

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peptidyl-prolyl cis-trans isomerase FKBP1B (Homo sapiens (Human)) | BDBM50263407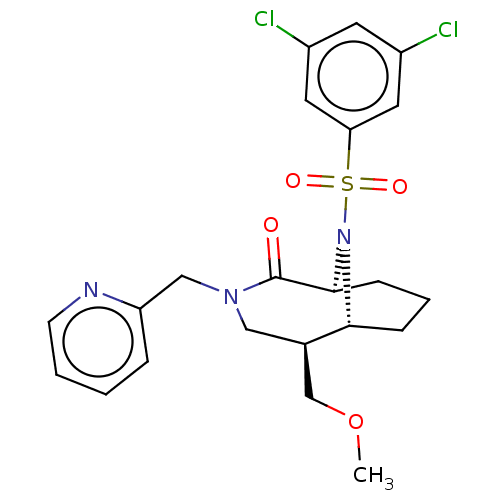 (CHEMBL4090599) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany. Curated by ChEMBL | Assay Description Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... | J Med Chem 61: 3660-3673 (2018) Article DOI: 10.1021/acs.jmedchem.8b00137 BindingDB Entry DOI: 10.7270/Q2833VGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1B (Homo sapiens (Human)) | BDBM50029191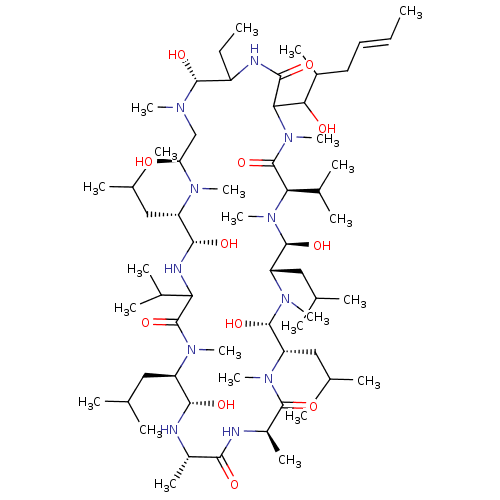 (15-Benzyl-30-ethyl-12-hydroxymethyl-33-(1-hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description The compound was tested for inhibition of Peptidyl-propyl isomerase (PPIase). | J Med Chem 38: 4164-70 (1995) BindingDB Entry DOI: 10.7270/Q2X34WGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1B (Homo sapiens (Human)) | BDBM50029190 (15-Benzyl-30-ethyl-33-(1-hydroxy-2-methyl-hex-4-en...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description The compound was tested for inhibition of Peptidyl-propyl isomerase (PPIase). | J Med Chem 38: 4164-70 (1995) BindingDB Entry DOI: 10.7270/Q2X34WGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1B (Homo sapiens (Human)) | BDBM50022815 ((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description The compound was tested for inhibition of Peptidyl-propyl isomerase (PPIase). | J Med Chem 38: 4164-70 (1995) BindingDB Entry DOI: 10.7270/Q2X34WGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1B (Homo sapiens (Human)) | BDBM50029192 (15-Benzyl-30-ethyl-12-hydroxymethyl-33-(1-hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description The compound was tested for inhibition of Peptidyl-propyl isomerase (PPIase). | J Med Chem 38: 4164-70 (1995) BindingDB Entry DOI: 10.7270/Q2X34WGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1B (Homo sapiens (Human)) | BDBM50029194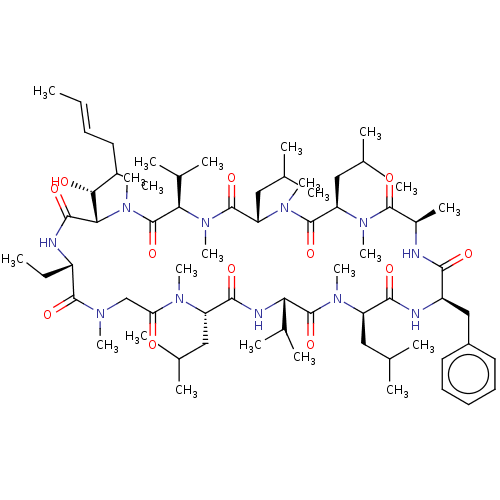 (15-Benzyl-30-ethyl-33-(1-hydroxy-2-methyl-hex-4-en...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description The compound was tested for inhibition of Peptidyl-propyl isomerase (PPIase). | J Med Chem 38: 4164-70 (1995) BindingDB Entry DOI: 10.7270/Q2X34WGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||