

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Botulinum neurotoxin type A2 [1-425] (Clostridium botulinum) | BDBM23299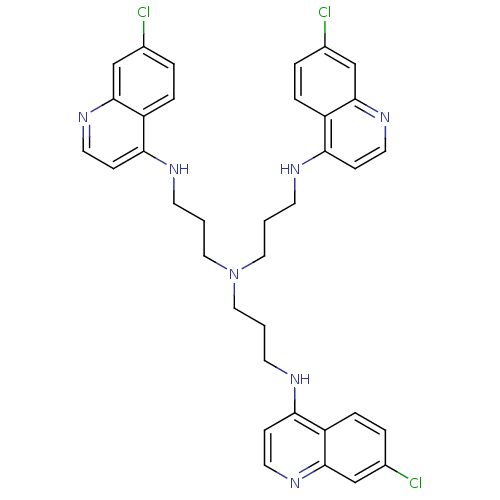 (4-amino-7-chloroquinoline (ACQ)-based compound, 4 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | 7.3 | 22 |
National Cancer Institute at Frederick | Assay Description Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA... | J Med Chem 50: 2127-36 (2007) Article DOI: 10.1021/jm061446e BindingDB Entry DOI: 10.7270/Q2VQ3105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A2 [1-425] (Clostridium botulinum) | BDBM23298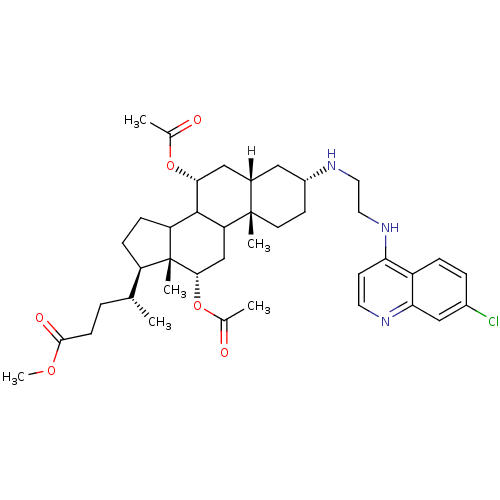 (4-amino-7-chloroquinoline (ACQ)-based compound, 3 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | 7.3 | 22 |
National Cancer Institute at Frederick | Assay Description Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA... | J Med Chem 50: 2127-36 (2007) Article DOI: 10.1021/jm061446e BindingDB Entry DOI: 10.7270/Q2VQ3105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A2 [1-425] (Clostridium botulinum) | BDBM23296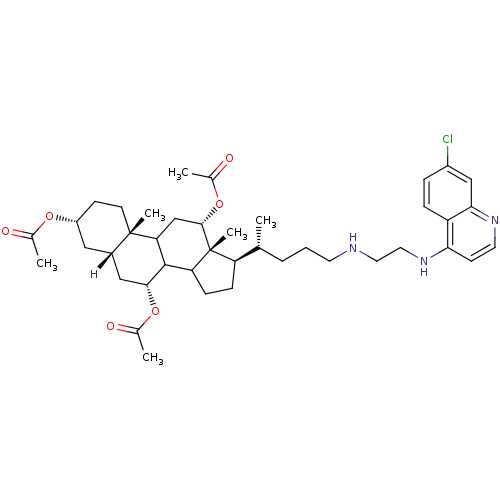 ((2S,5R,7S,9R,14R,15R,16S)-14-[(1R)-4-({2-[(7-chlor...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | 7.3 | 22 |
National Cancer Institute at Frederick | Assay Description Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA... | J Med Chem 50: 2127-36 (2007) Article DOI: 10.1021/jm061446e BindingDB Entry DOI: 10.7270/Q2VQ3105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A2 [1-425] (Clostridium botulinum) | BDBM23297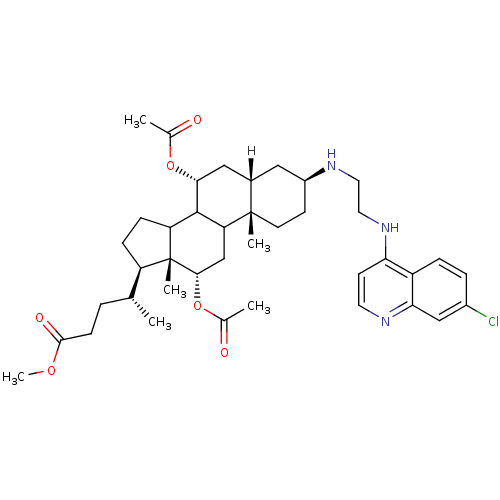 (4-amino-7-chloroquinoline (ACQ)-based compound, 2 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | 7.3 | 22 |
