

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50177318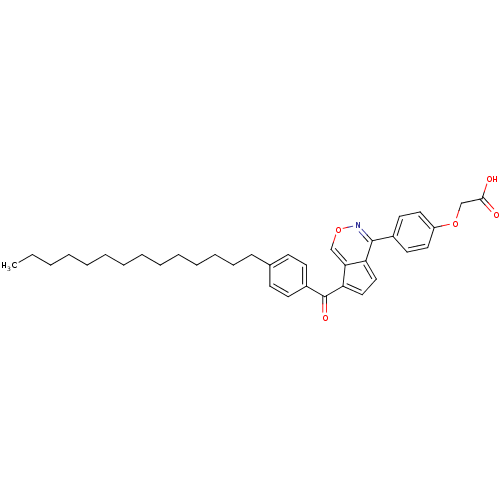 (2-(4-(7-(4-tetradecylbenzoyl)cyclopenta[d][1,2]oxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against CD45 phosphatase | Bioorg Med Chem Lett 16: 499-502 (2005) Article DOI: 10.1016/j.bmcl.2005.10.062 BindingDB Entry DOI: 10.7270/Q2319VFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50099692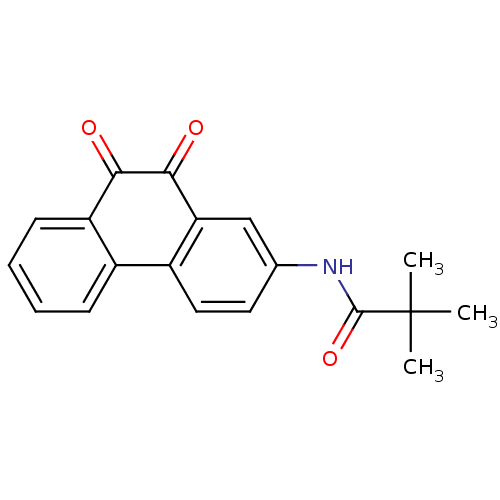 (CHEMBL51314 | N-(9,10-Dioxo-9,10-dihydro-phenanthr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against the cytosolic portion of CD45 protein-tyrosine phosphatase using pNPP as the substrate | J Med Chem 44: 1777-93 (2001) BindingDB Entry DOI: 10.7270/Q2X34WQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50094938 (5-Isopropoxy-2-(4-methylsulfanyl-pyridin-2-ylmetha...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity towards Protein tyrosine phosphatase of CD45 | Bioorg Med Chem Lett 10: 2657-60 (2000) BindingDB Entry DOI: 10.7270/Q2MK6C54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50262986 (CHEMBL515797 | [5-Hydroxy-3',5'-bis-trifluoromethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of CD45 (unknown origin) | Bioorg Med Chem 16: 7399-409 (2008) Article DOI: 10.1016/j.bmc.2008.06.014 BindingDB Entry DOI: 10.7270/Q2PC325T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50303173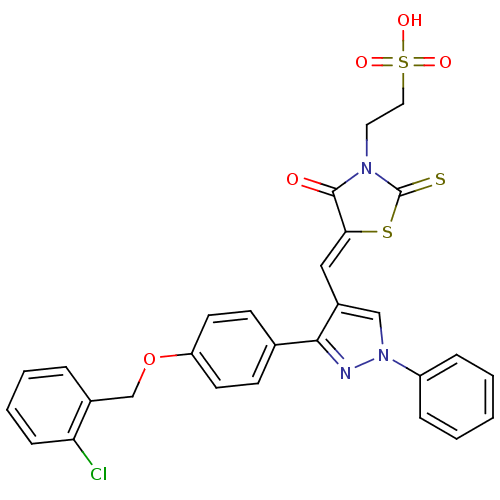 (2-(5-((3-(4-(2-chlorobenzyloxy)phenyl)-1-phenyl-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of recombinant CD45 | J Med Chem 52: 6716-23 (2009) Article DOI: 10.1021/jm901016k BindingDB Entry DOI: 10.7270/Q2VX0GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM22857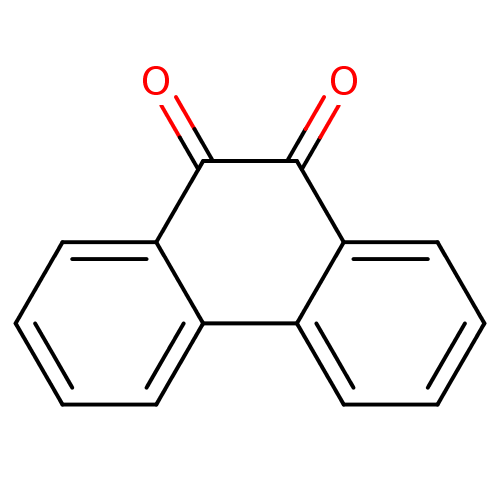 (1,2-Dione-Based Compound, 14 | 9,10-dihydrophenant...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
United States Army Medical Research Institute of Infectious Diseases | Assay Description Protein phosphatases were purchased from Upstate Biotechnology (Lake Placid, NY). | J Biol Chem 284: 12874-85 (2009) Article DOI: 10.1074/jbc.M809633200 BindingDB Entry DOI: 10.7270/Q2J67FJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50099700 (CHEMBL51776 | N-[7-(3-Methoxycarbonyl-propionylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against CD45 protein-tyrosine phosphatase using lck-10 mer as substrate | J Med Chem 44: 1777-93 (2001) BindingDB Entry DOI: 10.7270/Q2X34WQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50099700 (CHEMBL51776 | N-[7-(3-Methoxycarbonyl-propionylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against CD45 protein-tyrosine phosphatase using lck-10 mer as substrate | J Med Chem 44: 1777-93 (2001) BindingDB Entry DOI: 10.7270/Q2X34WQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50099772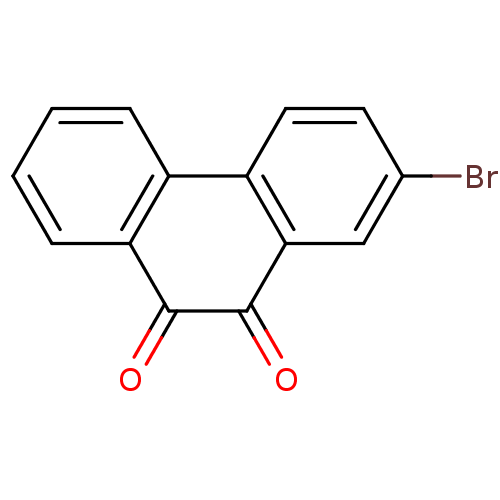 (2-Bromo-phenanthrene-9,10-dione | CHEMBL52491) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against the cytosolic portion of CD45 protein-tyrosine phosphatase using pNPP as the substrate | J Med Chem 44: 1777-93 (2001) BindingDB Entry DOI: 10.7270/Q2X34WQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50099730 (CHEMBL51781 | N-(9,10-Dioxo-9,10-dihydro-phenanthr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against the cytosolic portion of CD45 protein-tyrosine phosphatase using pNPP as the substrate | J Med Chem 44: 1777-93 (2001) BindingDB Entry DOI: 10.7270/Q2X34WQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50099746 (CHEMBL51579 | N-(9,10-Dioxo-9,10-dihydro-phenanthr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against the cytosolic portion of CD45 protein-tyrosine phosphatase using pNPP as the substrate | J Med Chem 44: 1777-93 (2001) BindingDB Entry DOI: 10.7270/Q2X34WQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50099737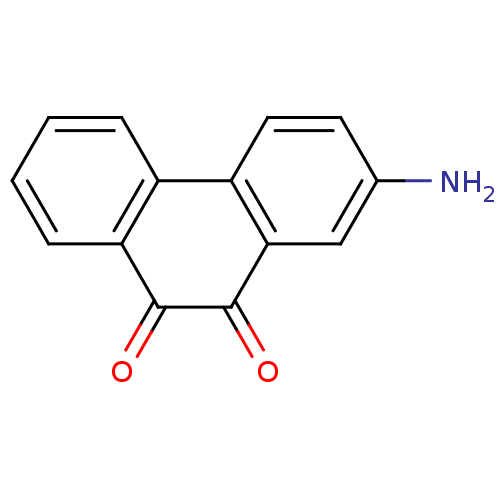 (2-Amino-phenanthrene-9,10-dione | CHEMBL417727 | U...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against the cytosolic portion of CD45 protein-tyrosine phosphatase using pNPP as the substrate | J Med Chem 44: 1777-93 (2001) BindingDB Entry DOI: 10.7270/Q2X34WQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50099692 (CHEMBL51314 | N-(9,10-Dioxo-9,10-dihydro-phenanthr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
