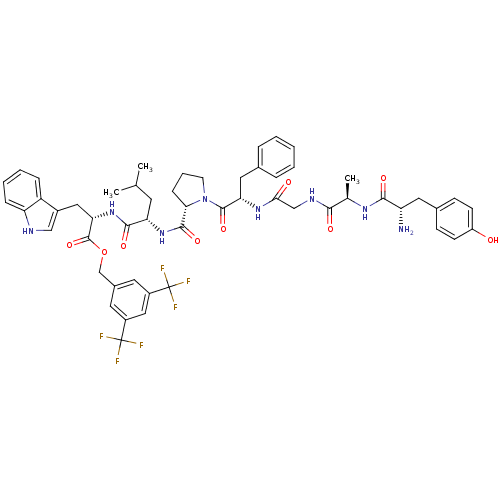 (C-terminal modified bifunctional peptide, 1 | H-Ty...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 50 | -41.7 | n/a | n/a | 35 | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 51: 1369-76 (2008) Article DOI: 10.1021/jm070332f BindingDB Entry DOI: 10.7270/Q2X63K7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21012 ((2S)-2-{[(2S)-1-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 72 | -40.8 | n/a | n/a | 80 | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 51: 1369-76 (2008) Article DOI: 10.1021/jm070332f BindingDB Entry DOI: 10.7270/Q2X63K7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21011 ((2S)-2-{[(2S)-1-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 77 | -40.6 | n/a | n/a | 150 | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 51: 1369-76 (2008) Article DOI: 10.1021/jm070332f BindingDB Entry DOI: 10.7270/Q2X63K7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21007 (C-terminal modified bifunctional peptide, 1 | H-Ty...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 180 | -38.5 | n/a | n/a | 140 | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 51: 1369-76 (2008) Article DOI: 10.1021/jm070332f BindingDB Entry DOI: 10.7270/Q2X63K7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21015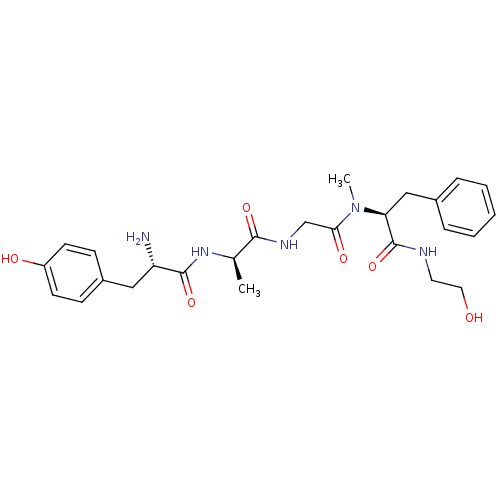 ((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 0.5 | 37 | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 51: 1369-76 (2008) Article DOI: 10.1021/jm070332f BindingDB Entry DOI: 10.7270/Q2X63K7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21008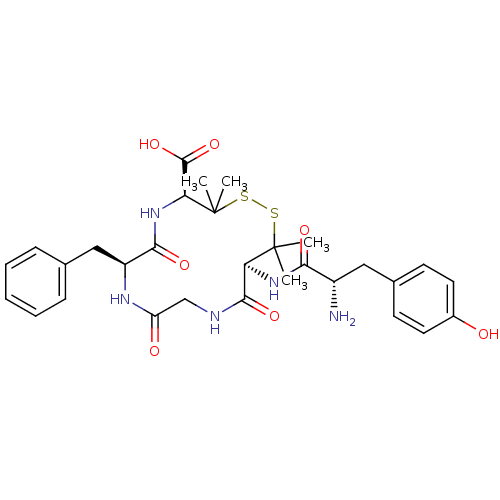 ((4S,7S,13S)-13-[(2S)-2-amino-3-(4-hydroxyphenyl)pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 0.450 | 1.60 | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 51: 1369-76 (2008) Article DOI: 10.1021/jm070332f BindingDB Entry DOI: 10.7270/Q2X63K7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||