 Found 70 hits of ic50 data for polymerid = 1023,50000926,50006986
Found 70 hits of ic50 data for polymerid = 1023,50000926,50006986 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 11B2, mitochondrial
(Rattus norvegicus) | BDBM50444550

(CHEMBL3099704)Show SMILES Fc1ccc(cc1)-c1cc(ccc1[C@H]1CCCCc2cncn12)C#N |r| Show InChI InChI=1S/C21H18FN3/c22-17-8-6-16(7-9-17)20-11-15(12-23)5-10-19(20)21-4-2-1-3-18-13-24-14-25(18)21/h5-11,13-14,21H,1-4H2/t21-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant CYP11B2 using 11-deoxycorticosterone as substrate by cell-based assay |
ACS Med Chem Lett 4: 1203-7 (2013)
Article DOI: 10.1021/ml400324c
BindingDB Entry DOI: 10.7270/Q2NZ8938 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Rattus norvegicus) | BDBM50249047

(CHEMBL4080420)Show InChI InChI=1S/C16H16FN3O/c1-10-15-13(17)5-4-6-14(15)20(19-10)12-7-11(8-18-9-12)16(2,3)21/h4-9,21H,1-3H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat CYP11B2-1F4 expressed in Chinese hamster V79 cells using 11-deoxycorticosterone as substrate preincubated for 1 hr followed by subs... |
Bioorg Med Chem Lett 27: 2384-2388 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.021
BindingDB Entry DOI: 10.7270/Q2MC92F2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Rattus norvegicus) | BDBM50249044
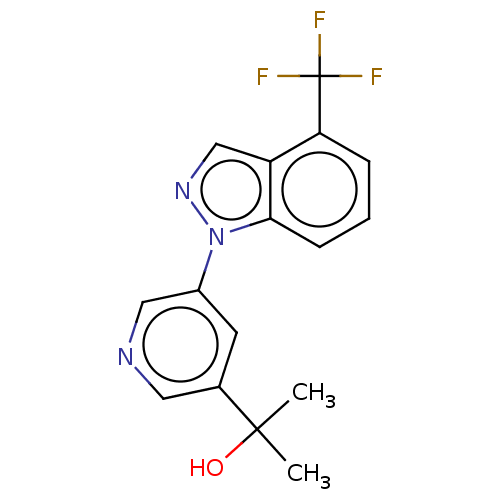
(CHEMBL4067000)Show InChI InChI=1S/C16H14F3N3O/c1-15(2,23)10-6-11(8-20-7-10)22-14-5-3-4-13(16(17,18)19)12(14)9-21-22/h3-9,23H,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat CYP11B2-1F4 expressed in Chinese hamster V79 cells using 11-deoxycorticosterone as substrate preincubated for 1 hr followed by subs... |
Bioorg Med Chem Lett 27: 2384-2388 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.021
BindingDB Entry DOI: 10.7270/Q2MC92F2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Mus musculus) | BDBM50238115
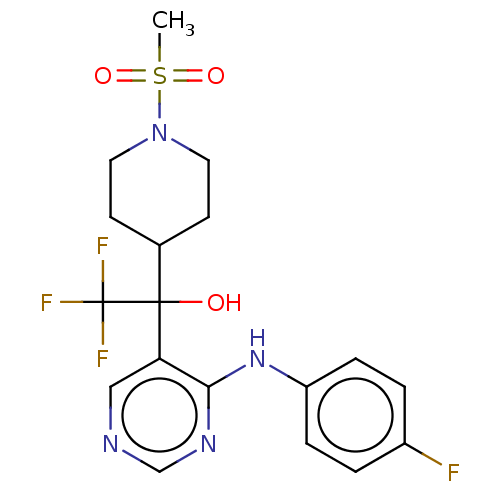
(CHEMBL4082403)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)(c1cncnc1Nc1ccc(F)cc1)C(F)(F)F Show InChI InChI=1S/C18H20F4N4O3S/c1-30(28,29)26-8-6-12(7-9-26)17(27,18(20,21)22)15-10-23-11-24-16(15)25-14-4-2-13(19)3-5-14/h2-5,10-12,27H,6-9H2,1H3,(H,23,24,25) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of mouse CYP11B2 expressed in HEK293 cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition after... |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Mus musculus) | BDBM50238115

(CHEMBL4082403)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)(c1cncnc1Nc1ccc(F)cc1)C(F)(F)F Show InChI InChI=1S/C18H20F4N4O3S/c1-30(28,29)26-8-6-12(7-9-26)17(27,18(20,21)22)15-10-23-11-24-16(15)25-14-4-2-13(19)3-5-14/h2-5,10-12,27H,6-9H2,1H3,(H,23,24,25) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of mouse CYP11B2 expressed in HEK293 cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition after... |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Rattus norvegicus) | BDBM50249045
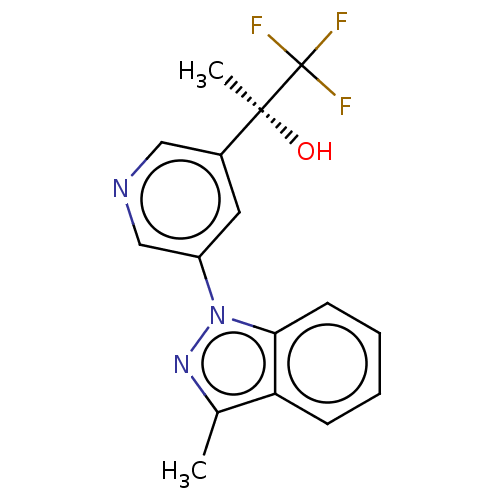
(CHEMBL4098297)Show SMILES Cc1nn(-c2cncc(c2)[C@](C)(O)C(F)(F)F)c2ccccc12 |r| Show InChI InChI=1S/C16H14F3N3O/c1-10-13-5-3-4-6-14(13)22(21-10)12-7-11(8-20-9-12)15(2,23)16(17,18)19/h3-9,23H,1-2H3/t15-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat CYP11B2-1F4 expressed in Chinese hamster V79 cells using 11-deoxycorticosterone as substrate preincubated for 1 hr followed by subs... |
Bioorg Med Chem Lett 27: 2384-2388 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.021
BindingDB Entry DOI: 10.7270/Q2MC92F2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Mus musculus) | BDBM50238114