United States Army Medical Research Institute of Infectious Diseases | Assay Description Protein phosphatases were purchased from Upstate Biotechnology (Lake Placid, NY). | J Biol Chem 284: 12874-85 (2009) Article DOI: 10.1074/jbc.M809633200 BindingDB Entry DOI: 10.7270/Q2J67FJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50335865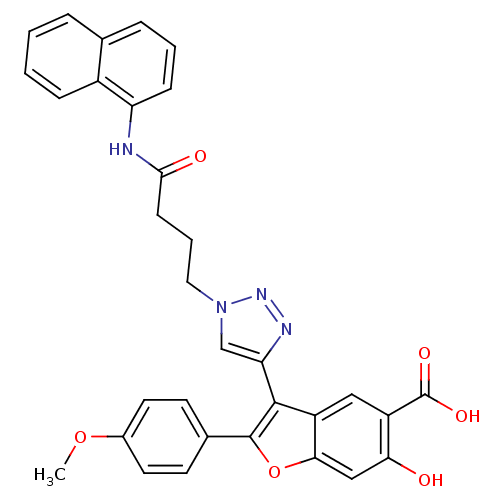 (6-Hydroxy-2-(4-methoxyphenyl)-3-(1-(4-(naphthalen-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of CD45 | J Med Chem 54: 562-71 (2011) Article DOI: 10.1021/jm101004d BindingDB Entry DOI: 10.7270/Q2N016T4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM28879 (2-[(5Z)-5-[(3-{4-[(4-chlorophenyl)methoxy]phenyl}-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of recombinant CD45 | J Med Chem 52: 6716-23 (2009) Article DOI: 10.1021/jm901016k BindingDB Entry DOI: 10.7270/Q2VX0GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50303174 (2-(5-((3-(4-(2-fluorobenzyloxy)phenyl)-1-phenyl-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of recombinant CD45 | J Med Chem 52: 6716-23 (2009) Article DOI: 10.1021/jm901016k BindingDB Entry DOI: 10.7270/Q2VX0GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50099704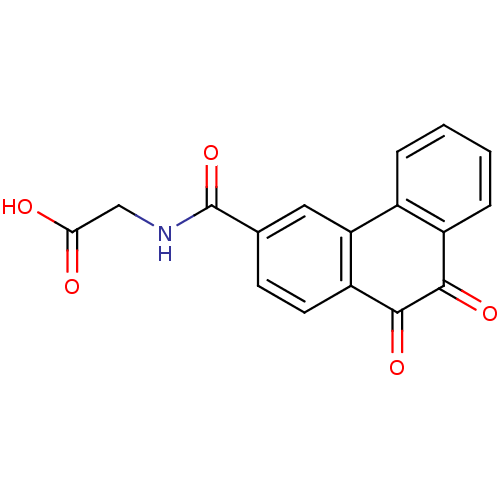 (CHEMBL301801 | [(9,10-Dioxo-9,10-dihydro-phenanthr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against the cytosolic portion of CD45 protein-tyrosine phosphatase using pNPP as the substrate | J Med Chem 44: 1777-93 (2001) BindingDB Entry DOI: 10.7270/Q2X34WQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50099742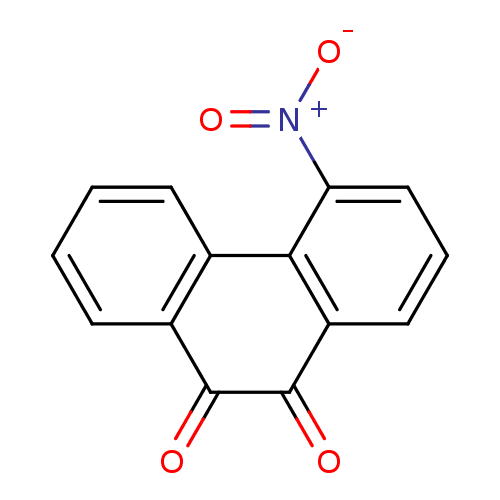 (4-Nitro-phenanthrene-9,10 dione | 4-Nitro-phenanth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against the cytosolic portion of CD45 protein-tyrosine phosphatase using pNPP as the substrate | J Med Chem 44: 1777-93 (2001) BindingDB Entry DOI: 10.7270/Q2X34WQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50099700 (CHEMBL51776 | N-[7-(3-Methoxycarbonyl-propionylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against the cytosolic portion of CD45 protein-tyrosine phosphatase using pNPP as the substrate | J Med Chem 44: 1777-93 (2001) BindingDB Entry DOI: 