National Cancer Institute at Frederick | Assay Description Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA... | J Med Chem 50: 2127-36 (2007) Article DOI: 10.1021/jm061446e BindingDB Entry DOI: 10.7270/Q2VQ3105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A2 [1-425] (Clostridium botulinum) | BDBM23304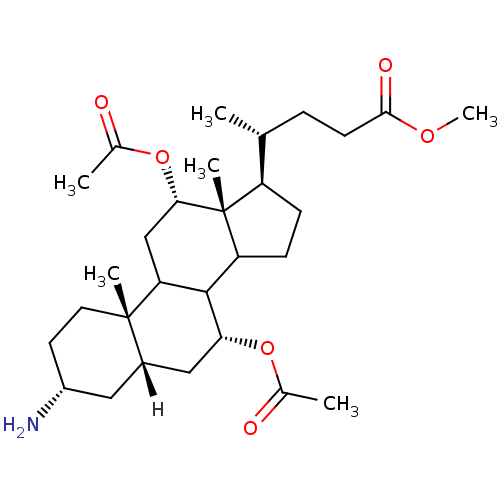 (bis(acetyloxy)-3-aminocholan-24-oate | bis(acetylo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.3 | 22 |
National Cancer Institute at Frederick | Assay Description Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA... | J Med Chem 50: 2127-36 (2007) Article DOI: 10.1021/jm061446e BindingDB Entry DOI: 10.7270/Q2VQ3105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A2 [1-425] (Clostridium botulinum) | BDBM23306 (4-amino-7-chloroquinoline (ACQ)-based compound, 11...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.3 | 22 |
National Cancer Institute at Frederick | Assay Description Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA... | J Med Chem 50: 2127-36 (2007) Article DOI: 10.1021/jm061446e BindingDB Entry DOI: 10.7270/Q2VQ3105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A2 [1-425] (Clostridium botulinum) | BDBM23303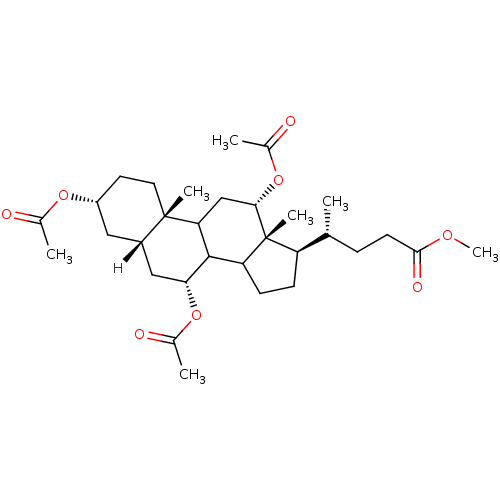 (methyl (4R)-4-[(2S,5R,7S,9R,14R,15R,16S)-5,9,16-tr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.3 | 22 |
National Cancer Institute at Frederick | Assay Description Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA... | J Med Chem 50: 2127-36 (2007) Article DOI: 10.1021/jm061446e BindingDB Entry DOI: 10.7270/Q2VQ3105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A2 [1-425] (Clostridium botulinum) | BDBM23302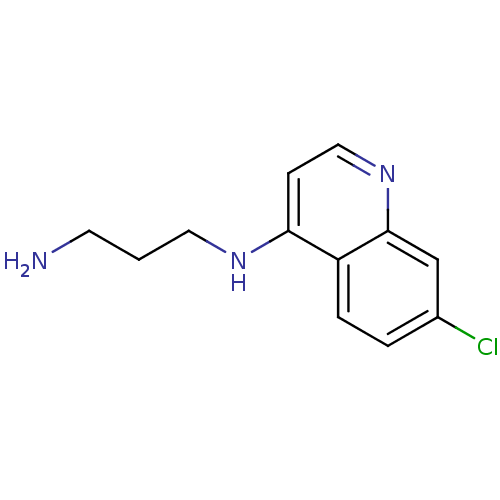 (4-amino-7-chloroquinoline (ACQ)-based compound, 7 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.3 | 22 |