(CHEMBL4093575)Show SMILES CC(C)C(O)(C1CCN(CC1)S(C)(=O)=O)c1cncnc1Nc1ccc(F)cc1 Show InChI InChI=1S/C20H27FN4O3S/c1-14(2)20(26,15-8-10-25(11-9-15)29(3,27)28)18-12-22-13-23-19(18)24-17-6-4-16(21)5-7-17/h4-7,12-15,26H,8-11H2,1-3H3,(H,22,23,24) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Rattus norvegicus) | BDBM50444548
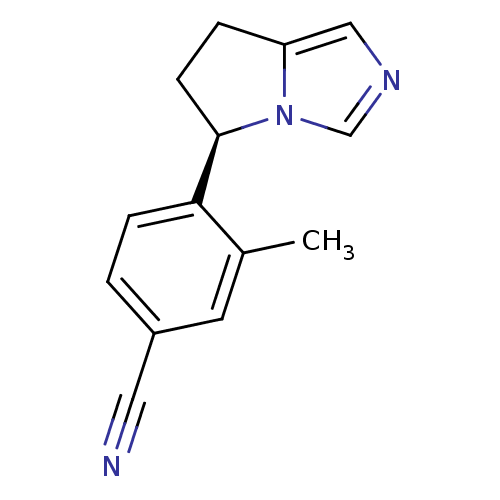
(CHEMBL3099696)Show InChI InChI=1S/C14H13N3/c1-10-6-11(7-15)2-4-13(10)14-5-3-12-8-16-9-17(12)14/h2,4,6,8-9,14H,3,5H2,1H3/t14-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant CYP11B2 using 11-deoxycorticosterone as substrate by cell-based assay |
ACS Med Chem Lett 4: 1203-7 (2013)
Article DOI: 10.1021/ml400324c
BindingDB Entry DOI: 10.7270/Q2NZ8938 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Rattus norvegicus) | BDBM50249048
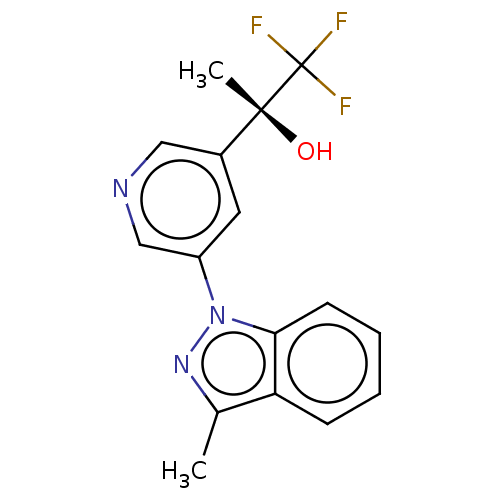
(CHEMBL4080256)Show SMILES Cc1nn(-c2cncc(c2)[C@@](C)(O)C(F)(F)F)c2ccccc12 |r| Show InChI InChI=1S/C16H14F3N3O/c1-10-13-5-3-4-6-14(13)22(21-10)12-7-11(8-20-9-12)15(2,23)16(17,18)19/h3-9,23H,1-2H3/t15-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat CYP11B2-1F4 expressed in Chinese hamster V79 cells using 11-deoxycorticosterone as substrate preincubated for 1 hr followed by subs... |
Bioorg Med Chem Lett 27: 2384-2388 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.021
BindingDB Entry DOI: 10.7270/Q2MC92F2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Rattus norvegicus) | BDBM50249046
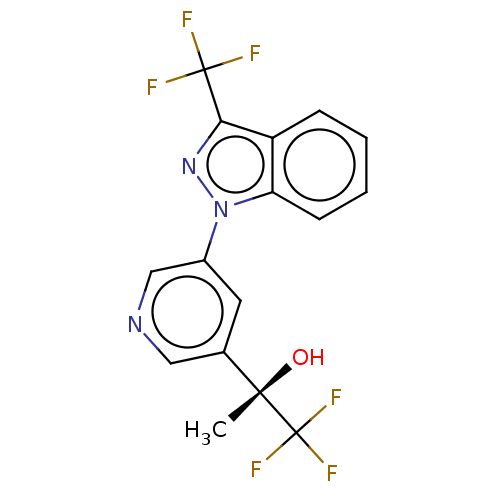
(CHEMBL4071689)Show SMILES C[C@](O)(c1cncc(c1)-n1nc(c2ccccc12)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C16H11F6N3O/c1-14(26,16(20,21)22)9-6-10(8-23-7-9)25-12-5-3-2-4-11(12)13(24-25)15(17,18)19/h2-8,26H,1H3/t14-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat CYP11B2-1F4 expressed in Chinese hamster V79 cells using 11-deoxycorticosterone as substrate preincubated for 1 hr followed by subs... |
Bioorg Med Chem Lett 27: 2384-2388 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.021
BindingDB Entry DOI: 10.7270/Q2MC92F2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Mus musculus) | BDBM50238115

(CHEMBL4082403)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)(c1cncnc1Nc1ccc(F)cc1)C(F)(F)F Show InChI InChI=1S/C18H20F4N4O3S/c1-30(28,29)26-8-6-12(7-9-26)17(27,18(20,21)22)15-10-23-11-24-16(15)25-14-4-2-13(19)3-5-14/h2-5,10-12,27H,6-9H2,1H3,(H,23,24,25) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of mouse CYP11B2 expressed in HEK293 cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition after... |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Mus musculus) | BDBM50238103

(CHEMBL4070323)Show SMILES CC(O)(C1CCN(CC1)S(C)(=O)=O)c1cncnc1Nc1ccc(F)cc1 Show InChI InChI=1S/C18H23FN4O3S/c1-18(24,13-7-9-23(10-8-13)27(2,25)26)16-11-20-12-21-17(16)22-15-5-3-14(19)4-6-15/h3-6,11-13,24H,7-10H2,1-2H3,(H,20,21,22) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Rattus norvegicus) | BDBM50444549