10.7270/Q2X34WQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50099697 (7-(9,10-Dioxo-9,10-dihydro-phenanthren-2-ylcarbamo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against the cytosolic portion of CD45 protein-tyrosine phosphatase using pNPP as the substrate | J Med Chem 44: 1777-93 (2001) BindingDB Entry DOI: 10.7270/Q2X34WQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50099722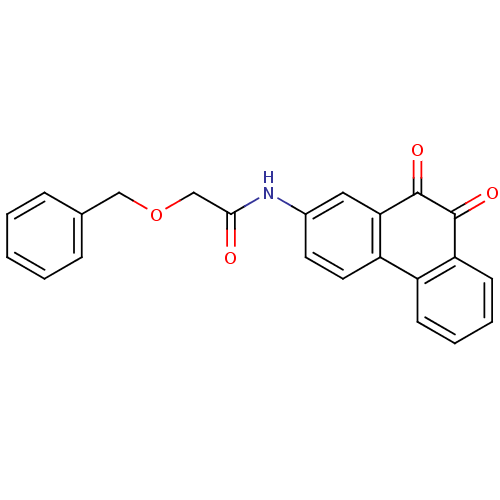 (2-Benzyloxy-N-(9,10-dioxo-9,10-dihydro-phenanthren...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against the cytosolic portion of CD45 protein-tyrosine phosphatase using pNPP as the substrate | J Med Chem 44: 1777-93 (2001) BindingDB Entry DOI: 10.7270/Q2X34WQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50099771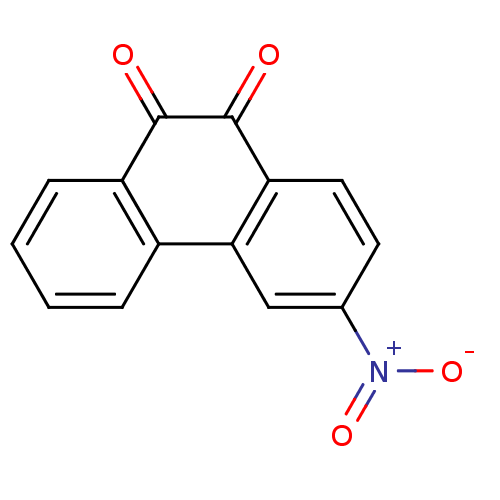 (3-Nitro-phenanthrene-9,10-dione | CHEMBL50973) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against the cytosolic portion of CD45 protein-tyrosine phosphatase using pNPP as the substrate | J Med Chem 44: 1777-93 (2001) BindingDB Entry DOI: 10.7270/Q2X34WQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50099708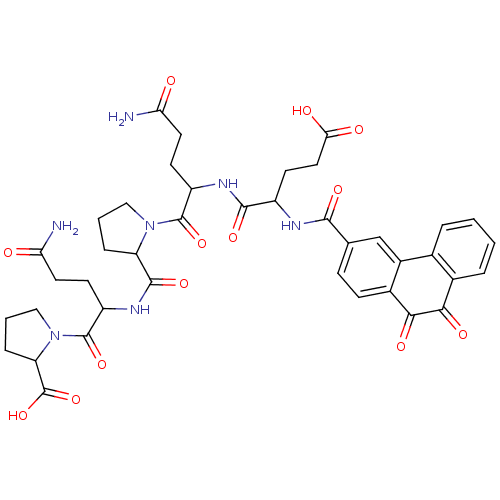 (1-(4-Carbamoyl-2-{[1-(4-carbamoyl-2-{4-carboxy-2-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against the cytosolic portion of CD45 protein-tyrosine phosphatase using pNPP as the substrate | J Med Chem 44: 1777-93 (2001) BindingDB Entry DOI: 10.7270/Q2X34WQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50099713 (CHEMBL50878 | N-(9,10-Dioxo-9,10-dihydro-phenanthr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against CD45 protein-tyrosine phosphatase pNPP | J Med Chem 44: 1777-93 (2001) BindingDB Entry DOI: 10.7270/Q2X34WQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50099713 (CHEMBL50878 | N-(9,10-Dioxo-9,10-dihydro-phenanthr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against the cytosolic portion of CD45 protein-tyrosine phosphatase using pNPP as the substrate | J Med Chem 44: 1777-93 (2001) BindingDB Entry DOI: 10.7270/Q2X34WQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50099757 (4-(Carboxymethyl-carbamoyl)-4-{[1-(2-{2-[(9,10-dio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against