

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Squalene synthase (Rattus norvegicus) | BDBM50292333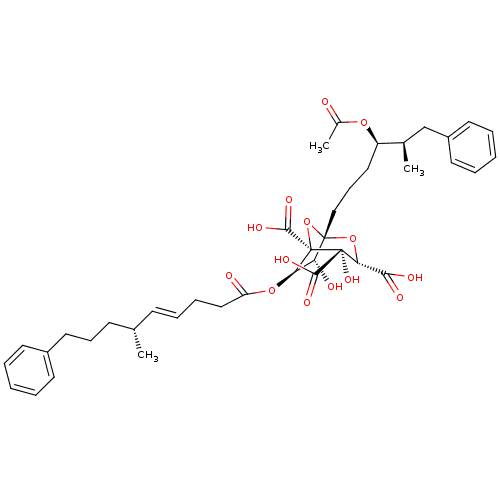 (CHEMBL505374 | zaragozic acid C) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of rat squalene synthase | J Nat Prod 59: 52-54 (1996) Article DOI: 10.1021/np960003i BindingDB Entry DOI: 10.7270/Q2Q52PND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50038096 ((6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-3-methylene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibitory activity of the compound was evaluated against squalene synthase in rat liver. | Bioorg Med Chem Lett 5: 1643-1646 (1995) Article DOI: 10.1016/0960-894X(95)00283-Y BindingDB Entry DOI: 10.7270/Q2DB81T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50038096 ((6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-3-methylene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of squalene synthase in rat liver. | Bioorg Med Chem Lett 3: 2029-2034 (1993) Article DOI: 10.1016/S0960-894X(01)81008-8 BindingDB Entry DOI: 10.7270/Q22J6BSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50038096 ((6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-3-methylene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against squalene synthase in rat liver squalene synthase (RLSS) enzyme assay | Bioorg Med Chem Lett 4: 1591-1594 (1994) Article DOI: 10.1016/S0960-894X(01)80572-2 BindingDB Entry DOI: 10.7270/Q2TB16T4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50291312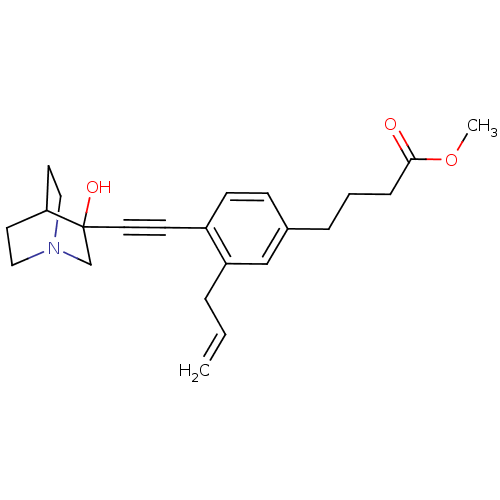 (4-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50075719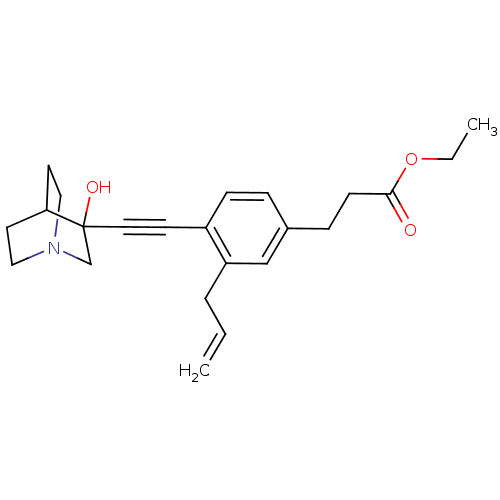 (3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50291315 (5-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50291311 (6-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50291316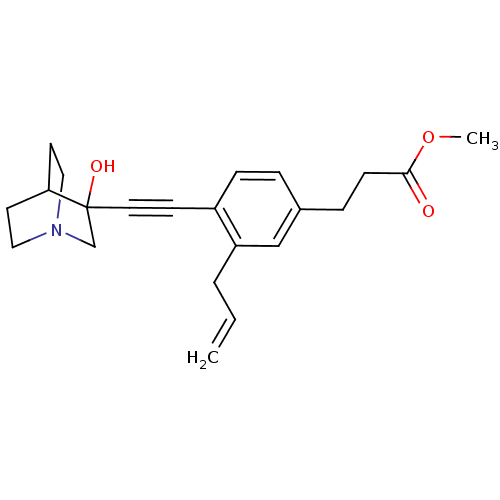 (3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50281603 ([Hydroxy-((2E,6E)-3,7,11-trimethyl-dodeca-2,6,10-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibitory potency against rat liver microsomal squalene synthase | Bioorg Med Chem Lett 3: 595-600 (1993) Article DOI: 10.1016/S0960-894X(01)81236-1 BindingDB Entry DOI: 10.7270/Q25Q4WMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50075719 (3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against human microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50291317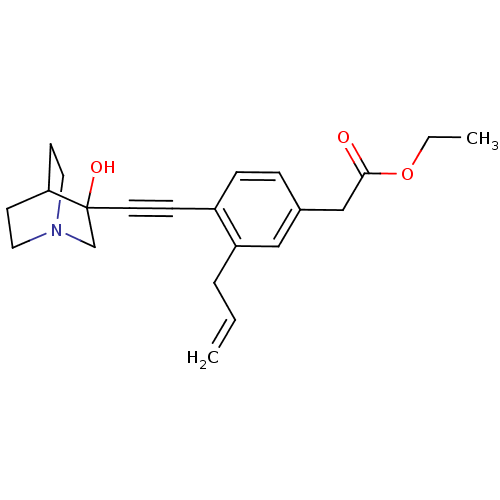 (CHEMBL154472 | [3-Allyl-4-(3-hydroxy-1-aza-bicyclo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50291313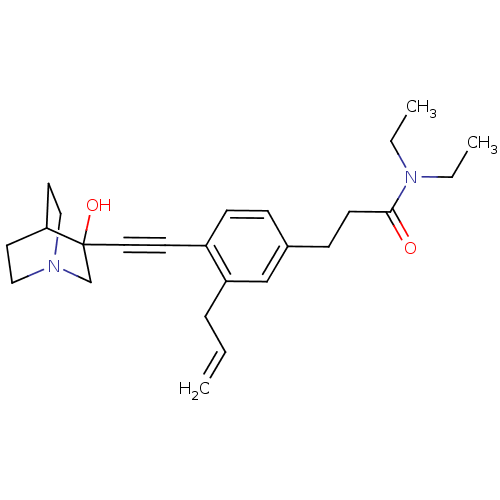 (3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | >250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50291314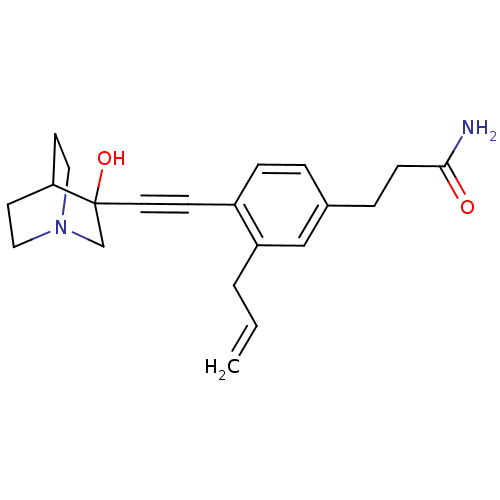 (3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | >250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50291318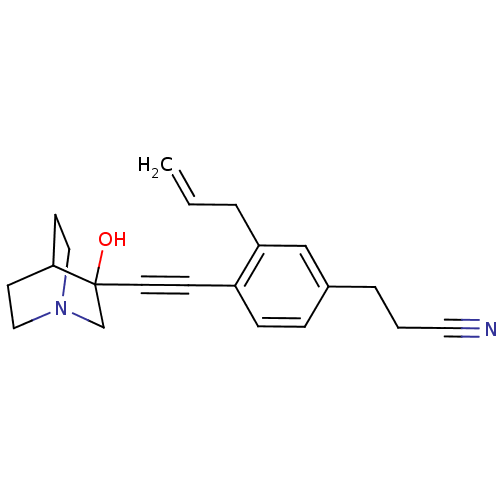 (3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | >250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50291319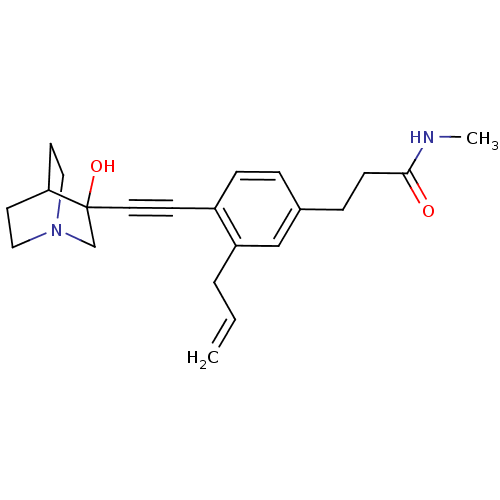 (3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | >250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268627 (CHEMBL496801 | N-Hydroxy-2-phosphono-5-(3-phenoxyp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268625 (CHEMBL447414 | N-[3-(3-Phenoxyphenyl)propyl]phosph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268510 (CHEMBL495624 | N-[3-(3-Phenoxyphenyl)propyl]phosph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268509 (CHEMBL495623 | N-[3-(3-(3,4-Dichlorophenoxy)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50388398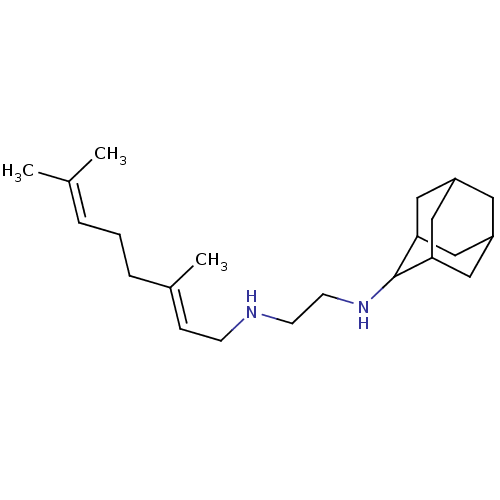 (CHEMBL561057 | SQ-109) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem | Article PubMed | 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human squalene synthase | J Med Chem 55: 4367-72 (2012) Article DOI: 10.1021/jm300208p BindingDB Entry DOI: 10.7270/Q2Z320QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268566 (CHEMBL497618 | N-Methyl-N-[3-(3-phenoxyphenyl)prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268511 (CHEMBL497634 | N-[3-(3-(4-Chlorophenoxy)phenyl)pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268562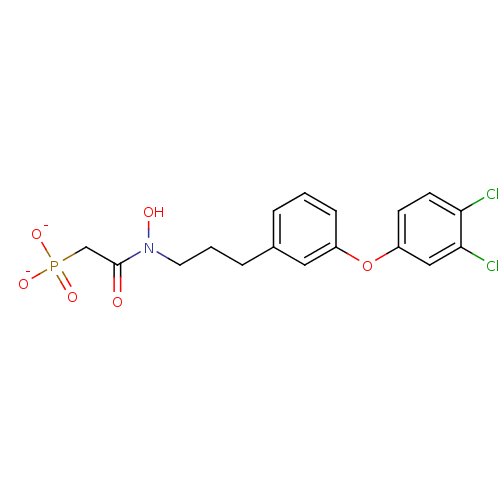 (CHEMBL497616 | N-Hydroxy-N-[3-(3-(3,4-dichlorophen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50366477 (CHEMBL69330) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against microsomal Squalene synthase | J Med Chem 34: 1912-4 (1991) Checked by Author BindingDB Entry DOI: 10.7270/Q2S75GXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268626 (CHEMBL524084 | N-[2-(3-Phenoxyphenyl)ethyl]phospho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50059865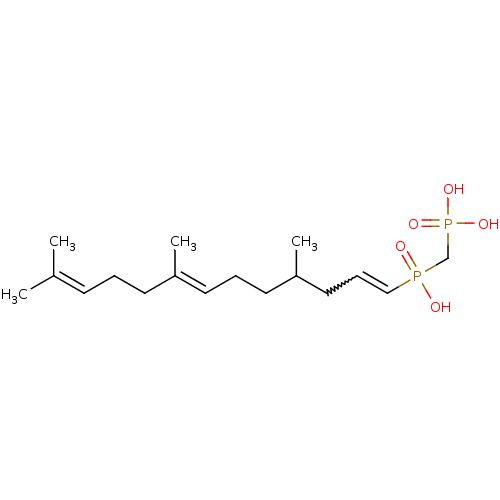 (4,8,12-trimethyl-(3E,7E)-3,7,11-tridecatrienylhydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against microsomal squalene synthase | J Med Chem 34: 1912-4 (1991) Checked by Author BindingDB Entry DOI: 10.7270/Q2S75GXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268676 (2-Oxo-6-(4-phenoxyphenyl)hexylphosphonic Acid Dipo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268677 (CHEMBL498628 | N-[3-(3-Phenoxyphenyl)propyl]phosph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268629 (3-(3-Phenoxyphenyl)propyl Phosphonoacetate Dipotas...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268679 (CHEMBL523897 | N-[3-(3-Phenoxyphenyl)propyl]phosph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268565 (CHEMBL497410 | N-[3-(3-Phenoxyphenyl)propyl]sulfoa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268564 (CHEMBL497815 | N-[3-(4-Biphenyl)propyl]phosphonoac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268563 (CHEMBL497617 | N-Hydroxy-N-[3-(3-phenoxyphenyl)pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268512 (3-(3-Phenoxyphenyl)propylphosphinylmethylphosphoni...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268678 (CHEMBL525377 | N-Hydroxy-N-[3-(4-methylbiphenyl)pr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268628 (CHEMBL496802 | N-[4-(3-Phenoxyphenyl)butyl]phospho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||