(CHEMBL3099695)Show InChI InChI=1S/C13H10FN3/c14-12-5-9(6-15)1-3-11(12)13-4-2-10-7-16-8-17(10)13/h1,3,5,7-8,13H,2,4H2/t13-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 111 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant CYP11B2 using 11-deoxycorticosterone as substrate by cell-based assay |
ACS Med Chem Lett 4: 1203-7 (2013)
Article DOI: 10.1021/ml400324c
BindingDB Entry DOI: 10.7270/Q2NZ8938 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Rattus norvegicus) | BDBM50047262
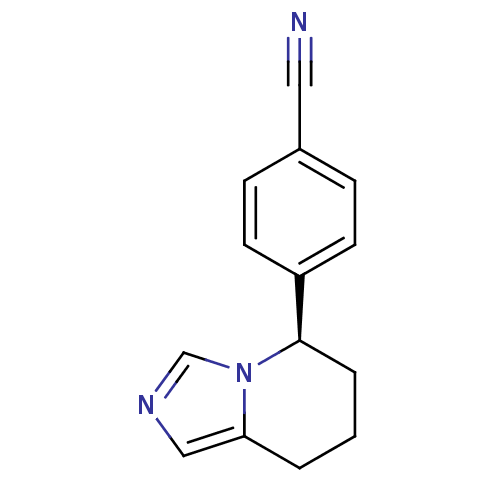
((R)-4-(5,6,7,8-tetrahydroimidazo[1,5-a]pyridin-5-y...)Show InChI InChI=1S/C14H13N3/c15-8-11-4-6-12(7-5-11)14-3-1-2-13-9-16-10-17(13)14/h4-7,9-10,14H,1-3H2/t14-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 118 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant CYP11B2 using 11-deoxycorticosterone as substrate by cell-based assay |
ACS Med Chem Lett 4: 1203-7 (2013)
Article DOI: 10.1021/ml400324c
BindingDB Entry DOI: 10.7270/Q2NZ8938 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Mus musculus) | BDBM50238104

(CHEMBL4072871)Show SMILES CCCCC(O)(C1CCN(CC1)S(C)(=O)=O)c1cncnc1Nc1ccc(F)cc1 Show InChI InChI=1S/C21H29FN4O3S/c1-3-4-11-21(27,16-9-12-26(13-10-16)30(2,28)29)19-14-23-15-24-20(19)25-18-7-5-17(22)6-8-18/h5-8,14-16,27H,3-4,9-13H2,1-2H3,(H,23,24,25) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of mouse CYP11B2 expressed in HEK293 cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition after... |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Mus musculus) | BDBM8611

(4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...)Show InChI InChI=1S/C14H13N3/c15-8-11-4-6-12(7-5-11)14-3-1-2-13-9-16-10-17(13)14/h4-7,9-10,14H,1-3H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research and Early Development (pRED)
Curated by ChEMBL
| Assay Description
Inhibition of mouse CYP11B2 expressed in renal leiomyoblastoma cells |
J Med Chem 58: 8054-65 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00851
BindingDB Entry DOI: 10.7270/Q2K0763C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Mus musculus) | BDBM50444549

(CHEMBL3099695)Show InChI InChI=1S/C13H10FN3/c14-12-5-9(6-15)1-3-11(12)13-4-2-10-7-16-8-17(10)13/h1,3,5,7-8,13H,2,4H2/t13-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research and Early Development (pRED)
Curated by ChEMBL
| Assay Description
Inhibition of mouse CYP11B2 expressed in renal leiomyoblastoma cells |
J Med Chem 58: 8054-65 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00851
BindingDB Entry DOI: 10.7270/Q2K0763C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Mus musculus) | BDBM50238110
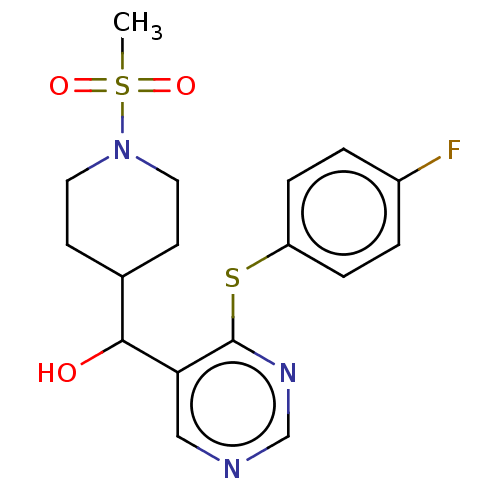
(CHEMBL4088766)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)c1cncnc1Sc1ccc(F)cc1 Show InChI InChI=1S/C17H20FN3O3S2/c1-26(23,24)21-8-6-12(7-9-21)16(22)15-10-19-11-20-17(15)25-14-4-2-13(18)3-5-14/h2-5,10-12,16,22H,6-9H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 257 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of mouse CYP11B2 expressed in HEK293 cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition after... |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Rattus norvegicus) | BDBM50238105

(CHEMBL4099824)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)c1cncnc1Nc1ccc(F)cc1 Show InChI InChI=1S/C17H21FN4O3S/c1-26(24,25)22-8-6-12(7-9-22)16(23)15-10-19-11-20-17(15)21-14-4-2-13(18)3-5-14/h2-5,10-12,16,23H,6-9H2,1H3,(H,19,20,21) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of rat CYP11B2 |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Mus musculus) | BDBM50238105