the cytosolic portion of CD45 protein-tyrosine phosphatase using pNPP as the substrate | J Med Chem 44: 1777-93 (2001) BindingDB Entry DOI: 10.7270/Q2X34WQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50099764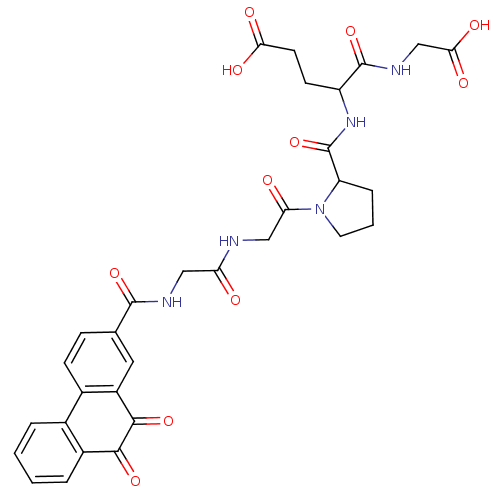 (4-(Carboxymethyl-carbamoyl)-4-{[1-(2-{2-[(9,10-dio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against the cytosolic portion of CD45 protein-tyrosine phosphatase using pNPP as the substrate | J Med Chem 44: 1777-93 (2001) BindingDB Entry DOI: 10.7270/Q2X34WQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50099707 (CHEMBL49992 | [(9,10-Dioxo-9,10-dihydro-phenanthre...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against the cytosolic portion of CD45 protein-tyrosine phosphatase using pNPP as the substrate | J Med Chem 44: 1777-93 (2001) BindingDB Entry DOI: 10.7270/Q2X34WQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50099747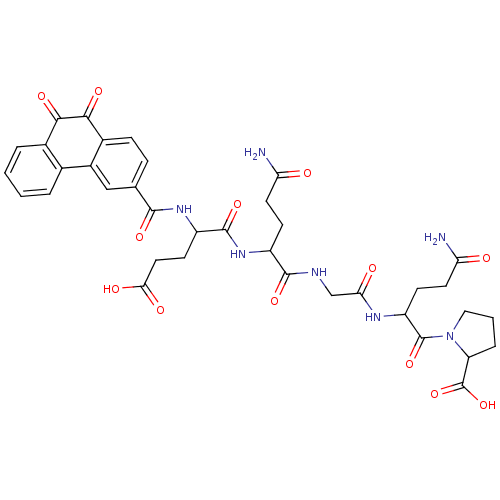 (1-{4-Carbamoyl-2-[2-(4-carbamoyl-2-{4-carboxy-2-[(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against the cytosolic portion of CD45 protein-tyrosine phosphatase using pNPP as the substrate | J Med Chem 44: 1777-93 (2001) BindingDB Entry DOI: 10.7270/Q2X34WQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50099752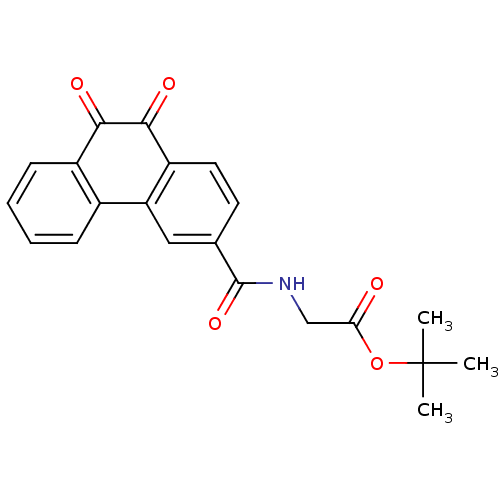 (CHEMBL54131 | [(9,10-Dioxo-9,10-dihydro-phenanthre...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against the cytosolic portion of CD45 protein-tyrosine phosphatase using pNPP as the substrate | J Med Chem 44: 1777-93 (2001) BindingDB Entry DOI: 10.7270/Q2X34WQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50303175 (2-(5-((3-(4-(benzyloxy)phenyl)-1-phenyl-1H-pyrazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of recombinant CD45 | J Med Chem 52: 6716-23 (2009) Article DOI: 10.1021/jm901016k BindingDB Entry DOI: 10.7270/Q2VX0GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50335868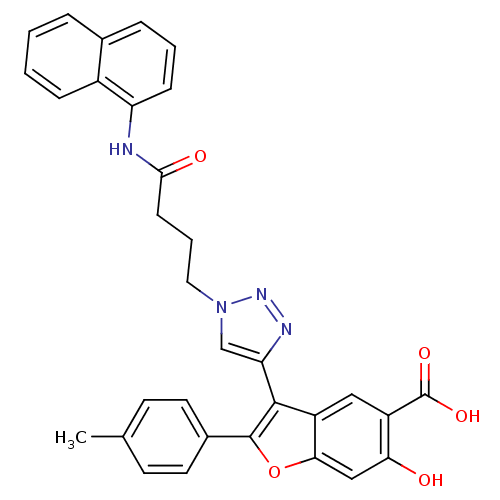 (6-Hydroxy-3-(1-(4-(naphthalen-1-ylamino)-4-oxobuty...