National Cancer Institute at Frederick | Assay Description Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA... | J Med Chem 50: 2127-36 (2007) Article DOI: 10.1021/jm061446e BindingDB Entry DOI: 10.7270/Q2VQ3105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A2 [1-425] (Clostridium botulinum) | BDBM23301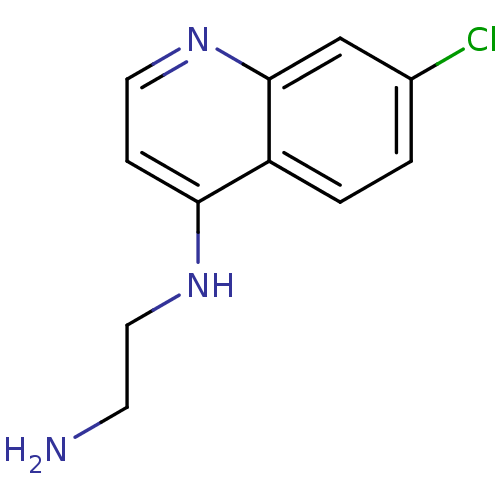 (4-amino-7-chloroquinoline (ACQ)-based compound, 6 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.3 | 22 |
National Cancer Institute at Frederick | Assay Description Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA... | J Med Chem 50: 2127-36 (2007) Article DOI: 10.1021/jm061446e BindingDB Entry DOI: 10.7270/Q2VQ3105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A2 [1-425] (Clostridium botulinum) | BDBM23300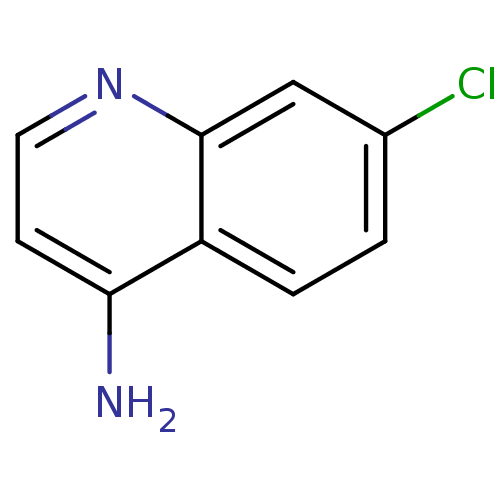 (4-amino-7-chloroquinoline (ACQ)-based compound, 5 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.3 | 22 |
National Cancer Institute at Frederick | Assay Description Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA... | J Med Chem 50: 2127-36 (2007) Article DOI: 10.1021/jm061446e BindingDB Entry DOI: 10.7270/Q2VQ3105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A2 [1-425] (Clostridium botulinum) | BDBM23305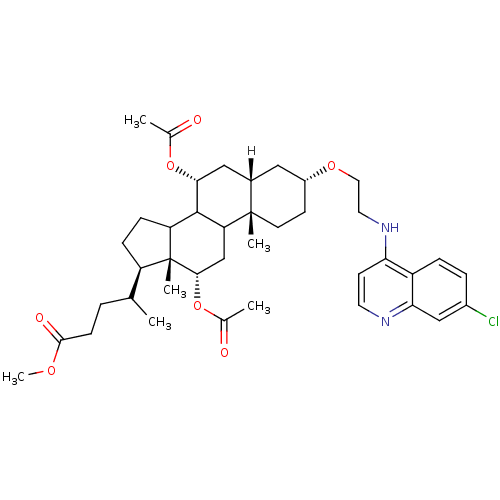 (4-amino-7-chloroquinoline (ACQ)-based compound, 10...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.3 | 22 |
National Cancer Institute at Frederick | Assay Description Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA... | J Med Chem 50: 2127-36 (2007) Article DOI: 10.1021/jm061446e BindingDB Entry DOI: 10.7270/Q2VQ3105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||