(CHEMBL4099824)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)c1cncnc1Nc1ccc(F)cc1 Show InChI InChI=1S/C17H21FN4O3S/c1-26(24,25)22-8-6-12(7-9-22)16(23)15-10-19-11-20-17(15)21-14-4-2-13(18)3-5-14/h2-5,10-12,16,23H,6-9H2,1H3,(H,19,20,21) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of mouse CYP11B2 expressed in HEK293 cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition after... |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Mus musculus) | BDBM50238102
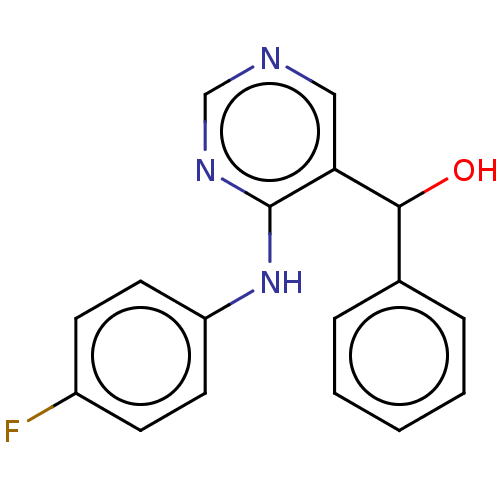
(CHEMBL4065476)Show InChI InChI=1S/C17H14FN3O/c18-13-6-8-14(9-7-13)21-17-15(10-19-11-20-17)16(22)12-4-2-1-3-5-12/h1-11,16,22H,(H,19,20,21) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Mus musculus) | BDBM50125049
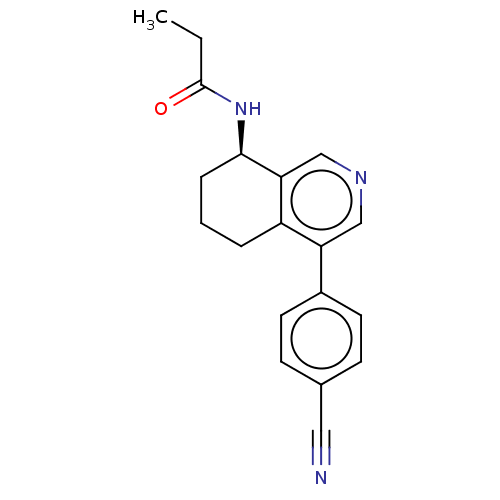
(CHEMBL3623830)Show SMILES CCC(=O)N[C@@H]1CCCc2c1cncc2-c1ccc(cc1)C#N |r| Show InChI InChI=1S/C19H19N3O/c1-2-19(23)22-18-5-3-4-15-16(11-21-12-17(15)18)14-8-6-13(10-20)7-9-14/h6-9,11-12,18H,2-5H2,1H3,(H,22,23)/t18-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research and Early Development (pRED)
Curated by ChEMBL
| Assay Description
Inhibition of mouse CYP11B2 expressed in human renal leiomyoblastoma cells using 11-deoxycorticosterone as substrate assessed as formation of aldoste... |
J Med Chem 58: 8054-65 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00851
BindingDB Entry DOI: 10.7270/Q2K0763C |
More data for this
Ligand-Target Pair | |
PI3K/PI4K catalytic domain-containing protein
() | BDBM533865
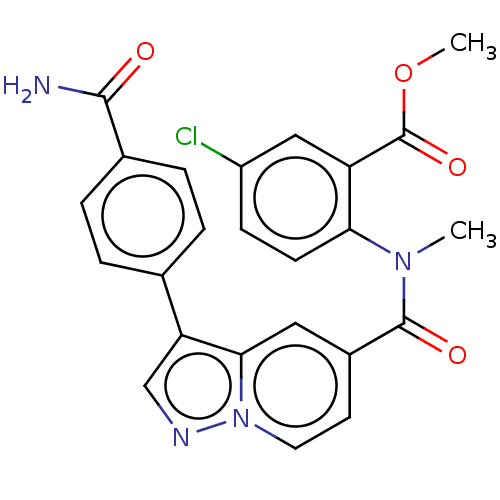
(Methyl 2-(3-(4-carbamoylphenyl)-N-methylpyrazolo[1...)Show SMILES COC(=O)c1cc(Cl)ccc1N(C)C(=O)c1ccn2ncc(-c3ccc(cc3)C(N)=O)c2c1 | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| WIPO WO2022079616
| n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q241717D |
More data for this
Ligand-Target Pair | |
PI3K/PI4K catalytic domain-containing protein
() | BDBM533764
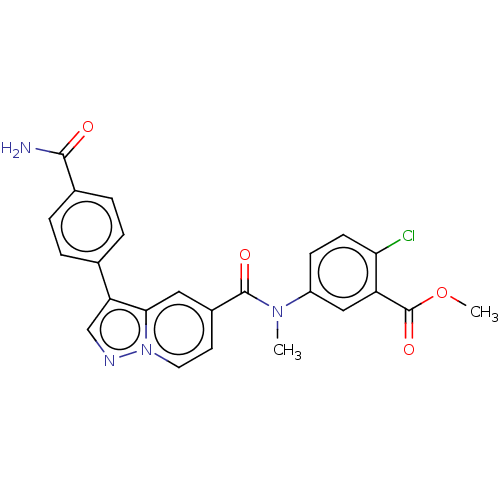
(N-(4-cyanophenyl)-N-methyl-3-(4-(trifluoromethyl)p...)Show SMILES COC(=O)c1cc(ccc1Cl)N(C)C(=O)c1ccn2ncc(-c3ccc(cc3)C(N)=O)c2c1 | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| WIPO WO2022079616
| n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q241717D |
More data for this
Ligand-Target Pair | |
PI3K/PI4K catalytic domain-containing protein
() | BDBM533790
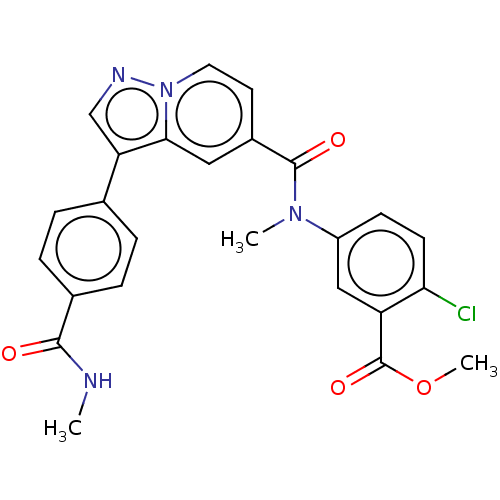
(Methyl 2-chloro-5-(N-methyl-3-(4-(methylcarbamoyl)...)Show SMILES CNC(=O)c1ccc(cc1)-c1cnn2ccc(cc12)C(=O)N(C)c1ccc(Cl)c(c1)C(=O)OC | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| WIPO WO2022079616
| n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q241717D |
More data for this
Ligand-Target Pair | |
PI3K/PI4K catalytic domain-containing protein
() | BDBM533793
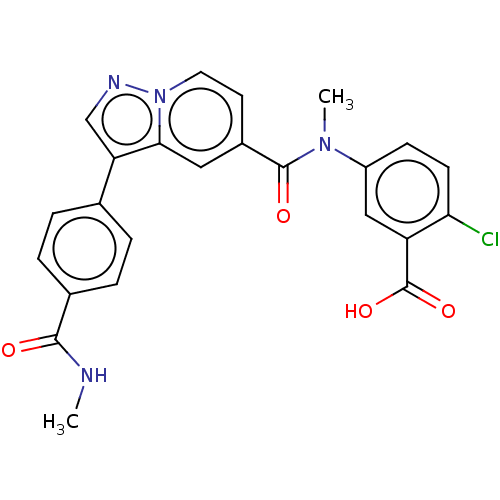
(2-Chloro-5-(N-methyl-3-(4-(methylcarbamoyl)phenyl)...)Show SMILES CNC(=O)c1ccc(cc1)-c1cnn2ccc(cc12)C(=O)N(C)c1ccc(Cl)c(c1)C(O)=O | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| WIPO WO2022079616
| n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q241717D |
More data for this
Ligand-Target Pair | |
PI3K/PI4K catalytic domain-containing protein
() | BDBM533812
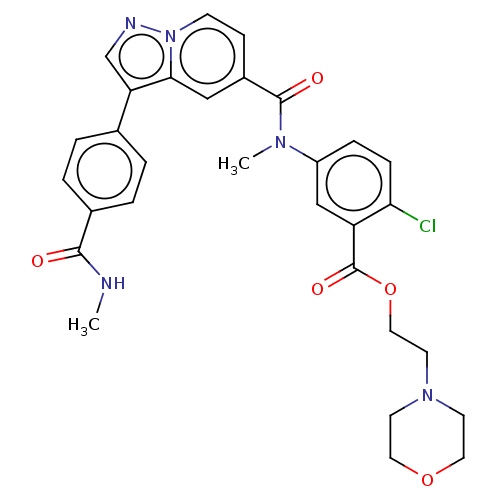
(2-Morpholinoethyl 2-chloro-5-(N-methyl-3-(4-(methy...)Show SMILES CNC(=O)c1ccc(cc1)-c1cnn2ccc(cc12)C(=O)N(C)c1ccc(Cl)c(c1)C(=O)OCCN1CCOCC1 | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| WIPO WO2022079616
| n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q241717D |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Rattus norvegicus) | BDBM8611