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 681 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of CD45 | J Med Chem 54: 562-71 (2011) Article DOI: 10.1021/jm101004d BindingDB Entry DOI: 10.7270/Q2N016T4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50099773 (1-{4-Carbamoyl-2-[2-(4-carboxy-2-{2-[(9,10-dioxo-9...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against the cytosolic portion of CD45 protein-tyrosine phosphatase using pNPP as the substrate | J Med Chem 44: 1777-93 (2001) BindingDB Entry DOI: 10.7270/Q2X34WQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50099768 (4-(2-{2-[1-(Carboxymethyl-carbamoyl)-4-guanidino-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against the cytosolic portion of CD45 protein-tyrosine phosphatase using pNPP as the substrate | J Med Chem 44: 1777-93 (2001) BindingDB Entry DOI: 10.7270/Q2X34WQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50099784 (4-{[1-(4-Carbamoyl-2-{2-[(9,10-dioxo-9,10-dihydro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against the cytosolic portion of CD45 protein-tyrosine phosphatase using pNPP as the substrate | J Med Chem 44: 1777-93 (2001) BindingDB Entry DOI: 10.7270/Q2X34WQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50099766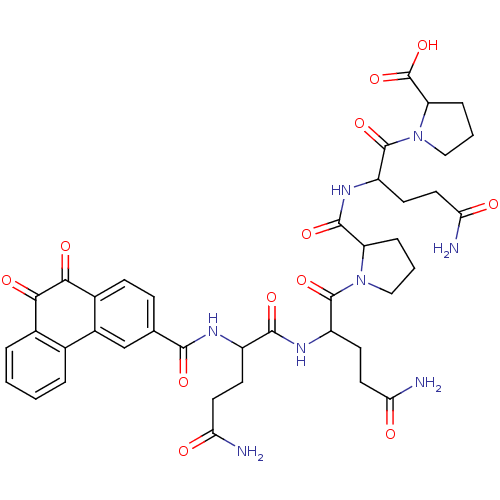 (1-(4-Carbamoyl-2-{[1-(4-carbamoyl-2-{4-carbamoyl-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against the cytosolic portion of CD45 protein-tyrosine phosphatase using pNPP as the substrate | J Med Chem 44: 1777-93 (2001) BindingDB Entry DOI: 10.7270/Q2X34WQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50118096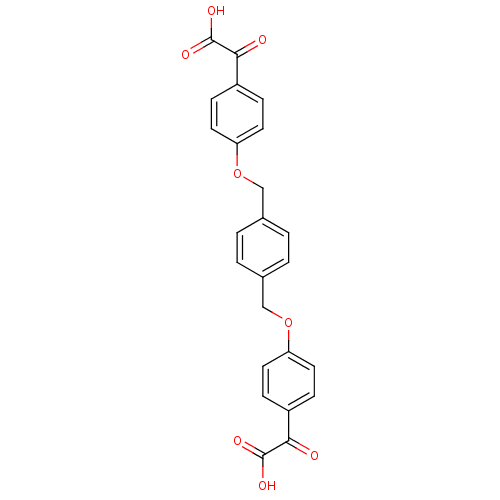 (2,2'-(4,4'-(1,4-phenylenebis(methylene))bis(oxy)bi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University Curated by ChEMBL | Assay Description Concentration required for 50% Inhibition of activity against Yersinia Protein-tyrosine phosphatase | J Med Chem 45: 3946-52 (2002) BindingDB Entry DOI: 10.7270/Q2F76BW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50099767 (4-(1-{2-[3-Carboxy-1-(carboxymethyl-carbamoyl)-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against the cytosolic portion of CD45 protein-tyrosine phosphatase using pNPP as the substrate | J Med Chem 44: 1777-93 (2001) BindingDB Entry DOI: 