

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036480 (2,6-Dichloro-3-(2-morpholin-4-yl-ethoxy)-benzoic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Inc. Curated by ChEMBL | Assay Description Potency of inhibition against human leukocyte elastase (HLE) expressed as an apparent binding constant | J Med Chem 38: 739-44 (1995) BindingDB Entry DOI: 10.7270/Q2W66JTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036476 (2,6-Dichloro-3-(4-methyl-piperazine-1-sulfonyl)-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Inc. Curated by ChEMBL | Assay Description Potency of inhibition against human leukocyte elastase (HLE) expressed as an apparent binding constant | J Med Chem 38: 739-44 (1995) BindingDB Entry DOI: 10.7270/Q2W66JTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036478 (3-Carboxymethoxy-2,6-dichloro-benzoic acid 4-isopr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Inc. Curated by ChEMBL | Assay Description Potency of inhibition against human leukocyte elastase (HLE) expressed as an apparent binding constant | J Med Chem 38: 739-44 (1995) BindingDB Entry DOI: 10.7270/Q2W66JTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036477 (2,6-Dichloro-3-(2-morpholin-4-yl-ethoxy)-benzoic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Inc. Curated by ChEMBL | Assay Description Potency of inhibition against human leukocyte elastase (HLE) expressed as an apparent binding constant | J Med Chem 38: 739-44 (1995) BindingDB Entry DOI: 10.7270/Q2W66JTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036481 (2,6-Dichloro-3-[(2-dimethylamino-ethyl)-methyl-sul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Inc. Curated by ChEMBL | Assay Description Potency of inhibition against human leukocyte elastase (HLE) expressed as an apparent binding constant | J Med Chem 38: 739-44 (1995) BindingDB Entry DOI: 10.7270/Q2W66JTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036475 (2,6-Dichloro-3-(2-pyrrolidin-1-yl-ethoxy)-benzoic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Inc. Curated by ChEMBL | Assay Description Potency of inhibition against human leukocyte elastase (HLE) expressed as an apparent binding constant | J Med Chem 38: 739-44 (1995) BindingDB Entry DOI: 10.7270/Q2W66JTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036482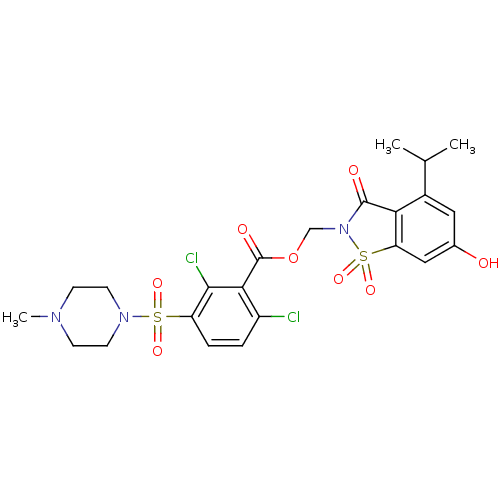 (2,6-Dichloro-3-(4-methyl-piperazine-1-sulfonyl)-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Inc. Curated by ChEMBL | Assay Description Potency of inhibition against human leukocyte elastase (HLE) expressed as an apparent binding constant | J Med Chem 38: 739-44 (1995) BindingDB Entry DOI: 10.7270/Q2W66JTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036479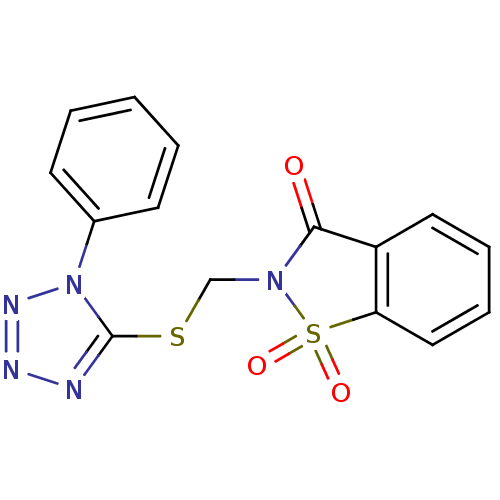 (1,1-Dioxo-2-(1-phenyl-1H-tetrazol-5-ylsulfanylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Inc. Curated by ChEMBL | Assay Description Potency of inhibition against human leukocyte elastase (HLE) expressed as an apparent binding constant | J Med Chem 38: 739-44 (1995) BindingDB Entry DOI: 10.7270/Q2W66JTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||