

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 2E1 (Rattus norvegicus (rat)) | BDBM31652 (imidazole-dioxolane, 3) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Queen's University | Assay Description CYP2E1 hydroxylation of p-nitrophenol was determined by the spectrophotometric measurement of 4-nitrocatechol. Briefly, reaction mixture consisting o... | Bioorg Med Chem 17: 2461-75 (2009) Article DOI: 10.1016/j.bmc.2009.01.078 BindingDB Entry DOI: 10.7270/Q2WQ0250 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Rattus norvegicus (rat)) | BDBM31656 (imidazole-dioxolane, 8) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Queen's University | Assay Description CYP2E1 hydroxylation of p-nitrophenol was determined by the spectrophotometric measurement of 4-nitrocatechol. Briefly, reaction mixture consisting o... | Bioorg Med Chem 17: 2461-75 (2009) Article DOI: 10.1016/j.bmc.2009.01.078 BindingDB Entry DOI: 10.7270/Q2WQ0250 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Rattus norvegicus (rat)) | BDBM31657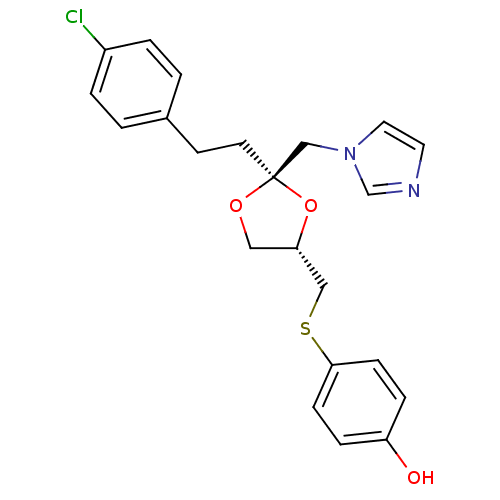 (imidazole-dioxolane, 9) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Queen's University | Assay Description CYP2E1 hydroxylation of p-nitrophenol was determined by the spectrophotometric measurement of 4-nitrocatechol. Briefly, reaction mixture consisting o... | Bioorg Med Chem 17: 2461-75 (2009) Article DOI: 10.1016/j.bmc.2009.01.078 BindingDB Entry DOI: 10.7270/Q2WQ0250 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Rattus norvegicus (rat)) | BDBM31658 (imidazole-dioxolane, 10) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Queen's University | Assay Description CYP2E1 hydroxylation of p-nitrophenol was determined by the spectrophotometric measurement of 4-nitrocatechol. Briefly, reaction mixture consisting o... | Bioorg Med Chem 17: 2461-75 (2009) Article DOI: 10.1016/j.bmc.2009.01.078 BindingDB Entry DOI: 10.7270/Q2WQ0250 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Rattus norvegicus (rat)) | BDBM31682 (imidazole-dioxolane, 32) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Queen's University | Assay Description CYP2E1 hydroxylation of p-nitrophenol was determined by the spectrophotometric measurement of 4-nitrocatechol. Briefly, reaction mixture consisting o... | Bioorg Med Chem 17: 2461-75 (2009) Article DOI: 10.1016/j.bmc.2009.01.078 BindingDB Entry DOI: 10.7270/Q2WQ0250 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Rattus norvegicus (rat)) | BDBM31665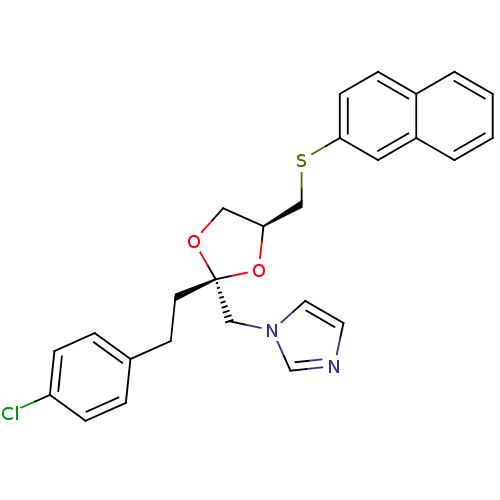 (imidazole-dioxolane, 17) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Queen's University | Assay Description CYP2E1 hydroxylation of p-nitrophenol was determined by the spectrophotometric measurement of 4-nitrocatechol. Briefly, reaction mixture consisting o... | Bioorg Med Chem 17: 2461-75 (2009) Article DOI: 10.1016/j.bmc.2009.01.078 BindingDB Entry DOI: 10.7270/Q2WQ0250 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Rattus norvegicus (rat)) | BDBM31669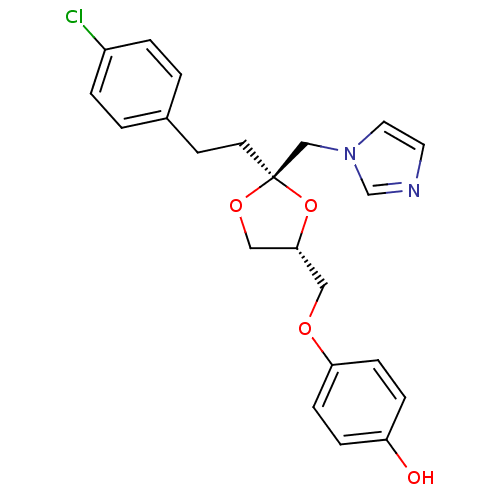 (imidazole-dioxolane, 21) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Queen's University | Assay Description CYP2E1 hydroxylation of p-nitrophenol was determined by the spectrophotometric measurement of 4-nitrocatechol. Briefly, reaction mixture consisting o... | Bioorg Med Chem 17: 2461-75 (2009) Article DOI: 10.1016/j.bmc.2009.01.078 BindingDB Entry DOI: 10.7270/Q2WQ0250 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Rattus norvegicus (rat)) | BDBM31672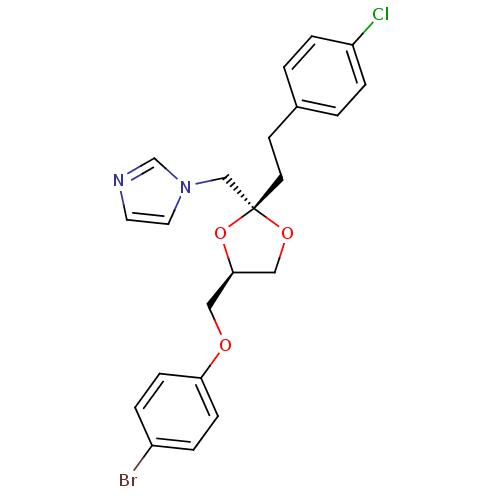 (imidazole-dioxolane, 24) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Queen's University | Assay Description CYP2E1 hydroxylation of p-nitrophenol was determined by the spectrophotometric measurement of 4-nitrocatechol. Briefly, reaction mixture consisting o... | Bioorg Med Chem 17: 2461-75 (2009) Article DOI: 10.1016/j.bmc.2009.01.078 BindingDB Entry DOI: 10.7270/Q2WQ0250 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Rattus norvegicus (rat)) | BDBM31664 (imidazole-dioxolane, 16) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Queen's University | Assay Description CYP2E1 hydroxylation of p-nitrophenol was determined by the spectrophotometric measurement of 4-nitrocatechol. Briefly, reaction mixture consisting o... | Bioorg Med Chem 17: 2461-75 (2009) Article DOI: 10.1016/j.bmc.2009.01.078 BindingDB Entry DOI: 10.7270/Q2WQ0250 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||