(4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...)Show InChI InChI=1S/C14H13N3/c15-8-11-4-6-12(7-5-11)14-3-1-2-13-9-16-10-17(13)14/h4-7,9-10,14H,1-3H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität des Saarlandes
Curated by ChEMBL
| Assay Description
In vitro inhibition of ACTH-stimulated aldosterone biosynthesis in rat adrenal slices |
J Med Chem 38: 2103-11 (1995)
BindingDB Entry DOI: 10.7270/Q2NV9H9S |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Mus musculus) | BDBM50238109
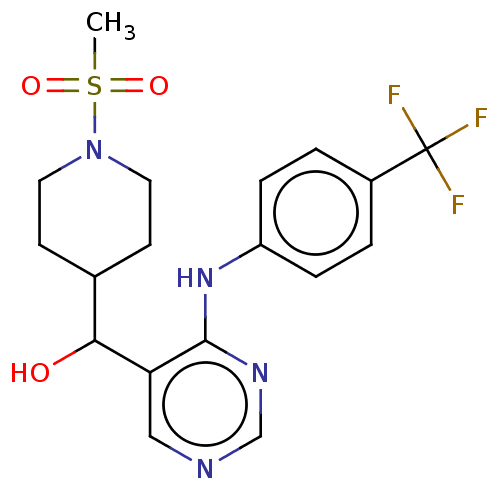
(CHEMBL4091167)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)c1cncnc1Nc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C18H21F3N4O3S/c1-29(27,28)25-8-6-12(7-9-25)16(26)15-10-22-11-23-17(15)24-14-4-2-13(3-5-14)18(19,20)21/h2-5,10-12,16,26H,6-9H2,1H3,(H,22,23,24) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 798 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of mouse CYP11B2 expressed in HEK293 cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition after... |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Mus musculus) | BDBM50238101
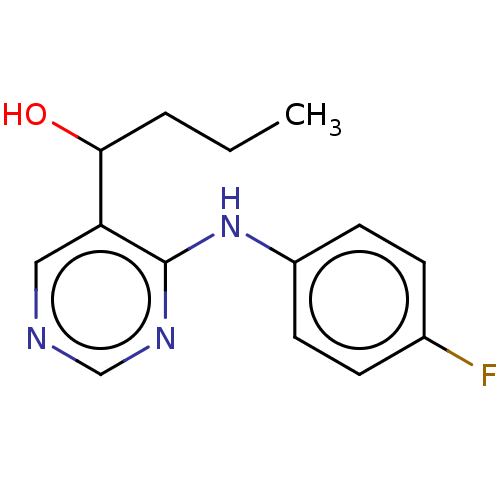
(CHEMBL4104118)Show InChI InChI=1S/C14H16FN3O/c1-2-3-13(19)12-8-16-9-17-14(12)18-11-6-4-10(15)5-7-11/h4-9,13,19H,2-3H2,1H3,(H,16,17,18) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 911 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of mouse CYP11B2 expressed in HEK293 cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition after... |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
PI3K/PI4K catalytic domain-containing protein
() | BDBM533862
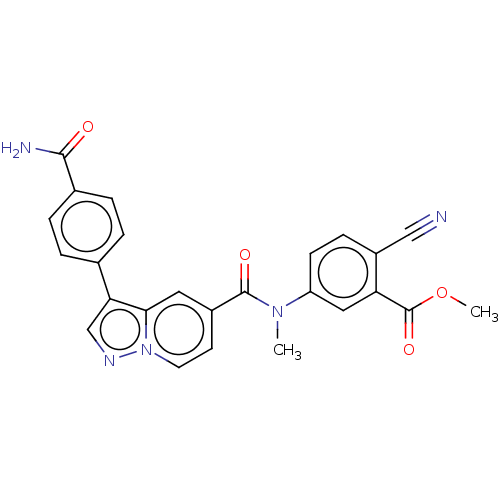
(Methyl 5-(3-(4-carbamoylphenyl)-N-methylpyrazolo[1...)Show SMILES COC(=O)c1cc(ccc1C#N)N(C)C(=O)c1ccn2ncc(-c3ccc(cc3)C(N)=O)c2c1 | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| WIPO WO2022079616
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q241717D |
More data for this
Ligand-Target Pair | |
PI3K/PI4K catalytic domain-containing protein
() | BDBM533860
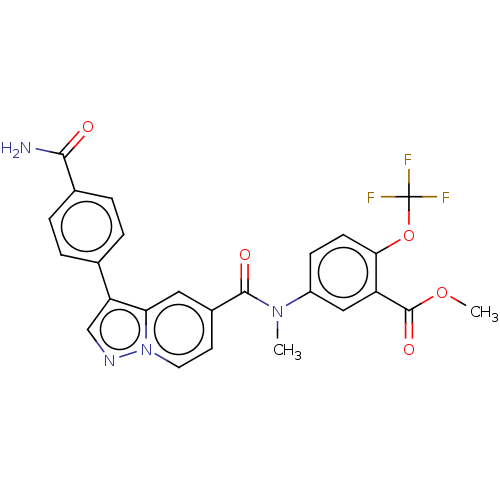
(Methyl 5-(3-(4-carbamoylphenyl)-N-methylpyrazolo[1...)Show SMILES COC(=O)c1cc(ccc1OC(F)(F)F)N(C)C(=O)c1ccn2ncc(-c3ccc(cc3)C(N)=O)c2c1 | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| WIPO WO2022079616
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q241717D |
More data for this
Ligand-Target Pair | |
PI3K/PI4K catalytic domain-containing protein
() | BDBM533857
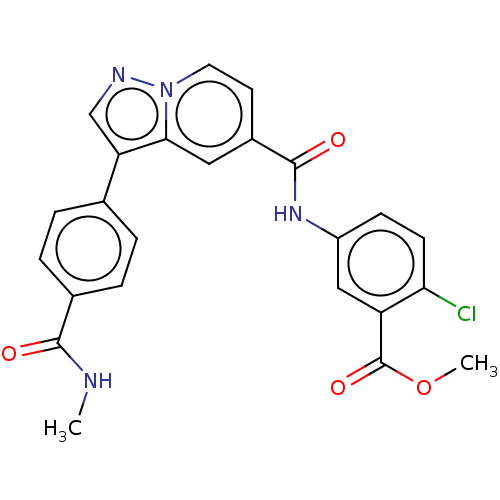
(Methyl 2-chloro-5-(3-(4-(methylcarbamoyl)phenyl)py...)Show SMILES CNC(=O)c1ccc(cc1)-c1cnn2ccc(cc12)C(=O)Nc1ccc(Cl)c(c1)C(=O)OC | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| WIPO WO2022079616
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q241717D |
More data for this
Ligand-Target Pair | |
PI3K/PI4K catalytic domain-containing protein
() | BDBM533856
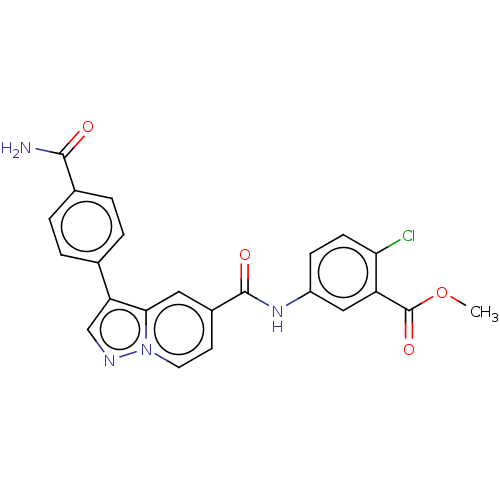
(Methyl 5-(3-(4-carbamoylphenyl)pyrazolo[1,5-a]pyri...)Show SMILES COC(=O)c1cc(NC(=O)c2ccn3ncc(-c4ccc(cc4)C(N)=O)c3c2)ccc1Cl | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| WIPO WO2022079616