10.7270/Q2X34WQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50099739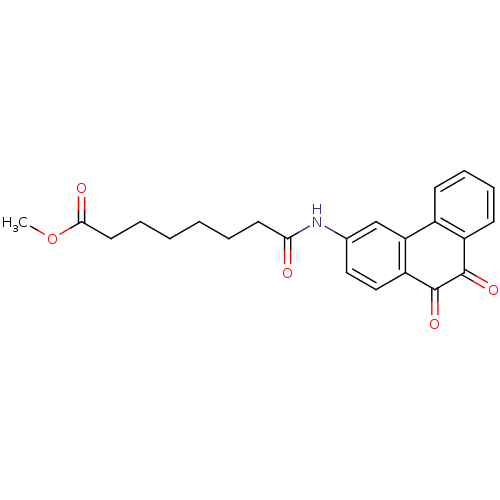 (7-(9,10-Dioxo-9,10-dihydro-phenanthren-3-ylcarbamo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against the cytosolic portion of CD45 protein-tyrosine phosphatase using pNPP as the substrate | J Med Chem 44: 1777-93 (2001) BindingDB Entry DOI: 10.7270/Q2X34WQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50099703 (1-(4-Carbamoyl-2-{[1-(4-carboxy-2-{2-[(9,10-dioxo-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against the cytosolic portion of CD45 protein-tyrosine phosphatase using pNPP as the substrate | J Med Chem 44: 1777-93 (2001) BindingDB Entry DOI: 10.7270/Q2X34WQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50099749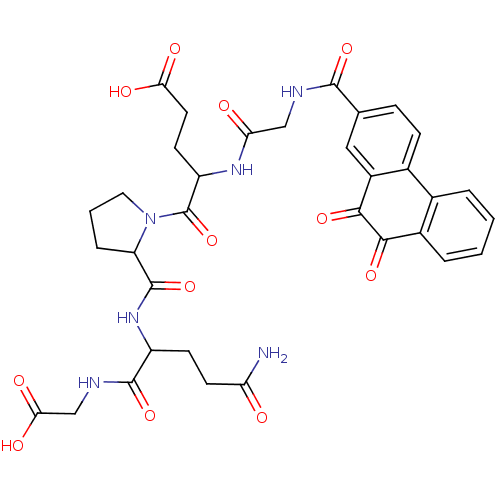 (5-{2-[3-Carbamoyl-1-(carboxymethyl-carbamoyl)-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against the cytosolic portion of CD45 protein-tyrosine phosphatase using pNPP as the substrate | J Med Chem 44: 1777-93 (2001) BindingDB Entry DOI: 10.7270/Q2X34WQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM22857 (1,2-Dione-Based Compound, 14 | 9,10-dihydrophenant...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against the cytosolic portion of CD45 protein-tyrosine phosphatase using pNPP as the substrate | J Med Chem 44: 1777-93 (2001) BindingDB Entry DOI: 10.7270/Q2X34WQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50099715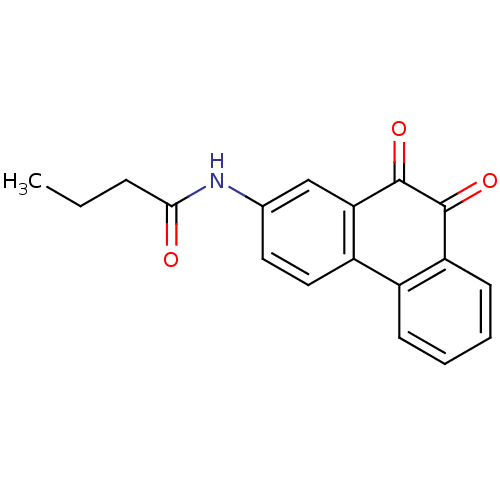 (CHEMBL51257 | N-(9,10-Dioxo-9,10-dihydro-phenanthr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against the cytosolic portion of CD45 protein-tyrosine phosphatase using pNPP as the substrate | J Med Chem 44: 1777-93 (2001) BindingDB Entry DOI: 10.7270/Q2X34WQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50099693 (5-{2-[1-(Carboxymethyl-carbamoyl)-4-guanidino-buty...