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q241717D |
More data for this
Ligand-Target Pair | |
PI3K/PI4K catalytic domain-containing protein
() | BDBM533837
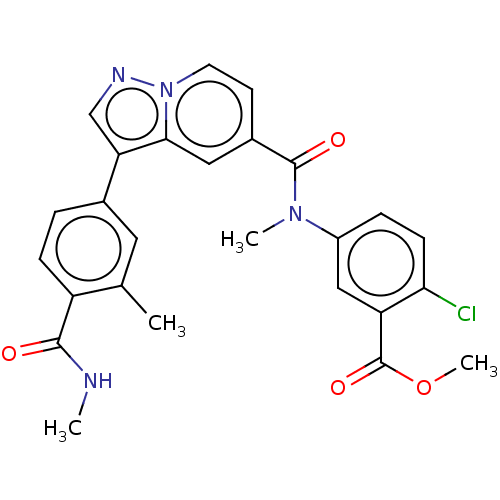
(Methyl 2-chloro-5-(N-methyl-3-(3-methyl-4-(carbamo...)Show SMILES CNC(=O)c1ccc(cc1C)-c1cnn2ccc(cc12)C(=O)N(C)c1ccc(Cl)c(c1)C(=O)OC | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| WIPO WO2022079616
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q241717D |
More data for this
Ligand-Target Pair | |
PI3K/PI4K catalytic domain-containing protein
() | BDBM533783
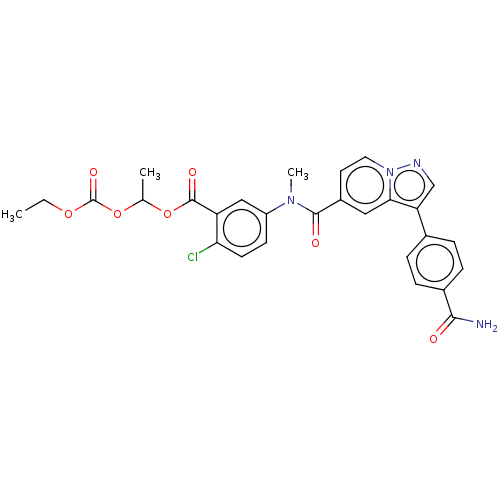
(WO2022079616, Example 1.14 | rac-1-((Ethoxycarbony...)Show SMILES CCOC(=O)OC(C)OC(=O)c1cc(ccc1Cl)N(C)C(=O)c1ccn2ncc(-c3ccc(cc3)C(N)=O)c2c1 | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| WIPO WO2022079616
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q241717D |
More data for this
Ligand-Target Pair | |
PI3K/PI4K catalytic domain-containing protein
() | BDBM533771
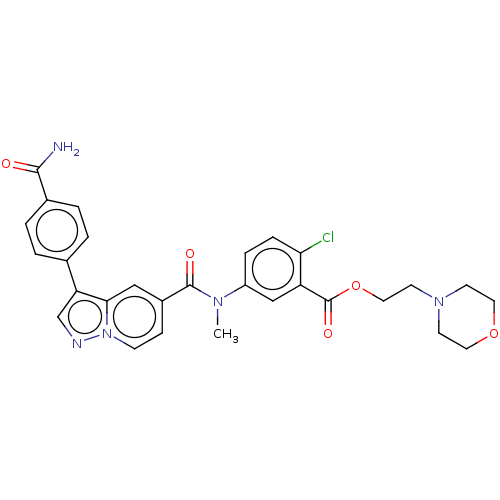
(2-Morpholinoethyl 5-(3-(4-carbamoylphenyl)-N-methy...)Show SMILES CN(C(=O)c1ccn2ncc(-c3ccc(cc3)C(N)=O)c2c1)c1ccc(Cl)c(c1)C(=O)OCCN1CCOCC1 | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| WIPO WO2022079616
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q241717D |
More data for this
Ligand-Target Pair | |
PI3K/PI4K catalytic domain-containing protein
() | BDBM533863
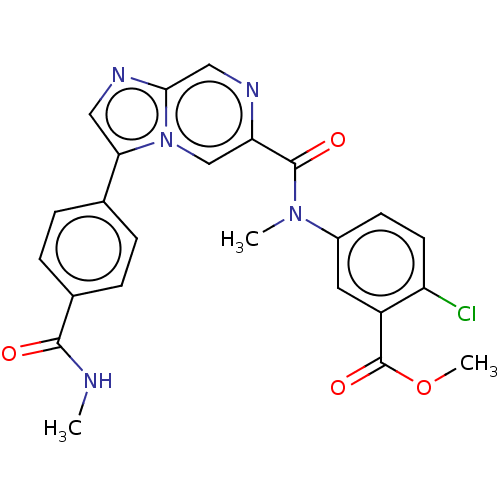
(WO2022079616, Example 2.1)Show SMILES CNC(=O)c1ccc(cc1)-c1cnc2cnc(cn12)C(=O)N(C)c1ccc(Cl)c(c1)C(=O)OC | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| WIPO WO2022079616
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q241717D |
More data for this
Ligand-Target Pair | |
PI3K/PI4K catalytic domain-containing protein
() | BDBM533813
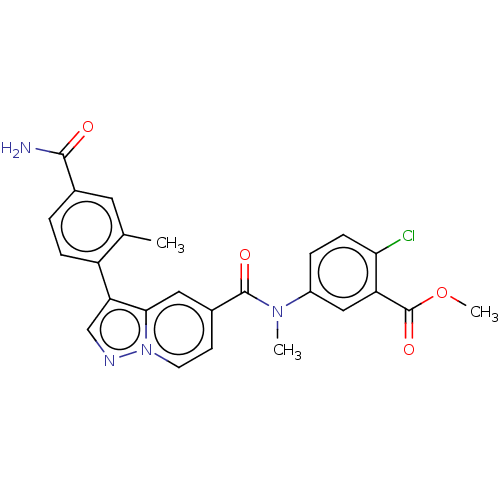
(Methyl 2-chloro-5-(N-methyl-3-(2-methyl-4-(carbamo...)Show SMILES COC(=O)c1cc(ccc1Cl)N(C)C(=O)c1ccn2ncc(-c3ccc(cc3C)C(N)=O)c2c1 | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| WIPO WO2022079616
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q241717D |
More data for this
Ligand-Target Pair | |
PI3K/PI4K catalytic domain-containing protein
() | BDBM533864
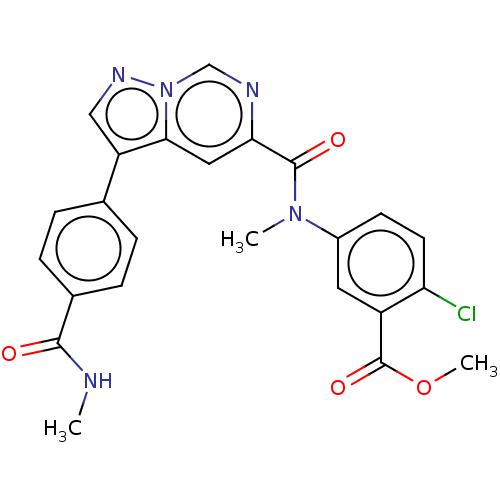
(Methyl 2-chloro-5-(N-methyl-3-(4-(methylcarbamoyl)...)Show SMILES CNC(=O)c1ccc(cc1)-c1cnn2cnc(cc12)C(=O)N(C)c1ccc(Cl)c(c1)C(=O)OC | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| WIPO WO2022079616
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q241717D |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Mus musculus) | BDBM50238106
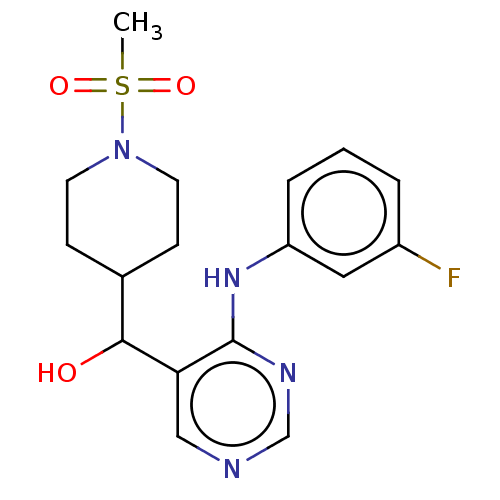
(CHEMBL4072943)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)c1cncnc1Nc1cccc(F)c1 Show InChI InChI=1S/C17H21FN4O3S/c1-26(24,25)22-7-5-12(6-8-22)16(23)15-10-19-11-20-17(15)21-14-4-2-3-13(18)9-14/h2-4,9-12,16,23H,5-8H2,1H3,(H,19,20,21) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of mouse CYP11B2 expressed in HEK293 cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition after... |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Mus musculus) | BDBM50238121

(CHEMBL4077051)Show InChI InChI=1S/C16H19FN4O/c17-12-1-3-13(4-2-12)21-16-14(9-19-10-20-16)15(22)11-5-7-18-8-6-11/h1-4,9-11,15,18,22H,5-8H2,(H,19,20,21) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of mouse CYP11B2 expressed in HEK293 cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition after... |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Mus musculus) | BDBM50238108
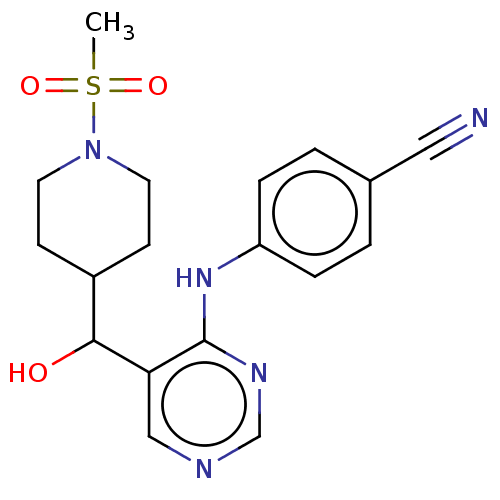
(CHEMBL4070230)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)c1cncnc1Nc1ccc(cc1)C#N Show InChI InChI=1S/C18H21N5O3S/c1-27(25,26)23-8-6-14(7-9-23)17(24)16-11-20-12-21-18(16)22-15-4-2-13(10-19)3-5-15/h2-5,11-12,14,17,24H,6-9H2,1H3,(H,20,21,22) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of mouse CYP11B2 expressed in HEK293 cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition after... |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Mus musculus) | BDBM50238111