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against the cytosolic portion of CD45 protein-tyrosine phosphatase using pNPP as the substrate | J Med Chem 44: 1777-93 (2001) BindingDB Entry DOI: 10.7270/Q2X34WQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50099698 (4-(1-{2-[1-(Carboxymethyl-carbamoyl)-4-guanidino-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against the cytosolic portion of CD45 protein-tyrosine phosphatase using pNPP as the substrate | J Med Chem 44: 1777-93 (2001) BindingDB Entry DOI: 10.7270/Q2X34WQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50099762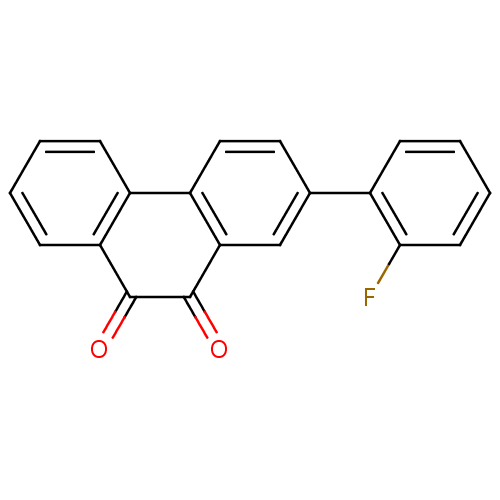 (2-(2-Fluoro-phenyl)-phenanthrene-9,10-dione | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against the cytosolic portion of CD45 protein-tyrosine phosphatase using pNPP as the substrate | J Med Chem 44: 1777-93 (2001) BindingDB Entry DOI: 10.7270/Q2X34WQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50099777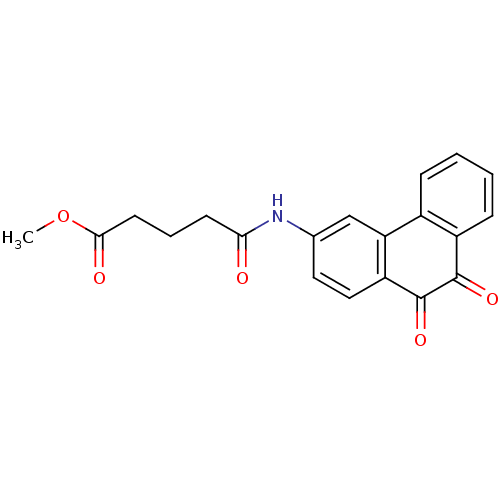 (4-(9,10-Dioxo-9,10-dihydro-phenanthren-3-ylcarbamo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against the cytosolic portion of CD45 protein-tyrosine phosphatase using pNPP as the substrate | J Med Chem 44: 1777-93 (2001) BindingDB Entry DOI: 10.7270/Q2X34WQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50099717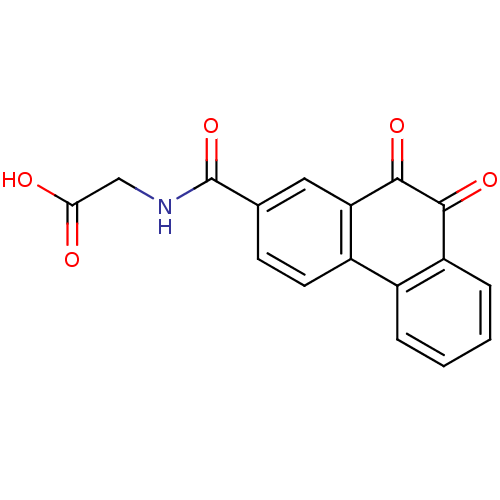 (CHEMBL300241 | [(9,10-Dioxo-9,10-dihydro-phenanthr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against the cytosolic portion of CD45 protein-tyrosine phosphatase using pNPP as the substrate | J Med Chem 44: 1777-93 (2001) BindingDB Entry DOI: 10.7270/Q2X34WQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50099689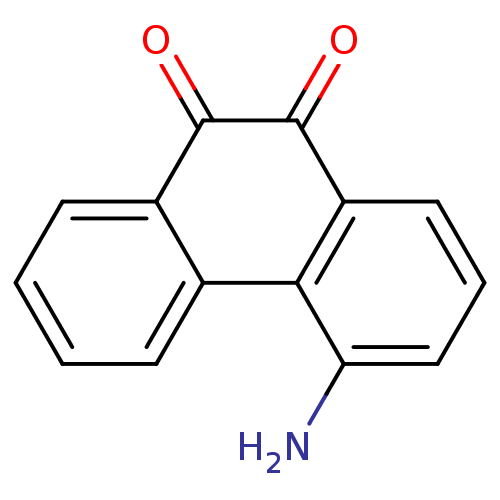 (4-Amino-phenanthrene-9,10-dione | CHEMBL49236) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against the cytosolic portion of CD45 protein-tyrosine phosphatase using pNPP as the substrate | J Med Chem 44: 1777-93 (2001) BindingDB Entry DOI: 10.7270/Q2X34WQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50099714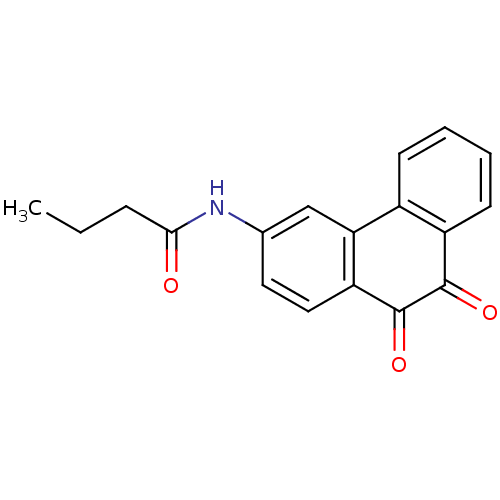 (CHEMBL50846 | N-(9,10-Dioxo-9,10-dihydro-phenanthr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against the cytosolic portion of CD45 protein-tyrosine phosphatase using pNPP as the substrate | J Med Chem 44: 1777-93 (2001) BindingDB Entry DOI: 10.7270/Q2X34WQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 355 total ) | Next | Last >> |