(CHEMBL4060559)Show SMILES CS(=O)(=O)N1CCC(O)(CC1)c1cncnc1Nc1ccc(F)cc1 Show InChI InChI=1S/C16H19FN4O3S/c1-25(23,24)21-8-6-16(22,7-9-21)14-10-18-11-19-15(14)20-13-4-2-12(17)3-5-13/h2-5,10-11,22H,6-9H2,1H3,(H,18,19,20) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Mus musculus) | BDBM50238119

(CHEMBL4075207)Show SMILES CS(=O)(=O)N1CCC(C1)C(O)c1cncnc1Nc1ccc(F)cc1 Show InChI InChI=1S/C16H19FN4O3S/c1-25(23,24)21-7-6-11(9-21)15(22)14-8-18-10-19-16(14)20-13-4-2-12(17)3-5-13/h2-5,8,10-11,15,22H,6-7,9H2,1H3,(H,18,19,20) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of mouse CYP11B2 expressed in HEK293 cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition after... |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Mus musculus) | BDBM50238116
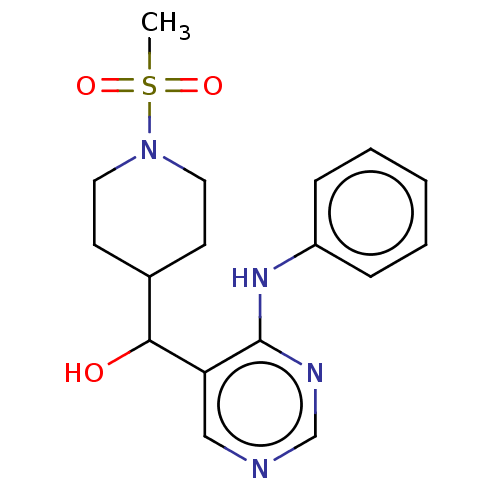
(CHEMBL4079006)Show InChI InChI=1S/C17H22N4O3S/c1-25(23,24)21-9-7-13(8-10-21)16(22)15-11-18-12-19-17(15)20-14-5-3-2-4-6-14/h2-6,11-13,16,22H,7-10H2,1H3,(H,18,19,20) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of mouse CYP11B2 expressed in HEK293 cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition after... |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Mus musculus) | BDBM50238117
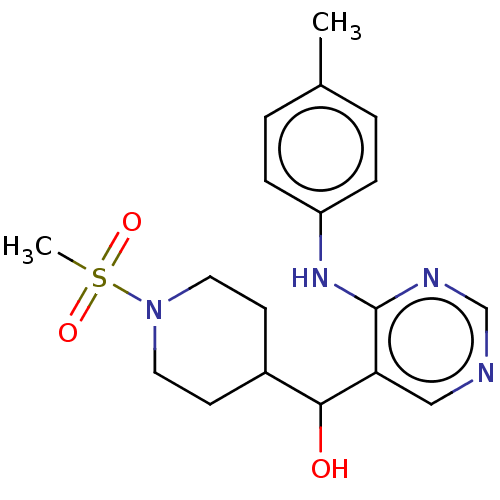
(CHEMBL4092081)Show SMILES Cc1ccc(Nc2ncncc2C(O)C2CCN(CC2)S(C)(=O)=O)cc1 Show InChI InChI=1S/C18H24N4O3S/c1-13-3-5-15(6-4-13)21-18-16(11-19-12-20-18)17(23)14-7-9-22(10-8-14)26(2,24)25/h3-6,11-12,14,17,23H,7-10H2,1-2H3,(H,19,20,21) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Rattus norvegicus) | BDBM50500189
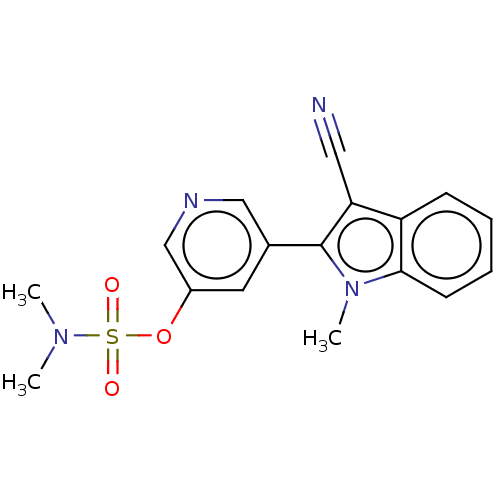
(CHEMBL3746175)Show SMILES CN(C)S(=O)(=O)Oc1cncc(c1)-c1c(C#N)c2ccccc2n1C Show InChI InChI=1S/C17H16N4O3S/c1-20(2)25(22,23)24-13-8-12(10-19-11-13)17-15(9-18)14-6-4-5-7-16(14)21(17)3/h4-8,10-11H,1-3H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat CYP11B2 using 11-deoxycorticosterone as substrate after 2 hrs by scintillation proximity assay |
J Med Chem 58: 9382-94 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01545
BindingDB Entry DOI: 10.7270/Q2CC13QV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Mus musculus) | BDBM50047262

((R)-4-(5,6,7,8-tetrahydroimidazo[1,5-a]pyridin-5-y...)Show InChI InChI=1S/C14H13N3/c15-8-11-4-6-12(7-5-11)14-3-1-2-13-9-16-10-17(13)14/h4-7,9-10,14H,1-3H2/t14-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Mus musculus) | BDBM50238123

(CHEMBL4061565)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)c1cncnc1Nc1cccc2ccccc12 Show InChI InChI=1S/C21H24N4O3S/c1-29(27,28)25-11-9-16(10-12-25)20(26)18-13-22-14-23-21(18)24-19-8-4-6-15-5-2-3-7-17(15)19/h2-8,13-14,16,20,26H,9-12H2,1H3,(H,22,23,24) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of mouse CYP11B2 expressed in HEK293 cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition after... |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data